Abstract
The Na/K-ATPase is the primary force regulating renal sodium handling and plays a key role in both ion homeostasis and blood pressure regulation. Recently, cardiotonic steroids (CTS)-mediated Na/K-ATPase signaling has been shown to regulate fibrosis, renal proximal tubule (RPT) sodium reabsorption, and experimental Dahl salt-sensitive hypertension in response to a high-salt diet. Reactive oxygen species (ROS) are an important modulator of nephron ion transport. As there is limited knowledge regarding the role of ROS-mediated fibrosis and RPT sodium reabsorption through the Na/K-ATPase, the focus of this review is to examine the possible role of ROS in the regulation of Na/K-ATPase activity, its signaling, fibrosis, and RPT sodium reabsorption.
1. Introduction
According to the American Heart Association (AHA), over 70 million people in the United States aged 20 and older have high blood pressure (BP). The cause of 90–95 percent of the cases of high BP is unknown, yet in the last decade the associated morbidity and mortality from high BP has increased precipitously. In the 2008 AHA Scientific Statement [1], excessive dietary salt intake is listed as one of the major lifestyle factors which significantly contributes to the development of hypertension and tends to be more pronounced in typical salt-sensitive patients. Modest dietary salt restriction and diuretic therapy, therefore, are recommended for treatment of resistant hypertension, especially in the salt-sensitive subgroup [1, 2]. Renal sodium handling is a key determinant of long-term BP regulation [3]. The relationship between dietary sodium, salt sensitivity, and BP has been established on an epidemiological and clinical basis. It is estimated that hypertension affects 25% to 35% of the world population aged 18 and older [4], and more hypertensive subjects (~50%) are significantly salt sensitive than normotensive subjects (~25%) [5]. In the DASH-Sodium clinical trial, BP reduction was correlated with sodium restriction in the salt-sensitive subjects regardless of diet [6]. Interestingly, animal renal cross-transplantation experiments [7–10] and studies of human renal transplantation [11] demonstrate that BP levels “travel with the donor's kidney,” providing compelling evidence for the role of renal function in the pathogenesis of hypertension. In clinical and experimental models, renal proximal tubule (RPT) sodium handling accounts for over 60% reabsorption of filtered sodium and is an independent determinant of BP response to salt intake, playing a critical role in the pathogenesis of salt-sensitive hypertension. Recently, accumulating data indicate that cardiotonic steroids (CTS) signaling through the Na/K-ATPase contribute to RPT sodium handling and salt sensitivity [12–18].
2. CTS and Na/K-ATPase Signaling
CTS (also known as endogenous digitalis-like substances) include plant-derived digitalis drugs, such as digoxin and ouabain, and vertebrate-derived aglycones such as bufalin and marinobufagenin (MBG) [16, 18]. Recent studies have identified both ouabain and MBG as endogenous steroids whose production, and secretion are regulated by stimuli including angiotensin II (Ang II) [18–21]. The Na/K-ATPase belongs to the P-type ATPases family and consists of two noncovalently linked α and β subunits [22–24]. Several α and β isoforms, expressed in a tissue-specific manner, have been identified and functionally characterized [22–25]. The α1 subunit contains multiple structural motifs that interact with soluble, membrane and structural proteins including Src, caveolin-1, phospholipase C-γ, PI3 kinase, IP3 receptor, BiP, calnexin, cofilin, and ankyrin [26–36]. Binding to these proteins not only regulates the ion-pumping function of the enzyme, but it also conveys signal-transducing functions to the Na/K-ATPase [18, 32, 37–39]. In LLC-PK1 cells, the Na/K-ATPase α1 subunit and Src form a functional receptor in which the binding of CTS to the α1 subunit activates Src and consequent signaling cascade [39]. The signaling function of the Na/K-ATPase regulates numerous cell functions in various types of organs and cells including cell motility, cell proliferation, cancer, endothelin release, glycogen synthesis, apoptosis, hypertension, intracellular calcium signaling, cardiac hypertrophy, cardiac remodeling, renal remodeling, epithelial cell tight junction, vascular tone homeostasis, and sodium homeostasis [26, 40–59]. The topic of CTS-Na/K-ATPase signaling and its downstream pathophysiological implications has been extensively reviewed in the last few years [12–16, 18, 21, 32, 39, 59–64], and we will not discuss them in detail in this review.
3. CTS and Sodium Homeostasis
Endogenous CTS were first proposed many years ago to function as a natriuretic hormone. Although their pathophysiological significance has been a subject of debate for many years [65], the concept of a natriuretic hormone is supported by the experimental observations in animal models of ouabain-induced natriuresis [66, 67]. Recently, several elegant reports have confirmed this concept with different approaches. Gene replacement studies have unequivocally demonstrated an important role of endogenous CTS in regulation of renal sodium excretion and BP [68, 69]. In transgenic mice expressing ouabain-sensitive Na/K-ATPase α 1 subunit, a significant observation is an augmented natriuretic response to both acute salt load and ouabain infusion, indicating that the ouabain-binding site of Na/K-ATPase α 1 subunit participates in the natriuretic response to salt load by responding to endogenous ouabain. Moreover, the augmented natriuretic response in the ouabain-sensitive α 1 isoform mice can be blocked with administration of an anti-digoxin antibody fragment [68, 69]. In normal male Wistar rats, endogenous circulatory ouabain has physiological roles controlling vasculature tone and sodium homeostasis, showing endogenous ouabain regulates renal sodium excretion in normal animals [70]. A significant inhibition of natriuresis is observed in rats that were passively immunized with anti-ouabain antibody and in rats that were actively immunized with ouabain-albumin antigen, in which endogenous ouabain levels were reduced in both cases. Like ouabain, MBG has both natriuretic and vasoconstrictor effects [12, 71, 72]. High salt intake induced an initial transient stimulation of ouabain and a subsequent progressive increase of MBG both in Dahl salt-sensitive (S) rats and in humans [12, 73]. In Dahl S rats, the increase in natriuresis stimulated by an acute salt loading is prevented by administration of both an anti-ouabain antibody and an anti-MBG antibody [72]. Furthermore, endogenous CTS have also been implied in age-related increases in salt sensitivity of BP in human normotensive subjects [73]. It is estimated that approximately 50% of humans with untreated essential hypertension have significantly elevated levels of endogenous ouabain [74] which is involved in the regulation of vascular tone homeostasis through stimulation of interaction between the Na/K-ATPase and sodium/calcium exchanger [59]. In normotensive human males, both a high salt diet and systematic sodium depletion (by hydrochlorothiazide) significantly increase plasma ouabain concentration [75].
Release of endogenous ouabain from the brain hypothalamus stimulated by a high salt diet leads to inhibition of the Na/K-ATPase activity and central sympathetic activation (reviewed in [76, 77]), which plays a crucial role in the pressor effects of high salt intake in spontaneously hypertensive rats (SHR) and Dahl S rats. In Dahl S rats, elevation in brain ouabain increased MBG secretion from the adrenal cortex, and this effect was blocked by the AT1 receptor antagonist losartan [78, 79]. The data suggest that the observed CTS-induced natriuretic effect might be a result of the combined effects of ouabain and MBG [60]. Furthermore, CTS not only induced hypertension in rats but also caused significant cardiovascular remodeling and natriuresis independent of their effect on BP [49, 51, 70, 80, 81].
4. CTS and Oxidative Stress in Cardiac and Renal Fibrosis
CTS binding to the Na/K-ATPase induces cellular reactive oxygen species (ROS) production and its downstream effects, such as cardiac and renal fibrosis, and these effects can be prevented by ROS scavenging [80, 82–84]. In kidney and heart, the central role of CTS in the development of fibrosis has been demonstrated both in vivo, in the partial (5/6th) nephrectomy model, and in vitro cell culture, including cardiac and renal fibroblasts. 5/6th nephrectomy increases circulating levels of MBG and stimulates cardiac fibrosis in both rat and mouse [80, 84, 85]. Rats subjected to 5/6th nephrectomy develop systemic oxidant stress that is similar to that seen in rats subjected to MBG infusion as evidenced by significant elevation of plasma carbonylated protein. Active immunization against MBG and reduction of circulating levels of MBG by adrenalectomy substantially attenuate 5/6th nephrectomy and MBG infusion-mediated cardiac fibrosis and oxidant stress, an effect that is independent of BP [80, 84]. In primary culture of rat cardiac and human dermal fibroblasts as well as a cell line derived from rat renal fibroblasts, MBG and ouabain stimulate [3H]proline incorporation as well as gene and protein expression of collagen [86]. MBG induced a PLC-dependant translocation of PKC-δ to the nucleus, resulting in the phosphorylation and degradation of transcription factor Friend leukemia integration-1 (Fli-1), a negative regulator of collagen synthesis [86]. Both CTS-induced Na/K-ATPase signaling and oxidative stress are necessary in the pathogenesis of cardiac and renal fibrosis as evidenced by CTS-stimulated phosphorylation of both Src and MAPK which is effectively blocked not only by ROS scavenging and Src inhibition but also through possible competitive inhibition of CTS binding to Na/K-ATPase by spironolactone and canrenone [80, 84, 87, 88]. MBG infusion causes renal fibrosis mainly in the cortex of the kidney by stimulation of the transcription factor Snail expression and its nuclear localization in the tubular epithelia, which is associated with epithelial-to-mesenchymal transition (EMT) during renal fibrosis [89]. This EMT phenomenon is also demonstrated in LLC-PK1 cells, indicating that MBG may cause damage of renal proximal tubules. CTS-induced fibrosis in heart and kidney might shift the cardiac and renal function curve to favor a higher set point of pressure natriuresis (Figure 1).
Figure 1.
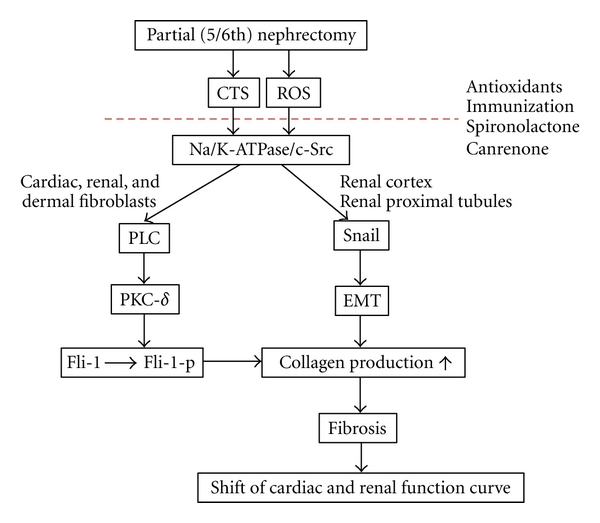
Schematic illustration of the effect of CTS on fibrosis.
