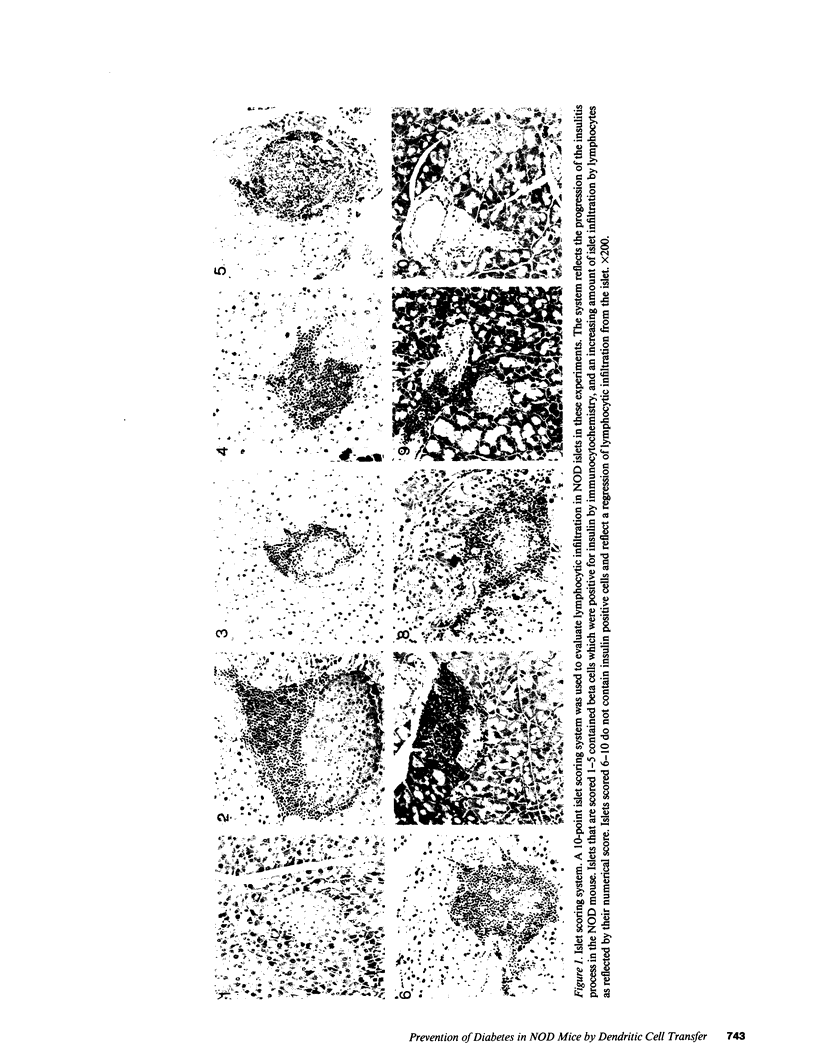
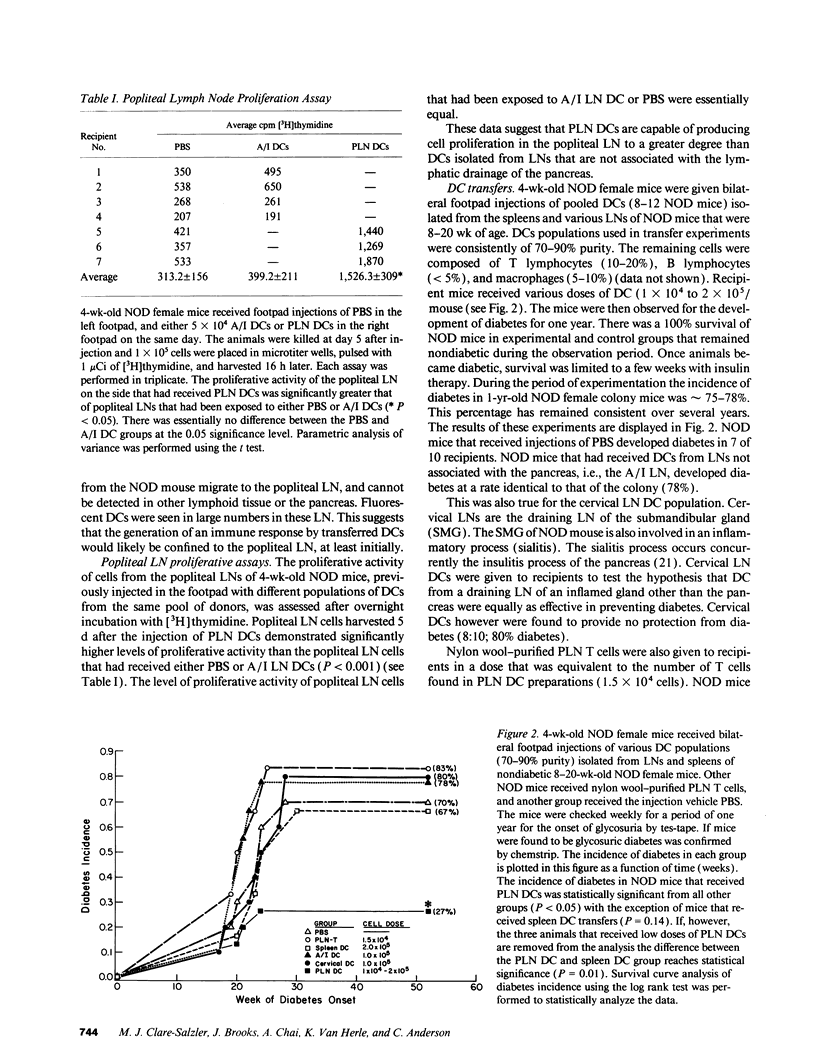
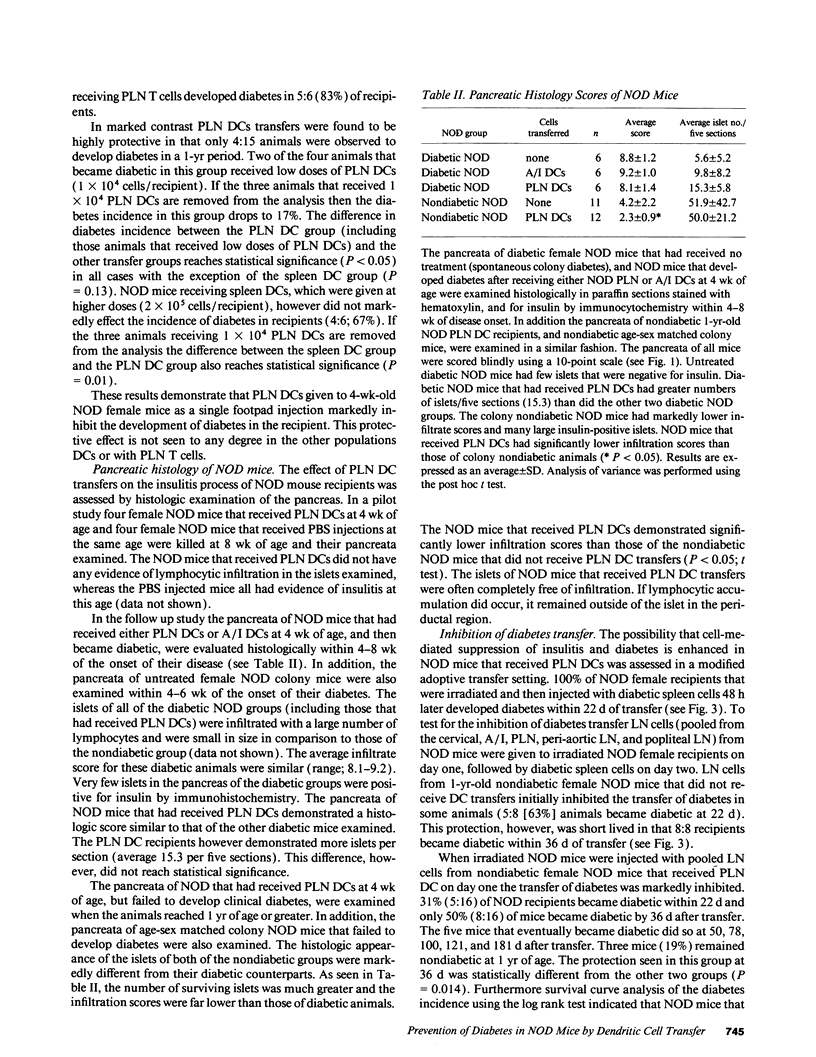
Abstract
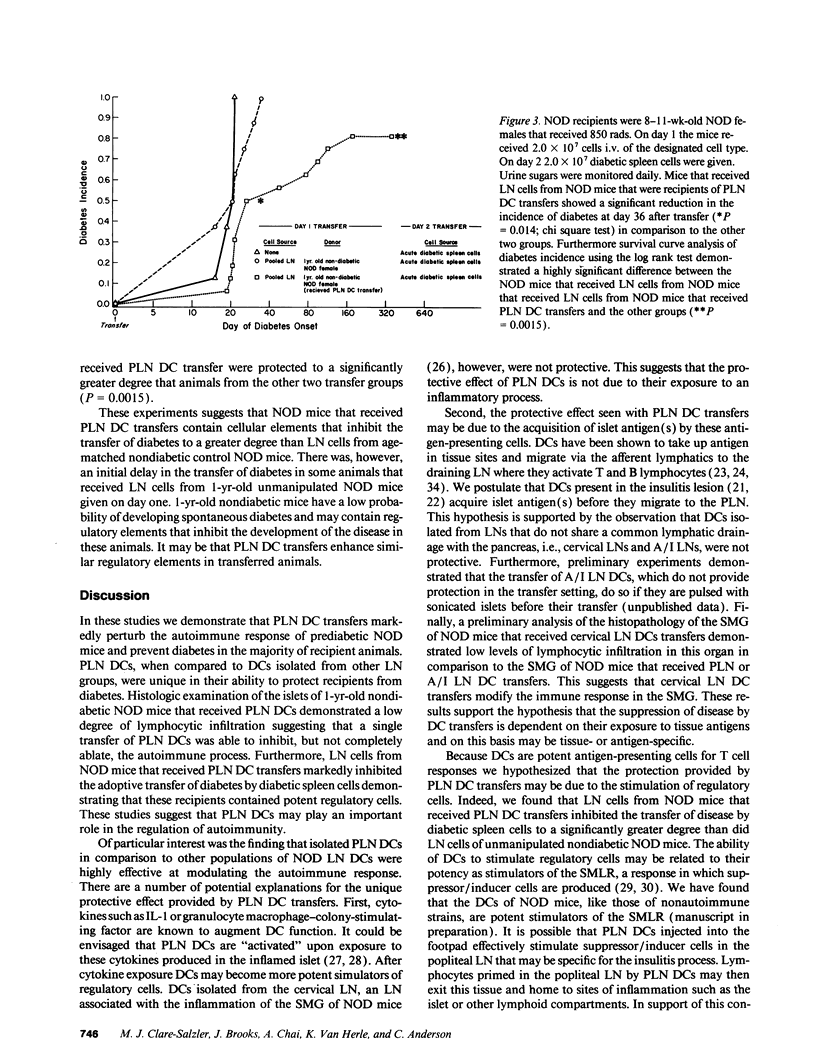
The purpose of this study was to determine the effect of dendritic cell (DC) transfers on the incidence of diabetes in female nonobese diabetic (NOD) mice. Groups of 4-wk-old NOD female mice were given a single foot pad of DCs (70-90% purity) isolated from the draining lymph nodes (LN) of the pancreas (PLN), the cervical LNs, or the axillary/inguinal LNs. In addition, other groups of NOD mice received purified spleen DCs, purified PLN T cells (the major contaminating population in DC preparations), or the injection vehicle PBS. All groups were monitored for diabetes for one year. Significant protection from diabetes was observed in NOD mice receiving greater than 1 x 10(4) PLN DCs in comparison to mice receiving other DCs populations, PLN T cells, or PBS (P less than 0.05). The pancreata of NOD mice that received PLN DCs demonstrated significantly lower levels of lymphocytic infiltration in the islets that age-sex matched nondiabetic female NOD control mice (P less than 0.05). LN cells from nondiabetic NOD mice that received PLN DC protected irradiated female recipients from the adoptive transfer of diabetes to a greater degree than LN cells from age and sex matched nondiabetic female NOD mice that did not receive PLN DC transfers at 36 d (P = 0.014) and at 1 yr (P = 0.0015) after transfer. These data suggest that the PLN DC transfers are able to modulate autoimmunity and limit diabetes expression in the NOD mouse. PLN DCs transfers may regulate autoimmunity by the induction of regulatory cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter A. G., Adams M. A., Mandel T. E. Comparison of high- and low-diabetes-incidence NOD mouse strains. Diabetes. 1989 Oct;38(10):1296–1300. doi: 10.2337/diab.38.10.1296. [DOI] [PubMed] [Google Scholar]