5. Na/K-ATPase Signaling and RPT Sodium Handling
It has been postulated for decades that endogenous CTS stimulated by increased sodium intake increases natriuresis and diuresis by directly inhibiting renal tubular Na/K-ATPase to prevent renal reabsorption of filtered sodium [90–92]. There is accumulating evidence supporting this idea under conditions such as high salt intake, chronic renal sodium retention, renal ischemia, uremic cardiomyopathy, and volume expansion in various animal models and human beings [12, 13, 15, 60, 62, 75, 93–107]. Although the direct inhibition of the Na/K-ATPase enzymatic activity and sodium reabsorption in RPTs by CTS has not been validated, recent observations indicate that ligand-mediated RPT sodium reabsorption via Na/K-ATPase/c-Src signaling counterbalances the sodium retention-mediated increases in BP, such as that seen in salt-sensitive hypertension [108–114]. Ouabain, a ligand of the Na/K-ATPase, activates the Na/K-ATPase/c-Src signaling pathway and subsequently redistributes basolateral Na/K-ATPase and apical sodium/hydrogen exchanger isoform 3 (NHE3) in RPTs, leading to reduced RPT sodium reabsorption and increased urinary sodium excretion.
In LLC-PK1 cells, ouabain activates Na/K-ATPase/c-Src signaling pathways and reduces cell surface Na/K-ATPase and NHE3, leading to a significant inhibition of active transcellular 22Na+ flux from the apical to basolateral compartment [108–112]. MBG, an important CTS species, and deproteinated extract of serum (derived from patients with chronic renal failure) also induce Na/K-ATPase endocytosis and inhibition of active transepithelial 22Na+ flux. However, it is still not clear how ouabain-activated Na/K-ATPase signaling regulates NHE3, since, at concentrations of ouabain used, no significant change in intracellular Na+ concentration was observed [108]. Interestingly, this phenomenon is either not observed or is much less significant in MDCK cells (a canine renal distal tubule cell line). These in vitro data suggest that CTS-Na/K-ATPase signaling has a profound effect on RPT sodium handling, but not in distal tubules.
Different species of endogenous CTS show differences in the kinetics and tissue actions in response to salt loading in both animal models and in human salt-sensitive hypertensive patients [16, 18, 60, 75, 79, 105]. The Dahl salt-resistant (R) and salt-sensitive (S) strains were developed by selective breeding of the outbred Sprague-Dawley rat strain for resistance or susceptibility to the hypertensive effects of high dietary sodium [115]. RPT sodium handling is a critical determinant of the different BP responses in these strains [7, 116–118], and there is no Na/K-ATPase α1 gene (Atp1a1) difference between R and S rats [119]. In comparison to Dahl S rats that eliminate excessive sodium mainly through pressure natriuresis at the expense of an elevated systolic BP, a major response to salt loading in the Dahl R rats is a greater reduction in renal sodium reabsorption to eliminate excessive sodium without raising BP. Our recent in vivo observation [114] is in agreement with this hypothesis and our in vitro observations in LLC-PK1 cells. Specifically in isolated RPTs, both a high salt diet and ouabain are able to activate Na/K-ATPase/c-Src signaling pathways, leading to the redistribution of Na/K-ATPase and NHE3 in the Dahl R but not in the S rats. The R rats show significant increases in total urinary sodium excretion and RPT-mediated fractional sodium excretion, without BP elevation [114]. While the BP response to salt loading in R and S rats involves many regulatory factors [117], our data indicate that impairment of the RPT Na/K-ATPase/c-Src signaling contributes to salt sensitivity. Since it failed to confirm the possible difference of Na/K-ATPase α1 gene (Atp1a1) between R and S rats [119], other factor(s) must be present to prevent activation of Na/K-ATPase/c-Src signaling in the S rats. The possible effect of CTS on RPT Na/K-ATPase signaling and sodium reabsorption is summarized in Figure 2.
Figure 2.
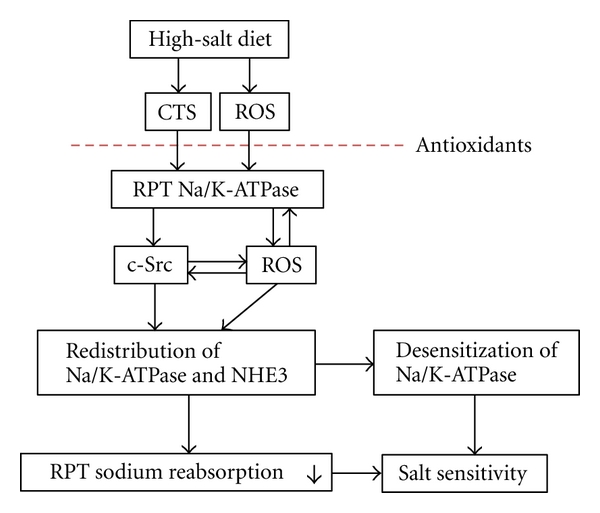
Schematic illustration of the effects of ROS and Na/K-ATPase signaling on RPT sodium reabsorption. CTS: cardiotonic steroids; RPT: renal proximal tubules.
The SHR rat is an established model of human essential hypertension with the characteristic of vascular resistance. SHR rat develops hypertension spontaneously at the age of 7–15 weeks, regardless of salt loading, mainly through increase in peripheral vascular resistance [120] including renal vascular resistance. Interestingly, either reduction of dietary vitamin E or caloric restriction without sodium restriction prevents the development of hypertension [121, 122]. Like Dahl S rats, SHR rats eliminate excessive sodium via a pressure-natriuresis mechanism but have significant lower BP response to a high salt diet and higher systolic BP to eliminate the same amount of sodium when compared to Dahl S rats [123–126]. Most interestingly, comparing to control Wistar-Kyoto (WKY) rats, regulation of the Na/K-ATPase and NHE3 with both aging and oxidative stress have been shown contributed to the development and maintenance of hypertension in the SHR [127–135]. In renal cortical (proximal) tubules, activity and protein levels of NHE-3 are significantly higher in SHR than age-matched WKY rats at all stages during the development and maintenance of hypertension. When NHE3 function is determined by the rate of bicarbonate reabsorption by in vivo stationary microperfusion in RPT, young SHR rats show higher NHE3 activity than adult SHR and WKY rats, and this is accompanied by changes in NHE3 phosphorylation and distribution. In young SHR rats, the RPT Na/K-ATPase activity is significantly higher than in age-matched WKY, which can be prevented by treatment of the diuretic drug hydrochlorothiazide. However, the Na-K-ATPase activity in medullary thick ascending limb, cortical thick ascending limb, and distal tubule is significantly lower in young SHR rats than in age-matched WKY rats. There were no significant differences in Na/K-ATPase activity in these nephron segments in adult SHR and WKY rats, nor in collecting duct segments of young and adult SHR and age-matched WKY rats. Interestingly, however, the Na/K-ATPase α 1 subunit gene expression is lower in both young and adult SHR rats than age-matched WKY rats, indicating a posttranscriptional regulation.
Recent study indicates that SHR rats have enhanced renal superoxide generation and NADPH oxidase (NOX) expression in both vascular and renal tissue before and after development of hypertension [136]. Elevated basal level of superoxide inhibits proximal tubule NHE3 activity and fluid reabsorption in SHR rats in comparison to WKY rats. This effect is prevented by the NOX inhibitor apocynin or knockdown of the critical NOX subunit p22phox with small interfering RNA, indicating that increased basal level of superoxide impairs RPT function [137]. Furthermore, in Sprague-Dawley rats, oxidative stress impairs Ang II-mediated regulation of NHE3 [138].
6. Oxidative Stress and Regulation of Na/K-ATPase
Many stimuli including hypoxia and dopamine induce a cell- and tissue-specific endocytosis and exocytosis of Na/K-ATPase and a change in Na/K-ATPase activity [33, 61, 139]. Ouabain-stimulated ROS generation functions as a second messenger in ouabain-activated Na/K-ATPase signaling in isolated rat cardiac myocytes [82, 140–142]. Binding of ouabain to the Na/K-ATPase activates Src kinase, which in turn transactivates EGFR, leading to activation of the Ras-Raf-MEK-ERK pathway [32, 39, 63]. Ras activation leads to the activation of MAPK and increase in [Ca2+]i which result in opening of mitochondrial ATP-sensitive K+ channels [142] and generation of mitochondrial ROS [82, 141]. ROS subsequently activate NF-κB [141, 143] and slow [Ca2+]i oscillations at nanomolar ouabain concentrations [40]. Additionally, ouabain-induced generation of ROS in neonatal myocytes is antagonized by overexpression of a dominant negative Ras as well as myxothiazol/diphenyleneiodonium, indicating a mitochondrial origin of the Ras-dependent ROS generation [82]. Ouabain also stimulates ROS generation in other cell types [144–146]. Conversely, oxidative stress can activate the Na/K-ATPase signaling. Both a bolus of H2O2 and exogenously added glucose oxidase (which generates a sustained low level of H2O2 by consuming glucose) activates Na/K-ATPase signaling in cardiac myocytes [140]. Pretreatment with the antioxidant N-acetyl cysteine (NAC) prevents ouabain-Na/K-ATPase signaling and its downstream effects. Moreover, infusion of CTS causes ROS generation and protein oxidation in experimental animals [80, 147].
The redox sensitivity of the Na/K-ATPase was first demonstrated in electric eels with treatment of H2O2 [148]. This phenomenon was further observed in a wide range of animal species, tissues, and other species of ROS such as hypochlorous anion, hydroxyl radicals, superoxide, hyperchlorite anions, and singlet oxygen. It has been shown that the Na/K-ATPase in skeletal muscle is redox-sensitive and infusion of NAC attenuated ROS-mediated inhibition of maximal pump activity [149]. In dog kidney, oxidative modification of kidney Na/K-ATPase by H2O2 was accompanied by a decrease in the amount of sulfhydryl (SH) groups [150] and, importantly, oxidative modification can result in formation of Na/K-ATPase oligomeric structure [151]. The differences in the number, location, and accessibility of SH groups in Na/K-ATPase isozymes might predict their oxidative stability [152]. Different antioxidant enzymes, natural or synthetic antioxidants, and some inhibitors of oxidase activity can attenuate ROS mediated inhibition of Na/K-ATPase activity. ROS are known to inhibit Na/K-ATPase activity in different types of cells as well. Interestingly, the Na/K-ATPase in rat cerebellar granule cells are redox-sensitive with an “optimal redox potential range,” where ROS levels out of this “optimal range” are capable of inhibiting Na/K-ATPase activity [153]. While the Na/K-ATPase does not contain heme groups, it does contain cysteine residues located in α subunit cytosolic loops which may determine the redox sensitivity of the α subunit. The sensitivity of the Na/K-ATPase to redox and oxygen status, the regulatory factors which govern these interactions, and the implied molecular mechanism were recently reviewed [154].
Increases in ROS can oxidize the Na/K-ATPase α/ β subunits and its independent regulator FXYD proteins. This oxidation inhibits its activity and promotes its susceptibility to degradation by proteasomal and endosomal/lysosomal proteolytic pathways in different cell types including cardiac myocytes, vascular smooth muscle cells, and RPTs [155–166]. It appears that the oxidized modification of the Na/K-ATPase is a reversible, redox-sensitive modification. However, purified enzyme has also been shown to be irreversibly inhibited upon exposure to hydrogen peroxide, the superoxide anion, and the hydroxyl radical [158]. The regulation of renal Na/K-ATPase α 1 by oxidants is not dependent on the ouabain sensitivity of the α 1 subunit per se. It appears that the α 2 and α 3 subunits are more sensitive to oxidants than the α 1 subunit, and ouabain-sensitive α 1 (canine) and insensitive- α 1 (rat) have similar sensitivity to oxidants, suggesting the regulation of α 1 by ROS is not species specific. Furthermore, ROS accelerates degradation of the oxidatively damaged Na/K-ATPase and the α 2 and α 3 subunits, which also appear to be more susceptible to degradation than the α 1 subunit [159]. These studies indicate that differential oxidant sensitivities of the Na/K-ATPase subunits are dictated by the primary sequences of different subunits and different subunit compositions of the various tissues may contribute to their relative susceptibilities to oxidant stress. In the RPT cell line originated from WKY rats, cadmium, a ROS generator, stimulates ROS production and causes a toxic oxidative damage of the Na/K-ATPase. Oxidative damage increases Na/K-ATPase degradation through both the proteasomal and endo-/lysosomal proteolytic pathways [163]. In purified renal Na/K-ATPase, peroxynitrite (ONOO−) causes tyrosine nitration and cysteine thiol group modification of the Na/K-ATPase, but only cysteine thiol group modification is implied in the inhibition of the enzyme activity since glutathione is unable to reverse the inhibition [167]. In isolated rat renal RPTs, peroxynitrite and its signaling participates in Ang II-induced regulation of renal Na/K-ATPase activity [168].
Ang II inhibits the Na/K-ATPase via PKC-dependent NOX activation. The dependence of Ang II-induced Na/K-ATPase inhibition on NOX and superoxide, as well as reversible oxidative modification of the Na/K-ATPase, strongly suggests a role for redox signaling [164–166]. In cardiac myocytes and pig kidney, oxidative stress induces glutathionylation of the β1 subunit of the Na/K-ATPase. In purified pig renal Na/K-ATPase, peroxynitrite inhibits the enzymatic activity by stabilization of the enzyme in an E2-prone conformation. At the same time, FXYD proteins reverse oxidative stress-induced inhibition of the Na/K-ATPase by facilitating deglutathionylation of the β1 subunit. Moreover, both tyrosine kinase c-Src and cell membrane structural component lipid rafts, which are critical in Na/K-ATPase/c-Src signaling, are also critical in redox signaling platform formation [169–172]. These observations, along with the fact that c-Src is redox-sensitive [173] and its activation regulates NOX-derived superoxide generation [174], suggests a redox-sensitive Na/K-ATPase/c-Src signaling cascade and its possible role in ROS regulation, although the mechanism is not clear. Our unpublished data suggest that certain basal levels of ROS might be required for the initiation of ouabain-Na/K-ATPase/c-Src signaling. In LLC-PK1 cells, pretreatment with higher concentrations of NAC (5 and 10 mM for 30 min), but not with lower concentration of 1 mM, can prevent ouabain-stimulated c-Src activation and the redistribution of Na/K-ATPase and NHE3. This suggests that ROS may stabilize Na/K-ATPase in a certain conformational status in order to facilitate ouabain binding to the Na/K-ATPase α 1 subunit and favor ouabain-Na/K-ATPase/c-Src signaling. Some pertinent questions remain to be resolved, such as how ROS interacts with and influences the Na/K-ATPase/c-Src signaling cascade and whether ouabain-induced ROS boosts the Na/K-ATPase signaling by a positive feedback mechanism and chronically desensitizes the signaling cascade by stimulating Na/K-ATPase/c-Src endocytosis (Figure 2).
7. Oxidative Stress and Regulation of Renal Function
ROS function as important intracellular and extracellular second messengers to modulate many signaling molecules. Physiological concentrations of ROS play an important role in normal redox signaling, while pathological levels of ROS contribute to renal and vascular dysfunction and remodeling through oxidative damage [175–177]. Genetic factor(s) partially contribute to high basal ROS levels and the development of hypertension [178, 179].
Oxidative stress has been shown to regulate BP and sodium handling in various animal models. High salt intake increases oxidative stress, and this has important implications for the regulation of cardiovascular and renal systems. An increase in oxidative stress is both a cause and consequence of hypertension [177, 180–183]. Renal NOX is present in the renal cortex, medulla, and vasculature. NOX, the major source of superoxide in the kidney, is of particular interest because of its prominent expression and implication in pathophysiology. In the kidney, increased oxidative stress influences a number of physiologic processes including renal sodium handling in the proximal tubule [137, 168, 184, 185] and thick ascending limb [186], renal medulla blood flow [183, 187–190], descending vasa recta contraction [191, 192] in addition to interactions with other regulatory systems such as the dopamine signaling pathway [193]. Increased oxidative stress has been shown to contribute to salt sensitivity [194, 195]. In macula densa cells, NOX isoform Nox2 is responsible for salt-induced superoxide generation, while Nox4 regulates basal ROS [196]. In the medullar thick ascending limb of the loop of Henle, both exogenous and endogenous superoxides stimulate sodium absorption [197]. Also, in this nephron segment, NOX-induced generation of superoxide enhances sodium absorption by reduction of the bioavailability of nitric oxide (NO) to prevent NO-induced reduction of NaCl absorption [198], which contributes to salt-sensitive hypertension observed in Dahl salt-sensitive rats [190].
In PRTs, in particular, increases in ROS inhibit the Na/K-ATPase as well as the apical NHE3 and sodium/glucose cotransporter, in order to promote RPT sodium excretion under certain circumstances [137, 168, 184, 185]. While elevated basal level of superoxide inhibits proximal tubule NHE3 activity and fluid reabsorption in SHR rats in comparison to WKY rats [137], peroxynitrite and its signaling participates in Ang II-induced regulation of renal Na/K-ATPase activity in isolated rat renal RPTs [168]. In the pathogenesis of diabetic nephropathy, a high level of glucose-induced ROS generation induced by stimulation of mitochondrial metabolism and NOX activity in RPT primary cultures leads to inhibition of the expression and activity of the sodium/glucose cotransport system [185]. In male Wistar rats, a high salt diet (3% NaCl for 2 weeks) promotes sodium/water excretion and urinary 8-isoprostane excretion, a marker of oxidative stress, which can be attenuated by treatment with apocynin, an NOX inhibitor. The salt loading leads to increased generation of ROS and a state of oxidative stress in the cortex but not to such a degree in the medulla [199].
RPT sodium and/or fluid absorption in the normal rat is reduced by inhibition of NO synthesis, while NO promotes RPT Na+ and/or fluid reabsorption [200]. In immortalized and freshly isolated RPTs from the WKY and SHR rats, the basal level of membrane NOX activity is greater in SHRs [201]. Moreover, NOX-induced superoxide generation inhibits RPT fluid reabsorption in SHRs [137]. In Sprague-Dawley rats treated with the oxidant L-buthionine sulfoximine, Ang II overstimulates RPT Na/K-ATPase and NOX and leads to increased sodium reabsorption, which is prevented by administration of the superoxide scavenger Tempol [202].
High salt diet, which is well documented for its stimulation of systematic oxidative stress, regulates the activity and distribution of the Na/K-ATPase and NHE3 in different animal models [203–207]. PRT sodium reabsorption significantly affects water and sodium homeostasis by regulating redistribution of ion transporters in response to high salt intake [208]. Regulation of NHE3 activity and distribution as well as PRT fluid reabsorption contribute to the development and maintenance of hypertension in young and adult SHR rats [127, 137, 209].
It has become clear that antioxidant agents such as Tempol and enzymes such as heme oxygenase-1 (HO-1) exhibit a beneficial and protective effect on BP in various animal models of hypertension. As an example, inhibition of HO activity reduces renal medullary blood flow [84, 210, 211], total renal blood flow (RBF) [212], glomerular filtration rate (GFR), and renal production of nitric oxide [213]. Inhibition of HO-1 increases mean arterial pressure in Sprague-Dawley rats [214, 215] and SHR rats [216]. Induction of HO-1 reduces BP in SHR rats [217–220]. The effect of HO-1 on BP is presumably through the carbon monoxide (CO)/HO system and depression of cytochrome p-450-derived 20-HETE. In the Dahl salt-dependent model of systemic hypertension, induction of HO-1 occurred in the vasculature and is accompanied by endothelial dysfunction [221, 222]. In Dahl salt-sensitive rats, a high salt diet increases HO-1 expression and CO generation in aorta and coronary arteries [221, 223], and, in Dahl salt-sensitive rats fed low salt diet, induction of HO-1 expression attenuates oxidative stress and reduces proteinuria and renal injury [224]. However, most studies examining the contribution of HO-1 to BP regulation have focused on the vasculature, that is, pressure-natriuresis and arterial BP, and so the role of HO-1 in RPT-mediated sodium handling is still poorly understood. Nevertheless, increased HO expression in RPTs could result in an increased ability to buffer locally produced oxidants, leading to their neutralization.