- Ben-Nun A., Yossefi S. Reversal of autoimmune encephalomyelitis by membranes presenting myelin basic protein-associated class II MHC molecule as an approach to immunotherapy of organ-specific autoimmune diseases. Eur J Immunol. 1990 Feb;20(2):357–361. doi: 10.1002/eji.1830200219. [DOI] [PubMed] [Google Scholar]
- Bocchieri M. H. T-suppressor clones derived from murine AMLR. Immunology. 1985 Sep;56(1):93–102. [PMC free article] [PubMed] [Google Scholar]
- Boitard C., Yasunami R., Dardenne M., Bach J. F. T cell-mediated inhibition of the transfer of autoimmune diabetes in NOD mice. J Exp Med. 1989 May 1;169(5):1669–1680. doi: 10.1084/jem.169.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley M., Inaba K., Steinman R. M. Dendritic cells are the principal cells in mouse spleen bearing immunogenic fragments of foreign proteins. J Exp Med. 1990 Jul 1;172(1):383–386. doi: 10.1084/jem.172.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eynon E. E., Parker D. C. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992 Jan 1;175(1):131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant J., Bryant A. E., Chan J., Himsworth R. L. Thyroglobulin-treated blood dendritic cells induce IgG anti-thyroglobulin antibody in vitro in Hashimoto's thyroiditis. Clin Immunol Immunopathol. 1986 Dec;41(3):433–442. doi: 10.1016/0090-1229(86)90014-0. [DOI] [PubMed] [Google Scholar]
- Faustman D., Eisenbarth G., Daley J., Breitmeyer J. Abnormal T-lymphocyte subsets in type I diabetes. Diabetes. 1989 Nov;38(11):1462–1468. doi: 10.2337/diab.38.11.1462. [DOI] [PubMed] [Google Scholar]
- Heufler C., Koch F., Schuler G. Granulocyte/macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med. 1988 Feb 1;167(2):700–705. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Metlay J. P., Crowley M. T., Steinman R. M. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990 Aug 1;172(2):631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Bedford P., Hunt R. The role of dendritic cells in the initiation of immune responses to contact sensitizers. II. Studies in nude mice. Cell Immunol. 1985 Sep;94(2):435–439. doi: 10.1016/0008-8749(85)90267-9. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Farrant J., Chan J., Bryant A., Bedford P. A., Bateman C. Induction of autoimmunity with dendritic cells: studies on thyroiditis in mice. Clin Immunol Immunopathol. 1988 Sep;48(3):277–289. doi: 10.1016/0090-1229(88)90021-9. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Hunt R., Dore C., Medawar P. B. Influence of dendritic cells on tumor growth. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4495–4497. doi: 10.1073/pnas.82.13.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight S. C., Mertin J., Stackpoole A., Clark J. Induction of immune responses in vivo with small numbers of veiled (dendritic) cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6032–6035. doi: 10.1073/pnas.80.19.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide S. L., Inaba K., Steinman R. M. Interleukin 1 enhances T-dependent immune responses by amplifying the function of dendritic cells. J Exp Med. 1987 Feb 1;165(2):515–530. doi: 10.1084/jem.165.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens J. W., Drexhage H. A., Benson W., Balfour B. M. A study of cells present in lymph draining from a contact allergic reaction in pigs sensitized to DNFB. Immunology. 1983 Jul;49(3):415–422. [PMC free article] [PubMed] [Google Scholar]
- Lohse A. W., Mor F., Karin N., Cohen I. R. Control of experimental autoimmune encephalomyelitis by T cells responding to activated T cells. Science. 1989 May 19;244(4906):820–822. doi: 10.1126/science.2471264. [DOI] [PubMed] [Google Scholar]
- Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980 Jan;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- Maron R., Zerubavel R., Friedman A., Cohen I. R. T lymphocyte line specific for thyroglobulin produces or vaccinates against autoimmune thyroiditis in mice. J Immunol. 1983 Nov;131(5):2316–2322. [PubMed] [Google Scholar]
- Matzinger P., Guerder S. Does T-cell tolerance require a dedicated antigen-presenting cell? Nature. 1989 Mar 2;338(6210):74–76. doi: 10.1038/338074a0. [DOI] [PubMed] [Google Scholar]
- Miyagawa J., Hanafusa T., Miyazaki A., Yamada K., Fujino-Kurihara H., Nakajima H., Kono N., Nonaka K., Tochino Y., Tarui S. Ultrastructural and immunocytochemical aspects of lymphocytic submandibulitis in the non-obese diabetic (NOD) mouse. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(3):215–225. doi: 10.1007/BF02899031. [DOI] [PubMed] [Google Scholar]
- Miyazaki A., Hanafusa T., Yamada K., Miyagawa J., Fujino-Kurihara H., Nakajima H., Nonaka K., Tarui S. Predominance of T lymphocytes in pancreatic islets and spleen of pre-diabetic non-obese diabetic (NOD) mice: a longitudinal study. Clin Exp Immunol. 1985 Jun;60(3):622–630. [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M. Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med. 1980 May 1;151(5):1196–1212. doi: 10.1084/jem.151.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Viruses as therapeutic agents. I. Treatment of nonobese insulin-dependent diabetes mice with virus prevents insulin-dependent diabetes mellitus while maintaining general immune competence. J Exp Med. 1990 Jun 1;171(6):2077–2089. doi: 10.1084/jem.171.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadelain M. W., Qin H. Y., Lauzon J., Singh B. Prevention of type I diabetes in NOD mice by adjuvant immunotherapy. Diabetes. 1990 May;39(5):583–589. doi: 10.2337/diab.39.5.583. [DOI] [PubMed] [Google Scholar]