The beneficial effect of antioxidants is controversial and not seen in most clinic trials with administration of antioxidants (reviewed in [177, 225]). The Dietary Approaches to Stop Hypertension (DASH) study and subsequent studies have demonstrated that lower BP associated with reduced dietary salt intake may be related to reductions in oxidative stress [6, 226–228]. Interestingly, however, while a combination antioxidant supplement (with an ascorbic acid, synthetic vitamin E, and β-carotene) had no improvement on BP after 5-year treatment [229], another combination antioxidant supplement (zinc, ascorbic acid, α-tocopherol, and β-carotene) did result in a significant reduction in systolic BP [230]. Other studies have also shown that certain antioxidants, such as glutathione and vitamin C, have a blood-pressure-lowering effect [231, 232]. However, antioxidant supplementation may be ineffective or even dangerous [233] due to the possible “over-antioxidant-buffering” effect of excessive antioxidant supplementation. In this scenario, excess antioxidants might become pro-oxidants (by providing H+) if they cannot promptly be reduced by the following antioxidant in the biological antioxidant chain. Thus, it appears that the balance of the ROS status, within a physiological range, may be more important to maintain beneficial ROS signaling.
8. Perspective
Renal sodium handling is a key determinant of blood pressure. ROS status, among others, is an important regulator of vasculature and sodium handling. However, the effect of ROS and Na/K-ATPase, especially of their interaction, on RPT sodium reabsorption has only been explored in a limited fashion. Coordinated regulation of two major ion transporters, the basolateral Na/K-ATPase and the apical NHE3, has been implicated in the counterbalancing of high salt intake (volume expansion) mediated blood pressure increase. The Na/K-ATPase/c-Src signaling regulates this coordinated regulatory mechanism and impairment of this signaling cascade contributes to experimental Dahl salt-sensitive hypertension. Both the Na/K-ATPase (α and β subunit) and its proximal signaling partner c-Src are redox sensitive, suggesting a redox-sensitive Na/K-ATPase/c-Src signaling complex. However, the mechanisms remain largely to be elucidated since the available data is limited [184, 234]. Nevertheless, some pertinent questions remain to be addressed. In the future, it will be important to explore whether ROS signaling is a link between the Na/K-ATPase/c-Src cascade and NHE3 regulation and how oxidative stress stimulated by high salt and CTS regulates Na/K-ATPase/c-Src signaling in renal sodium handling and fibrosis.
References
- 1.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: siagnosis, evaluation, and treatment a scientific statement from the american heart association professional education committee of the council for high blood pressure research. Hypertension . 2008;51(6):1403–1419. doi: 10.1161/HYPERTENSIONAHA.108.189141. . [DOI] [PubMed] [Google Scholar]
- 2.He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database of Systematic Reviews . 2004;(3) doi: 10.1002/14651858.CD004937. Article ID CD004937. [DOI] [PubMed] [Google Scholar]
- 3.Guyton AC. Blood pressure control—special role of the kidneys and body fluids. Science . 1991;252(5014):1813–1816. doi: 10.1126/science.2063193. . [DOI] [PubMed] [Google Scholar]
- 4.Staessen JA, Wang J, Bianchi G, Birkenhäger WH. Essential hypertension. Lancet . 2003;361(9369):1629–1641. doi: 10.1016/S0140-6736(03)13302-8. . [DOI] [PubMed] [Google Scholar]
- 5.Weinberger MH, Miller JZ, Luft FC. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension . 1986;8(6):II127–II134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. . [DOI] [PubMed] [Google Scholar]
- 6.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. New England Journal of Medicine . 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. . [DOI] [PubMed] [Google Scholar]
- 7.Dahl LK, Heine M, Thompson K. Genetic influence of the kidneys on blood pressure. Evidence from chronic renal homografts in rats with opposite predispositions to hypertension. Circulation Research . 1974;40(4):94–101. doi: 10.1161/01.res.40.4.94. . [DOI] [PubMed] [Google Scholar]
- 8.Bianchi G, et al. The hypertensive role of the kidney in spontaneously hypertensive rats. Clinical Science and Molecular Medicine . 1973;23(45, Supplement 1):S135–S139. doi: 10.1042/cs045135s. . [DOI] [PubMed] [Google Scholar]
- 9.Heller J, Schubert G, Havlickova J, Thurau K. The role of the kidney in the development of hypertension: a transplantation study in the Prague hypertensive rat. Pflugers Archiv European Journal of Physiology . 1993;425(3-4):208–212. doi: 10.1007/BF00374168. . [DOI] [PubMed] [Google Scholar]
- 10.Morgan DA, DiBona GF, Mark AL. Effects of interstrain renal transplantation of NaCl-induced hypertension in Dahl rats. Hypertension . 1990;15(4):436–442. doi: 10.1161/01.hyp.15.4.436. . [DOI] [PubMed] [Google Scholar]
- 11.Curtis JJ, Luke RG, Dustan HP. Remission of essential hypertension after renal transplantation. New England Journal of Medicine . 1983;309(17):1009–1015. doi: 10.1056/NEJM198310273091702. . [DOI] [PubMed] [Google Scholar]
- 12.Bagrov AY, Shapiro JI. Endogenous digitalis: pathophysiologic roles and therapeutic applications. Nature Clinical Practice Nephrology . 2008;4(7):378–392. doi: 10.1038/ncpneph0848. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckalew VM. Endogenous digitalis-like factors. An historical overview. Frontiers in Bioscience . 2005;10(1):2325–2334. doi: 10.2741/1701. . [DOI] [PubMed] [Google Scholar]
- 14.Fedorova OV, Shapiro JI, Bagrov AY. Endogenous cardiotonic steroids and salt-sensitive hypertension. Biochimica et Biophysica Acta . 2010;1802(12):1230–1236. doi: 10.1016/j.bbadis.2010.03.011. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Xie ZJ. The sodium pump and cardiotonic steroids-induced signal transduction protein kinases and calcium-signaling microdomain in regulation of transporter trafficking. Biochimica et Biophysica Acta . 2010;1802(12):1237–1245. doi: 10.1016/j.bbadis.2010.01.013. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoner W, Scheiner-Bobis G. Role of endogenous cardiotonic steroids in sodium homeostasis. Nephrology Dialysis Transplantation . 2008;23(9):2723–2729. doi: 10.1093/ndt/gfn325. . [DOI] [PubMed] [Google Scholar]
- 17.Meneton P, Jeunemaitre X, De Wardener HE, Macgregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiological Reviews . 2005;85(2):679–715. doi: 10.1152/physrev.00056.2003. . [DOI] [PubMed] [Google Scholar]
- 18.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. American Journal of Physiology, Cell Physiology . 2007;293(2):C509–C536. doi: 10.1152/ajpcell.00098.2007. . [DOI] [PubMed] [Google Scholar]
- 19.Hamlyn JM, Blaustein MP, Bova S, et al. Identification and characterization of a ouabain-like compound from human plasma. Proceedings of the National Academy of Sciences of the United States of America . 1991;88(14):6259–6263. doi: 10.1073/pnas.88.14.6259. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laredo J, Shah JR, Lu ZR, Hamilton BP, Hamlyn JM. Angiotensin II stimulates secretion of endogenous ouabain from bovine adrenocortical cells via angiotensin type 2 receptors. Hypertension . 1997;29(1):401–407. doi: 10.1161/01.hyp.29.1.401. . [DOI] [PubMed] [Google Scholar]
- 21.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacological Reviews . 2009;61(1):9–38. doi: 10.1124/pr.108.000711. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweadner KJ. Isozymes of the Na+/K+-ATPase. Biochimica et Biophysica Acta . 1989;988(2):185–220. doi: 10.1016/0304-4157(89)90019-1. . [DOI] [PubMed] [Google Scholar]
- 23.Kaplan JH. Biochemistry of Na,K-ATPase. Annual Review of Biochemistry . 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. . [DOI] [PubMed] [Google Scholar]
- 24.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. American Journal of Physiology . 1998;275(5):F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. . [DOI] [PubMed] [Google Scholar]
- 25.Sanchez G, Nguyen ANT, Timmerberg B, Tash JS, Blanco G. The Na,K-ATPase α4 isoform from humans has distinct enzymatic properties and is important for sperm motility. Molecular Human Reproduction . 2006;12(9):565–576. doi: 10.1093/molehr/gal062. . [DOI] [PubMed] [Google Scholar]
- 26.Barwe SP, Anilkumar G, Moon SY, et al. Novel role for Na,K-ATPase in phosphatidylinositol 3-kinase signaling and suppression of cell motility. Molecular Biology of the Cell . 2005;16(3):1082–1094. doi: 10.1091/mbc.E04-05-0427. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beggah AT, Geering K. α and β subunits of Na,K-ATPase interact with BiP and calnexin. Annals of the New York Academy of Sciences . 1997;834:537–539. doi: 10.1111/j.1749-6632.1997.tb52311.x. . [DOI] [PubMed] [Google Scholar]
- 28.Feschenko MS, Wetzel RK, Sweadner KJ. Phosphorylation of Na,K-ATPase by protein kinases. Sites, susceptibility, and consequences. Annals of the New York Academy of Sciences . 1997;834:479–488. doi: 10.1111/j.1749-6632.1997.tb52306.x. . [DOI] [PubMed] [Google Scholar]
- 29.Jordan C, Püschel B, Koob R, Drenckhahn D. Identification of a binding motif for ankyrin on the α-subunit of Na+,K+-ATPase. Journal of Biological Chemistry . 1995;270(50):29971–29975. doi: 10.1074/jbc.270.50.29971. . [DOI] [PubMed] [Google Scholar]
- 30.Lee K, Jung J, Kim M, Guidotti G. Interaction of the α subunit of Na,K-ATPase with, cofilin. Biochemical Journal . 2001;353(2):377–385. doi: 10.1042/0264-6021:3530377. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian J, Cai T, Yuan Z, et al. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Molecular Biology of the Cell . 2006;17(1):317–326. doi: 10.1091/mbc.E05-08-0735. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Z, Cai T. Na+-K+–ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv . 2003;3(3):157–168. doi: 10.1124/mi.3.3.157. . [DOI] [PubMed] [Google Scholar]
- 33.Yudowski GA, Efendiev R, Pedemonte CH, Katz AI, Berggren PO, Bertorello AM. Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+,K+-ATPase α subunit and regulates its trafficking. Proceedings of the National Academy of Sciences of the United States of America . 2000;97(12):6556–6561. doi: 10.1073/pnas.100128297. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Devarajan P, Dorfman AL, Morrow JS. Structure of the ankyrin-binding domain of α-Na,K-ATPase. Journal of Biological Chemistry . 1998;273(30):18681–18684. doi: 10.1074/jbc.273.30.18681. . [DOI] [PubMed] [Google Scholar]
- 35.Song H, Lee MY, Kinsey SP, Weber DJ, Blaustein MP. An N-terminal sequence targets and tethers Na+ pump α2 subunits to specialized plasma membrane microdomains. Journal of Biological Chemistry . 2006;281(18):12929–12940. doi: 10.1074/jbc.M507450200. . [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Malmersjö S, Li J, et al. Distinct role of the N-terminal tail of the Na,K-ATPase catalytic subunit as a signal transducer. Journal of Biological Chemistry . 2006;281(31):21954–21962. doi: 10.1074/jbc.M601578200. . [DOI] [PubMed] [Google Scholar]
- 37.Kaplan JH. A moving new role for the sodium pump in epithelial cells and carcinomas. Science’s STKE . 2005;2005(289):p. pe31. doi: 10.1126/stke.2892005pe31. . [DOI] [PubMed] [Google Scholar]
- 38.Kaunitz JD. Membrane transport proteins: not just for transport anymore. American Journal of Physiology . 2006;290(5):F995–F996. doi: 10.1152/ajprenal.00515.2005. . [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Xie Z. The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflugers Archiv European Journal of Physiology . 2009;457(3):635–644. doi: 10.1007/s00424-008-0470-0. . [DOI] [PubMed] [Google Scholar]
- 40.Aizman O, Uhlén P, Lal M, Brismar H, Aperia A. Ouabain, a steroid hormone that signals with slow calcium oscillations. Proceedings of the National Academy of Sciences of the United States of America . 2001;98(23):13420–13424. doi: 10.1073/pnas.221315298. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aydemir-Koksoy A, Abramowitz J, Allen JC. Ouabain-induced signaling and vascular smooth muscle cell proliferation. Journal of Biological Chemistry . 2001;276(49):46605–46611. doi: 10.1074/jbc.M106178200. . [DOI] [PubMed] [Google Scholar]
- 42.Li J, Zelenin S, Aperia A, Aizman O. Low doses of ouabain protect from serum deprivation-triggered apoptosis and stimulate kidney cell proliferation via activation of NF-κB. Journal of the American Society of Nephrology . 2006;17(7):1848–1857. doi: 10.1681/ASN.2005080894. . [DOI] [PubMed] [Google Scholar]
- 43.Saunders R, Scheiner-Bobis G. Ouabain stimulates endothelin release and expression in human endothelial cells without inhibiting the sodium pump. European Journal of Biochemistry . 2004;271(5):1054–1062. doi: 10.1111/j.1432-1033.2004.04012.x. . [DOI] [PubMed] [Google Scholar]
- 44.Kotova O, Al-Khalili L, Talia S, et al. Cardiotonic steroids stimulate glycogen synthesis in human skeletal muscle cells via a Src- and ERK1/2-dependent mechanism. Journal of Biological Chemistry . 2006;281(29):20085–20094. doi: 10.1074/jbc.M601577200. . [DOI] [PubMed] [Google Scholar]
- 45.Khundmiri SJ, Metzler MA, Ameen M, Amin V, Rane MJ, Delamere NA. Ouabain induces cell proliferation through calcium-dependent phosphorylation of Akt (protein kinase B) in opossum kidney proximal tubule cells. American Journal of Physiology, Cell Physiology . 2006;291(6):C1247–C1257. doi: 10.1152/ajpcell.00593.2005. . [DOI] [PubMed] [Google Scholar]
- 46.Trevisi L, Visentin B, Cusinato F, Pighin I, Luciani S. Antiapoptotic effect of ouabain on human umbilical vein endothelial cells. Biochemical and Biophysical Research Communications . 2004;321(3):716–721. doi: 10.1016/j.bbrc.2004.07.027. . [DOI] [PubMed] [Google Scholar]
- 47.Ferrari P, Ferrandi M, Valentini G, Manunta P, Bianchi G. Targeting Ouabain- and Adducin-dependent mechanisms of hypertension and cardiovascular remodeling as a novel pharmacological approach. Medical Hypotheses . 2007;68(6):1307–1314. doi: 10.1016/j.mehy.2006.07.058. . [DOI] [PubMed] [Google Scholar]
- 48.Golden WC, Martin LJ. Low-dose ouabain protects against excitotoxic apoptosis and up-regulates nuclear Bcl-2 in vivo . Neuroscience . 2006;137(1):133–144. doi: 10.1016/j.neuroscience.2005.10.004. . [DOI] [PubMed] [Google Scholar]
- 49.Jiang X, Ren YP, Lü ZR. Ouabain induces cardiac remodeling in rats independent of blood pressure. Acta Pharmacologica Sinica . 2007;28(3):344–352. doi: 10.1111/j.1745-7254.2007.00496.x. . [DOI] [PubMed] [Google Scholar]
- 50.Li M, Wang Q, Guan L. Effects of ouabain on proliferation, intracellular free calcium and c-myc mRNA expression in vascular smooth muscle cells. Journal of Comparative Physiology B . 2007;177(5):589–595. doi: 10.1007/s00360-007-0157-4. . [DOI] [PubMed] [Google Scholar]
- 51.Skoumal R, Szokodi I, Aro J, et al. Involvement of endogenous ouabain-like compound in the cardiac hypertrophic process in vivo . Life Sciences . 2007;80(14):1303–1310. doi: 10.1016/j.lfs.2006.12.026. . [DOI] [PubMed] [Google Scholar]
- 52.Taguchi K, Kumanogoh H, Nakamura S, Maekawa S. Ouabain-induced isoform-specific localization change of the Na+,K+-ATPase α subunit in the synaptic plasma membrane of rat brain. Neuroscience Letters . 2007;413(1):42–45. doi: 10.1016/j.neulet.2006.11.061. . [DOI] [PubMed] [Google Scholar]
- 53.Thundathil JC, Anzar M, Buhr MM. Na+/K+ ATPase as a signaling molecule during bovine sperm capacitation. Biology of Reproduction . 2006;75(3):308–317. doi: 10.1095/biolreprod.105.047852. . [DOI] [PubMed] [Google Scholar]
- 54.Larre I, Ponce A, Fiorentino R, Shoshani L, Contreras RG, Cereijido M. Contacts and cooperation between cells depend on the hormone ouabain. Proceedings of the National Academy of Sciences of the United States of America . 2006;103(29):10911–10916. doi: 10.1073/pnas.0604496103. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen ANT, Wallace DP, Blanco G. Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. Journal of the American Society of Nephrology . 2007;18(1):46–57. doi: 10.1681/ASN.2006010086. . [DOI] [PubMed] [Google Scholar]
- 56.Dmitrieva RL, Doris PA. Ouabain is a potent promoter of growth and activator of ERK1/2 in ouabain-resistant rat renal epithelial cells. Journal of Biological Chemistry . 2003;278(30):28160–28166. doi: 10.1074/jbc.M303768200. . [DOI] [PubMed] [Google Scholar]
- 57.Larre I, Lazaro A, Contreras RG, et al. Ouabain modulates epithelial cell tight junction. Proceedings of the National Academy of Sciences of the United States of America . 2010;107(25):11387–11392. doi: 10.1073/pnas.1000500107. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajasekaran AK, Rajasekaran SA. Role of Na-K-ATPase in the assembly of tight junctions. American Journal of Physiology . 2003;285(3):F388–F396. doi: 10.1152/ajprenal.00439.2002. . [DOI] [PubMed] [Google Scholar]
- 59.Blaustein MP, Zhang J, Chen L, et al. The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension . 2009;53(2):291–298. doi: 10.1161/HYPERTENSIONAHA.108.119974. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bagrov AY, Fedorova OV. Cardenolide and bufadienolide ligands of the sodium pump. How they work together in NaCl sensitive hypertension. Frontiers in Bioscience . 2005;10(1):2250–2256. doi: 10.2741/1694. . [DOI] [PubMed] [Google Scholar]
- 61.Bertorello AM, Sznajder JI. The dopamine paradox in lung and kidney epithelia: sharing the same target but operating different signaling networks. American Journal of Respiratory Cell and Molecular Biology . 2005;33(5):432–437. doi: 10.1165/rcmb.2005-0297TR. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu J, Shapiro JI. Regulation of sodium pump endocytosis by cardiotonic steroids: molecular mechanisms and physiological implications. Pathophysiology . 2007;14(3-4):171–181. doi: 10.1016/j.pathophys.2007.09.008. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie Z, Askari A. Na+/K+-ATPase as a signal transducer. European Journal of Biochemistry . 2002;269(10):2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. . [DOI] [PubMed] [Google Scholar]
- 64.Aperia A. New roles for an old enzyme: Na,K-ATPase emerges as an interesting drug target. Journal of Internal Medicine . 2007;261(1):44–52. doi: 10.1111/j.1365-2796.2006.01745.x. . [DOI] [PubMed] [Google Scholar]
- 65.Kelly RA, Smith TW. The search for the endogenous digitalis: an alternative hypothesis. American Journal of Physiology . 1989;256(5):C937–C950. doi: 10.1152/ajpcell.1989.256.5.C937. . [DOI] [PubMed] [Google Scholar]