- Satoh J., Seino H., Abo T., Tanaka S., Shintani S., Ohta S., Tamura K., Sawai T., Nobunaga T., Oteki T. Recombinant human tumor necrosis factor alpha suppresses autoimmune diabetes in nonobese diabetic mice. J Clin Invest. 1989 Oct;84(4):1345–1348. doi: 10.1172/JCI114304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serreze D. V., Hamaguchi K., Leiter E. H. Immunostimulation circumvents diabetes in NOD/Lt mice. J Autoimmun. 1989 Dec;2(6):759–776. doi: 10.1016/0896-8411(89)90003-6. [DOI] [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H. Defective activation of T suppressor cell function in nonobese diabetic mice. Potential relation to cytokine deficiencies. J Immunol. 1988 Jun 1;140(11):3801–3807. [PubMed] [Google Scholar]
- Voorbij H. A., Jeucken P. H., Kabel P. J., De Haan M., Drexhage H. A. Dendritic cells and scavenger macrophages in pancreatic islets of prediabetic BB rats. Diabetes. 1989 Dec;38(12):1623–1629. doi: 10.2337/diab.38.12.1623. [DOI] [PubMed] [Google Scholar]
- Wicker L. S., Miller B. J., Mullen Y. Transfer of autoimmune diabetes mellitus with splenocytes from nonobese diabetic (NOD) mice. Diabetes. 1986 Aug;35(8):855–860. doi: 10.2337/diab.35.8.855. [DOI] [PubMed] [Google Scholar]
- Wilberz S., Partke H. J., Dagnaes-Hansen F., Herberg L. Persistent MHV (mouse hepatitis virus) infection reduces the incidence of diabetes mellitus in non-obese diabetic mice. Diabetologia. 1991 Jan;34(1):2–5. doi: 10.1007/BF00404016. [DOI] [PubMed] [Google Scholar]
- Yamashita U., Ono S., Nakamura H. The syngeneic mixed leukocyte reaction in mice. II. The I region control of suppressor T cell activity induced in the syngeneic mixed leukocyte reaction. J Immunol. 1982 Mar;128(3):1010–1017. [PubMed] [Google Scholar]
- Zamoyska R., Waldmann H., Matzinger P. Peripheral tolerance mechanisms prevent the development of autoreactive T cells in chimeras grafted with two minor incompatible thymuses. Eur J Immunol. 1989 Jan;19(1):111–117. doi: 10.1002/eji.1830190118. [DOI] [PubMed] [Google Scholar]