- 66.Foulkes R, Ferrario RG, Salvati P, Bianchi G. Differences in ouabain-induced natriuresis between isolated kidneys of Milan hypertensive and normotensive rats. Clinical Science . 1992;82(2):185–190. doi: 10.1042/cs0820185. . [DOI] [PubMed] [Google Scholar]
- 67.Yates NA, McDougall JG. Effect of volume expansion on the natriuretic response to ouabain infusion. Renal Physiology and Biochemistry . 1995;18(6):311–320. doi: 10.1159/000173932. . [DOI] [PubMed] [Google Scholar]
- 68.Dostanic-Larson I, van Huysse JW, Lorenz JN, Lingrel JB. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proceedings of the National Academy of Sciences of the United States of America . 2005;102(44):15845–15850. doi: 10.1073/pnas.0507358102. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loreaux EL, Kaul B, Lorenz JN, Lingrel JB. Ouabain-sensitive α1 Na,K-ATPase enhances natriuretic response to saline load. Journal of the American Society of Nephrology . 2008;19(10):1947–1954. doi: 10.1681/ASN.2008020174. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nesher M, Dvela M, Igbokwe VU, Rosen H, Lichtstein D. Physiological roles of endogenous ouabain in normal rats. American Journal of Physiology, Heart and Circulatory Physiology . 2009;297(6):H2026–H2034. doi: 10.1152/ajpheart.00734.2009. . [DOI] [PubMed] [Google Scholar]
- 71.Fedorova OV, Kolodkin NI, Agalakova NI, Lakatta EG, Bagrov AY. Marinobufagenin, an endogenous α-1 sodium pump ligand, in hypertensive Dahl salt-sensitive rats. Hypertension . 2001;37(2):462–466. doi: 10.1161/01.hyp.37.2.462. . [DOI] [PubMed] [Google Scholar]
- 72.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. Endogenous ligand of α1 sodium pump, marinobufagenin, is a novel mediator of sodium chloride-dependent hypertension. Circulation . 2002;105(9):1122–1127. doi: 10.1161/hc0902.104710. . [DOI] [PubMed] [Google Scholar]
- 73.Anderson DE, Fedorova OV, Morrell CH, et al. Endogenous sodium pump inhibitors and age-associated increases in salt sensitivity of blood pressure in normotensives. American Journal of Physiology, Regulatory Integrative and Comparative Physiology . 2008;294(4):R1248–R1254. doi: 10.1152/ajpregu.00782.2007. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manunta P, Stella P, Rivera R, et al. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension . 1999;34(3):450–456. doi: 10.1161/01.hyp.34.3.450. . [DOI] [PubMed] [Google Scholar]
- 75.Manunta P, Hamilton BP, Hamlyn JM. Salt intake and depletion increase circulating levels of endogenous ouabain in normal men. American Journal of Physiology, Regulatory Integrative and Comparative Physiology . 2006;290(3):R553–R559. doi: 10.1152/ajpregu.00648.2005. . [DOI] [PubMed] [Google Scholar]
- 76.Huang BS, Amin MS, Leenen FHH. The central role of the brain in salt-sensitive hypertension. Current Opinion in Cardiology . 2006;21(4):295–304. doi: 10.1097/01.hco.0000231398.64362.94. . [DOI] [PubMed] [Google Scholar]
- 77.van Huysse JW. Endogenous brain Na pumps, brain ouabain-like substance and the α2 isoform in salt-dependent hypertension. Pathophysiology . 2007;14(3-4):213–220. doi: 10.1016/j.pathophys.2007.09.010. . [DOI] [PubMed] [Google Scholar]
- 78.Fedorova OV, Zhuravin IA, Agalakova NI, et al. Intrahippocampal microinjection of an exquisitely low dose of ouabain mimics NaCl loading and stimulates a bufadienolide Na/K-ATPase inhibitor. Journal of Hypertension . 2007;25(9):1834–1844. doi: 10.1097/HJH.0b013e328200497a. . [DOI] [PubMed] [Google Scholar]
- 79.Fedorova OV, Agalakova NI, Talan MI, Lakatta EG, Bagrov AY. Brain ouabain stimulates peripheral marinobufagenin via angiotensin II signalling in NaCl-loaded Dahl-S rats. Journal of Hypertension . 2005;23(8):1515–1523. doi: 10.1097/01.hjh.0000174969.79836.8b. . [DOI] [PubMed] [Google Scholar]
- 80.Kennedy DJ, Vetteth S, Periyasamy SM, et al. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension . 2006;47(3):488–495. doi: 10.1161/01.HYP.0000202594.82271.92. . [DOI] [PubMed] [Google Scholar]
- 81.Ferrandi M, Molinari I, Barassi P, Minotti E, Bianchi G, Ferrari P. Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. Journal of Biological Chemistry . 2004;279(32):33306–33314. doi: 10.1074/jbc.M402187200. . [DOI] [PubMed] [Google Scholar]
- 82.Liu J, Tian J, Haas M, Shapiro JI, Askari A, Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. Journal of Biological Chemistry . 2000;275(36):27838–27844. doi: 10.1074/jbc.M002950200. . [DOI] [PubMed] [Google Scholar]
- 83.Priyadarshi S, Valentine B, Han C, et al. Effect of green tea extract on cardiac hypertrophy following 5/6 nephrectomy in the rat. Kidney International . 2003;63(5):1785–1790. doi: 10.1046/j.1523-1755.2003.00914.x. . [DOI] [PubMed] [Google Scholar]
- 84.Elkareh J, Kennedy DJ, Yashaswi B, et al. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension . 2007;49(1):215–224. doi: 10.1161/01.HYP.0000252409.36927.05. . [DOI] [PubMed] [Google Scholar]
- 85.Kennedy DJ, Elkareh J, Shidyak A, et al. Partial nephrectomy as a model for uremic cardiomyopathy in the mouse. American Journal of Physiology . 2008;294(2):F450–F454. doi: 10.1152/ajprenal.00472.2007. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Elkareh J, Periyasamy SM, Shidyak A, et al. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase C and Fli-1: implications for uremic cardiomyopathy. American Journal of Physiology . 2009;296(5):F1219–F1226. doi: 10.1152/ajprenal.90710.2008. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian J, Shidyak A, Periyasamy SM, et al. Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension . 2009;54(6):1313–1320. doi: 10.1161/HYPERTENSIONAHA.109.140038. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.El-Okdi N, Smaili S, Raju V, et al. Effects of cardiotonic steroids on dermal collagen synthesis and wound healing. Journal of Applied Physiology . 2008;105(1):30–36. doi: 10.1152/japplphysiol.00119.2008. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fedorova LV, Raju V, El-Okdi N, et al. The cardiotonic steroid hormone marinobufagenin induces renal fibrosis: implication of epithelial-to-mesenchymal transition. American Journal of Physiology . 2009;296(4):F922–F934. doi: 10.1152/ajprenal.90605.2008. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blaustein MP. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. American Journal of Physiology, Cell Physiology . 1977;232(5):C165–C173. doi: 10.1152/ajpcell.1977.232.5.C165. . [DOI] [PubMed] [Google Scholar]
- 91.Haddy FJ, Pamnani MB, Clough DL. Humoral factors and the sodium-potassium pump in volume expanded hypertension. Life Sciences . 1979;24(23):2105–2117. doi: 10.1016/0024-3205(79)90108-5. . [DOI] [PubMed] [Google Scholar]
- 92.de Wardener HE, Clarkson EM. Concept of natriuretic hormone. Physiological Reviews . 1985;65(3):658–759. doi: 10.1152/physrev.1985.65.3.658. . [DOI] [PubMed] [Google Scholar]
- 93.Haddy FJ, Overbeck HW. The role of humoral agents in volume expanded hypertension. Life Sciences . 1976;19(7):935–947. doi: 10.1016/0024-3205(76)90284-8. . [DOI] [PubMed] [Google Scholar]
- 94.de Wardener HE, Clarkson EM. Concept of natriuretic hormone. Physiological Reviews . 1985;65(3):658–759. doi: 10.1152/physrev.1985.65.3.658. . [DOI] [PubMed] [Google Scholar]
- 95.McDougall JG, Yates NA. Natriuresis and inhibition of Na+/K+-ATPase: modulation of response by physiological manipulation. Clinical and Experimental Pharmacology and Physiology . 1998;25(supplement):S57–S60. doi: 10.1111/j.1440-1681.1998.tb02302.x. . [DOI] [PubMed] [Google Scholar]
- 96.Yates NA, McDougall JG. Effects of direct renal arterial infusion of bufalin and ouabain in conscious sheep. British Journal of Pharmacology . 1993;108(3):627–630. doi: 10.1111/j.1476-5381.1993.tb12852.x. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yates NA, McDougall JG. Interaction of exogenous ouabain and chronic mineralocorticoid treatment in the kidney of the conscious sheep. Clinical and Experimental Pharmacology and Physiology . 1997;24(1):57–63. doi: 10.1111/j.1440-1681.1997.tb01783.x. . [DOI] [PubMed] [Google Scholar]
- 98.de Wardener HE, MacGregor GA. Dahl’s hypothesis that a saluretic substance may be responsible for a sustained rise in arterial pressure: its possible role in essential hypertension. Kidney International . 1980;18(1):1–9. doi: 10.1038/ki.1980.104. . [DOI] [PubMed] [Google Scholar]
- 99.Fedorova OV, Agalakova NI, Morrell CH, Lakatta EG, Bagrov AY. ANP differentially modulates marinobufagenin-induced sodium pump inhibition in kidney and aorta. Hypertension . 2006;48(6):1160–1168. doi: 10.1161/01.HYP.0000248129.20524.d0. . [DOI] [PubMed] [Google Scholar]
- 100.Fedorova OV, Lakatta EG, Bagrov AY. Endogenous Na,K pump ligands are differentially regulated during acute NaCl loading of dahl rats. Circulation . 2000;102(24):3009–3014. doi: 10.1161/01.cir.102.24.3009. . [DOI] [PubMed] [Google Scholar]
- 101.Schoner W, Scheiner-Bobis G. Role of endogenous cardiotonic steroids in sodium homeostasis. Nephrology Dialysis Transplantation . 2008;23(9):2723–2729. doi: 10.1093/ndt/gfn325. . [DOI] [PubMed] [Google Scholar]
- 102.Yoshika M, Komiyama Y, Konishi M, et al. Novel digitalis-like factor, marinobufotoxin, isolated from cultured Y-1 cells, and its hypertensive effect in rats. Hypertension . 2007;49(1):209–214. doi: 10.1161/01.HYP.0000250433.64202.78. . [DOI] [PubMed] [Google Scholar]
- 103.Ritz E, et al. Uremic Cardiomyopathy-An Endogenous Digitalis Intoxication? Journal of the American Society of Nephrology . 2006;17(6):1493–1497. . [Google Scholar]
- 104.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacological Reviews . 2009;61(1):9–38. doi: 10.1124/pr.108.000711. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haddy FJ, Pamnani MB. Role of ouabain-like factors and NA-K-ATPase inhibitors in hypertension—some old and recent findings. Clinical and Experimental Hypertension . 1998;20(5-6):499–508. doi: 10.3109/10641969809053228. . [DOI] [PubMed] [Google Scholar]
- 106.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. American Journal of Physiology, Cell Physiology . 2007;293(2):C509–C536. doi: 10.1152/ajpcell.00098.2007. . [DOI] [PubMed] [Google Scholar]
- 107.Tian J, Haller S, Periyasamy S, et al. Renal ischemia regulates marinobufagenin release in humans. Hypertension . 2010;56(5):914–919. doi: 10.1161/HYPERTENSIONAHA.110.155564. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cai H, Wu L, Qu W, et al. Regulation of apical NHE3 trafficking by ouabain-induced activation of the basolateral Na+,K+-ATPase receptor complex. American Journal of Physiology, Cell Physiology . 2008;294(2):C555–C563. doi: 10.1152/ajpcell.00475.2007. . [DOI] [PubMed] [Google Scholar]
- 109.Liu J, Kesiry R, Periyasamy SM, Malhotra D, Xie Z, Shapiro JI. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney International . 2004;66(1):227–241. doi: 10.1111/j.1523-1755.2004.00723.x. . [DOI] [PubMed] [Google Scholar]
- 110.Liu J, Liang M, Liu L, Malhotra D, Xie Z, Shapiro JI. Ouabain-induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney International . 2005;67(5):1844–1854. doi: 10.1111/j.1523-1755.2005.00283.x. . [DOI] [PubMed] [Google Scholar]
- 111.Liu J, Periyasamy SM, Gunning W, et al. Effects of cardiac glycosides on sodium pump expression and function in LLC-PK1 and MDCK cells. Kidney International . 2002;62(6):2118–2125. doi: 10.1046/j.1523-1755.2002.00672.x. . [DOI] [PubMed] [Google Scholar]
- 112.Oweis S, Wu L, Kiela PR, et al. Cardiac glycoside downregulates NHE3 activity and expression in LLC-PK 1 cells. American Journal of Physiology . 2006;290(5):F997–F1008. doi: 10.1152/ajprenal.00322.2005. . [DOI] [PubMed] [Google Scholar]
- 113.Periyasamy SM, Liu J, Tanta F, et al. Salt loading induces redistribution of the plasmalemmal Na/K-ATPase in proximal tubule cells. Kidney International . 2005;67(5):1868–1877. doi: 10.1111/j.1523-1755.2005.00285.x. . [DOI] [PubMed] [Google Scholar]
- 114.Liu J, Yan Y, Liu L, et al. Impairment of Na/K-ATPase signaling in renal proximal tubule contributes to Dahl salt-sensitive hypertension. Journal of Biological Chemistry . 2011;286(26):22806–22813. doi: 10.1074/jbc.M111.246249. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dahl LK, Heine M, Tassinari L. Role of genetic factors in susceptibility to experimental hypertension due to chronic excess salt ingestion. Nature . 1962;194(4827):480–482. doi: 10.1038/194480b0. . [DOI] [PubMed] [Google Scholar]
- 116.Dahl LK, Knudsen KD, Iwai J. Humoral transmission of hypertension: evidence from parabiosis. Circulation Research . 1969;24(5, supplement):21–33. . [PubMed] [Google Scholar]
- 117.Rapp JP. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension . 1982;4(6):753–763. doi: 10.1161/01.hyp.4.6.753. . [DOI] [PubMed] [Google Scholar]
- 118.Rapp JP, Dene H. Development and characteristics of inbred strains of Dahl salt-sensitive and salt-resistant rats. Hypertension . 1985;7(3):340–349. . [PubMed] [Google Scholar]
- 119.Mokry M, Cuppen E. The Atp1a1 gene from inbred Dahl salt sensitive rats does not contain the A1079T missense transversion. Hypertension . 2008;51(4):922–927. doi: 10.1161/HYPERTENSIONAHA.107.108415. . [DOI] [PubMed] [Google Scholar]
- 120.Yamori Y. The development of Spontaneously Hypertensive Rat (SHR) and of various spontaneous rat models, and their implications. In: Ganten D, de Jong W, editors. Experimental and Genetic Models of Hypertension . Vol. 4. New York, NY, USA: Elsevier; 1984. pp. 224–239. (Handbook of Hypertension). . [Google Scholar]
- 121.Pace-Asciak CR, Carrara MC. Reduction in dietary vitamin E prevents onset of hypertension in developing spontaneously hypertensive rats. Experientia . 1979;35(12):1561–1562. doi: 10.1007/BF01953192. . [DOI] [PubMed] [Google Scholar]
- 122.Young JB, Mullen D, Landsberg L. Caloric restriction lowers blood pressure in the spontaneously hypertensive rat. Metabolism . 1978;27(12):1711–1714. doi: 10.1016/0026-0495(78)90256-1. . [DOI] [PubMed] [Google Scholar]
- 123.Adams N, Blizard DA. Genetic and maternal influences in rat models of spontaneous and salt-induced hypertension. Developmental Psychobiology . 1991;24(7):507–519. doi: 10.1002/dev.420240705. . [DOI] [PubMed] [Google Scholar]
- 124.Arendshorst WJ, Beierwaltes WH. Renal tubular reabsorption in spontaneously hypertensive rats. The American Journal of Physiology . 1979;237(1):F38–47. doi: 10.1152/ajprenal.1979.237.1.F38. . [DOI] [PubMed] [Google Scholar]
- 125.Beierwaltes WH, Arendshorst WJ. Renal function of conscious spontaneously hypertensive rats. Circulation Research . 1978;42(5):721–726. doi: 10.1161/01.res.42.5.721. . [DOI] [PubMed] [Google Scholar]
- 126.Arendshorst WJ. Autoregulation of renal blood flow in spontaneously hypertensive rats. Circulation Research . 1979;44(3):344–349. doi: 10.1161/01.res.44.3.344. . [DOI] [PubMed] [Google Scholar]
- 127.Crajoinas RO, Lessa LMA, Carraro-Lacroix LR, et al. Posttranslational mechanisms associated with reduced NHE3 activity in adult vs. young prehypertensive SHR. American Journal of Physiology . 2010;299(4):F872–F881. doi: 10.1152/ajprenal.00654.2009. . [DOI] [PubMed] [Google Scholar]
- 128.LaPointe MS, Sodhi C, Sahai A, Batlle D. Na+/H+ exchange activity and NHE-3 expression in renal tubules from the spontaneously hypertensive rat. Kidney International . 2002;62(1):157–165. doi: 10.1046/j.1523-1755.2002.00406.x. . [DOI] [PubMed] [Google Scholar]
- 129.Garg LC, Narang N, McArdle S. Na-K-ATPase in nephron segments of rats developing spontaneous hypertension. The American Journal of Physiology . 1985;249(6):F863–869. doi: 10.1152/ajprenal.1985.249.6.F863. . [DOI] [PubMed] [Google Scholar]
- 130.Cangiano JL, Rodriguez-Sargent C, Opava-Stitzer S, Martinez-Maldonado M. Renal Na+,K+-ATPase in weanling and adult spontaneously hypertensive rats. Proceedings of the Society for Experimental Biology and Medicine . 1984;177(2):240–246. doi: 10.3181/00379727-177-41937. . [DOI] [PubMed] [Google Scholar]
- 131.Garg LC, Narang N. Differences in renal tubular Na-K-adenosine triphosphatase in spontaneously hypertensive and normotensive rats. Journal of Cardiovascular Pharmacology . 1986;8(1):186–189. doi: 10.1097/00005344-198601000-00027. . [DOI] [PubMed] [Google Scholar]
- 132.Garg LC, Narang N. Effects of hydrochlorothiazide on Na-K-ATPase activity along the rat nephron. Kidney International . 1987;31(4):918–922. doi: 10.1038/ki.1987.86. . [DOI] [PubMed] [Google Scholar]
- 133.Morduchowicz GA, Sheikh-Hamad D, Jo OD, Nord EP, Lee DBN, Yanagawa N. Increased Na+/H+ antiport activity in the renal brush border membrane of SHR. Kidney International . 1989;36(4):576–581. doi: 10.1038/ki.1989.233. . [DOI] [PubMed] [Google Scholar]
- 134.Hayward AL, Hinojos CA, Nurowska B, et al. Altered sodium pump alpha and gamma subunit gene expression in nephron segments from hypertensive rats. Journal of Hypertension . 1999;17(8):1081–1087. doi: 10.1097/00004872-199917080-00006. . [DOI] [PubMed] [Google Scholar]
- 135.Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. American Journal of Physiology, Cell Physiology . 1998;275(1):C1–C24. doi: 10.1152/ajpcell.1998.275.1.C1. . [DOI] [PubMed] [Google Scholar]
- 136.Chabrashvili T, Tojo A, Onozato ML, et al. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension . 2002;39(2):269–274. doi: 10.1161/hy0202.103264. . [DOI] [PubMed] [Google Scholar]
- 137.Panico C, Luo Z, Damiano S, Artigiano F, Gill P, Welch WJ. Renal proximal tubular reabsorption is reduced in adult spontaneously hypertensive rats: roles of superoxide and Na+/H+ exchanger 3. Hypertension . 2009;54(6):1291–1297. doi: 10.1161/HYPERTENSIONAHA.109.134783. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Banday AA, Lokhandwala MF. Angiotensin II-mediated biphasic regulation of proximal tubular Na+/H+ exchanger 3 is impaired during oxidative stress. American Journal of Physiology, Renal Physiology . 2011;301(2):F364–F370. doi: 10.1152/ajprenal.00121.2011. . [DOI] [PubMed] [Google Scholar]
- 139.Dada LA, Novoa E, Lecuona E, Sun H, Sznajder JI. Role of the small GTPase RhoA in the hypoxia-induced decrease of plasma membrane Na,K-ATPase in A549 cells. Journal of Cell Science . 2007;120(13):2214–2222. doi: 10.1242/jcs.003038. . [DOI] [PubMed] [Google Scholar]
- 140.Liu L, Li J, Liu J, et al. Involvement of Na+/K+-ATPase in hydrogen peroxide-induced hypertrophy in cardiac myocytes. Free Radical Biology and Medicine . 2006;41(10):1548–1556. doi: 10.1016/j.freeradbiomed.2006.08.018. . [DOI] [PubMed] [Google Scholar]
- 141.Xie Z, Kometiani P, Liu J, Li J, Shapiro JI, Askari A. Intracellular reactive oxygen species mediate the linkage of Na+/K+- ATPase to hypertrophy and its marker genes in cardiac myocytes. Journal of Biological Chemistry . 1999;274(27):19323–19328. doi: 10.1074/jbc.274.27.19323. . [DOI] [PubMed] [Google Scholar]
- 142.Tian J, Liu J, Garlid KD, Shapiro JI, Xie Z. Involvement of mitogen-activated protein kinases and reactive oxygen species in the inotropic action of ouabain on cardiac myocytes. A potential role for mitochondrial KATP channels. Molecular and Cellular Biochemistry . 2003;242(1-2):181–187. . [PubMed] [Google Scholar]
- 143.Miyakawa-Naito A, Uhlén P, Lal M, et al. Cell signaling microdomain with Na,K-ATPase and inositol 1,4,5-trisphosphate receptor generates calcium oscillations. Journal of Biological Chemistry . 2003;278(50):50355–50361. doi: 10.1074/jbc.M305378200. . [DOI] [PubMed] [Google Scholar]
- 144.Kajikawa M, Fujimoto S, Tsuura Y, et al. Ouabain suppresses glucose-induced mitochondrial ATP production and insulin release by generating reactive oxygen species in pancreatic islets. Diabetes . 2002;51(8):2522–2529. doi: 10.2337/diabetes.51.8.2522. . [DOI] [PubMed] [Google Scholar]
- 145.Huang YT, Chueh SC, Teng CM, Guh JH. Investigation of ouabain-induced anticancer effect in human androgen-independent prostate cancer PC-3 cells. Biochemical Pharmacology . 2004;67(4):727–733. doi: 10.1016/j.bcp.2003.10.013. . [DOI] [PubMed] [Google Scholar]
- 146.Boldyrev A, Bulygina E, Yuneva M, Schoner W. Na/K-ATPase regulates intracellular ROS level in cerebellum neurons. Annals of the New York Academy of Sciences . 2003;986:519–521. doi: 10.1111/j.1749-6632.2003.tb07238.x. . [DOI] [PubMed] [Google Scholar]
- 147.Kennedy DJ, Vetteth S, Xie M, et al. Ouabain decreases sarco(endo)plasmic reticulum calcium ATPase activity in rat hearts by a process involving protein oxidation. American Journal of Physiology, Heart and Circulatory Physiology . 2006;291(6):H3003–H3011. doi: 10.1152/ajpheart.00603.2006. . [DOI] [PubMed] [Google Scholar]
- 148.Glynn IM. “Transport adenosinetriphosphatase” in electric organ. The relation between ion transport and oxidative phosphorylation. The Journal of Physiology . 1963;169(2):452–465. doi: 10.1113/jphysiol.1963.sp007272. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McKenna MJ, Medved I, Goodman CA, et al. N-acetylcysteine attenuates the decline in muscle Na+, K+-pump activity and delays fatigue during prolonged exercise in humans. Journal of Physiology . 2006;576(1):279–288. doi: 10.1113/jphysiol.2006.115352. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boldyrev A, Kurella E. Mechanism of oxidative damage of dog kidney Na/K-ATPase. Biochemical and Biophysical Research Communications . 1996;222(2):483–487. doi: 10.1006/bbrc.1996.0770. . [DOI] [PubMed] [Google Scholar]
- 151.Dobrota D, Matejovicova M, Kurella EG, Boldyrev AA. Na/K-ATPase under oxidative stress: molecular mechanisms of injury. Cellular and Molecular Neurobiology . 1999;19(1):141–149. doi: 10.1023/A:1006928927480. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kurella EG, Tyulina OV, Boldyrev AA. Oxidative resistance of Na/K-ATPase. Cellular and Molecular Neurobiology . 1999;19(1):133–140. doi: 10.1023/A:1006976810642. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Petrushanko I, Bogdanov N, Bulygina E, et al. Na-K-ATPase in rat cerebellar granule cells is redox sensitive. American Journal of Physiology, Regulatory Integrative and Comparative Physiology . 2006;290(4):R916–R925. doi: 10.1152/ajpregu.00038.2005. . [DOI] [PubMed] [Google Scholar]
- 154.Bogdanova A, Boldyrev A, Gassmann M. Oxygen- and redox-induced regulaton of the Na/K ATPase. Current Enzyme Inhibition . 2006;2(1):37–59. . [Google Scholar]
- 155.Ellis DZ, Rabe J, Sweadner KJ. Global loss of Na,K-ATPase and its nitric oxide-mediated regulation in a transgenic mouse model of amyotrophic lateral sclerosis. Journal of Neuroscience . 2003;23(1):43–51. doi: 10.1523/JNEUROSCI.23-01-00043.2003. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim MS, Akera T. O2 free radicals: cause of ischemia-reperfusion injury to cardiac Na+,K+-ATPase. American Journal of Physiology, Heart and Circulatory Physiology . 1987;252(2):H252–H257. doi: 10.1152/ajpheart.1987.252.2.H252. . [DOI] [PubMed] [Google Scholar]
- 157.Xie Z, Wang Y, Askari A, Huang WH, Klaunig JE, Askari A. Studies on the specificity of the effects of oxygen metabolites on cardiac sodium pump. Journal of Molecular and Cellular Cardiology . 1990;22(8):911–920. doi: 10.1016/0022-2828(90)90122-i. . [DOI] [PubMed] [Google Scholar]
- 158.Huang WH, Wang Y, Askari A. (Na+ + K+)-ATPase: inactivation and degradation induced by oxygen radicals. International Journal of Biochemistry . 1992;24(4):621–626. doi: 10.1016/0020-711x(92)90337-z. . [DOI] [PubMed] [Google Scholar]
- 159.Huang W-H, Wang Y, Askari A, Zolotarjova N, Ganjeizadeh M. Different sensitivities of the Na+/K+-ATPase isoforms to oxidants. Biochimica et Biophysica Acta, Biomembranes . 1994;1190(1):108–114. doi: 10.1016/0005-2736(94)90039-6. . [DOI] [PubMed] [Google Scholar]
- 160.Zolotarjova N, Ho C, Mellgren RL, Askari A, Huang W-H. Different sensitivities of native and oxidized forms of Na+/K+-ATPase to intracellular proteinases. Biochimica et Biophysica Acta, Biomembranes . 1994;1192(1):125–131. doi: 10.1016/0005-2736(94)90152-x. . [DOI] [PubMed] [Google Scholar]
- 161.Xie Z, Jack-Hays M, Wang Y, et al. Different oxidant sensitivities of the α 1 and α 2 isoforms of Na+/K+-ATPase expressed in baculovirus-infected insect cells. Biochemical and Biophysical Research Communications . 1995;207(1):155–159. doi: 10.1006/bbrc.1995.1166. . [DOI] [PubMed] [Google Scholar]
- 162.Mense M, Stark G, Apell HJ. Effects of free radicals on partial reactions of the Na,K-ATPase. Journal of Membrane Biology . 1997;156(1):63–71. doi: 10.1007/s002329900188. . [DOI] [PubMed] [Google Scholar]
- 163.Thévenod F, Friedmann JM. Cadmium-mediated oxidative stress in kidney proximal tubule cells induces degradation of Na+/K+-ATPase through proteasomal and endo- /lysosomal proteolytic pathways. FASEB Journal . 1999;13(13):1751–1761. doi: 10.1096/fasebj.13.13.1751. . [DOI] [PubMed] [Google Scholar]
- 164.White CN, Figtree GA, Liu CC, et al. Angiotensin II inhibits the Na+-K+ pump via PKC-dependent activation of NADPH oxidase. American Journal of Physiology, Cell Physiology . 2009;296(4):C693–C700. doi: 10.1152/ajpcell.00648.2008. . [DOI] [PubMed] [Google Scholar]
- 165.Figtree GA, Liu CC, Bibert S, et al. Reversible oxidative modification: a key mechanism of Na+-K+ pump regulation. Circulation Research . 2009;105(2):185–193. doi: 10.1161/CIRCRESAHA.109.199547. . [DOI] [PubMed] [Google Scholar]
- 166.Bibert S, Liu C-C, Figtree GA, et al. FXYD proteins reverse inhibition of the Na+-K+ pump mediated by glutathionylation of its β1 subunit. Journal of Biological Chemistry . 2011;286(21):18562–18572. doi: 10.1074/jbc.M110.184101. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Reifenberger MS, Arnett KL, Gatto C, Milanick MA. The reactive nitrogen species peroxynitrite is a potent inhibitor of renal Na-K-ATPase activity. American Journal of Physiology . 2008;295(4):F1191–F1198. doi: 10.1152/ajprenal.90296.2008. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang C, Imam SZ, Ali SF, Mayeux PR. Peroxynitrite and the regulation of Na+,K+-ATPase activity by angiotensin II in the rat proximal tubule. Nitric Oxide . 2002;7(1):30–35. doi: 10.1016/s1089-8603(02)00003-4. . [DOI] [PubMed] [Google Scholar]
- 169.Zhang AY, Yi F, Zhang G, Gulbins E, Li PL. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension . 2006;47(1):74–80. doi: 10.1161/10.1161/01.HYP.0000196727.53300.62. . [DOI] [PubMed] [Google Scholar]
- 170.Zuo L, Ushio-Fukai M, Ikeda S, Hilenski L, Patrushev N, Alexander RW. Caveolin-1 is essential for activation of Rac1 and NAD(P)H oxidase after angiotensin II type 1 receptor stimulation in vascular smooth muscle cells: role in redox signaling and vascular hypertrophy. Arteriosclerosis, Thrombosis, and Vascular Biology . 2005;25(9):1824–1830. doi: 10.1161/01.ATV.0000175295.09607.18. . [DOI] [PubMed] [Google Scholar]
- 171.Touyz RM. Lipid rafts take center stage in endothelial cell redox signaling by death receptors. Hypertension . 2006;47(1):16–18. doi: 10.1161/01.HYP.0000196730.13216.f3. . [DOI] [PubMed] [Google Scholar]
- 172.Han W, Li H, Villar VAM, et al. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension . 2008;51(2):481–487. doi: 10.1161/HYPERTENSIONAHA.107.103275. . [DOI] [PubMed] [Google Scholar]
- 173.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circulation Research . 2002;91(5):406–413. doi: 10.1161/01.res.0000033523.08033.16. . [DOI] [PubMed] [Google Scholar]
- 174.Touyz RM, Yao G, Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology . 2003;23(6):981–987. doi: 10.1161/01.ATV.0000069236.27911.68. . [DOI] [PubMed] [Google Scholar]
- 175.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovascular Research . 2006;71(2):247–258. doi: 10.1016/j.cardiores.2006.05.001. . [DOI] [PubMed] [Google Scholar]
- 176.Wilcox CS, Gutterman D. Focus on oxidative stress in the cardiovascular and renal systems. American Journal of Physiology, Heart and Circulatory Physiology . 2005;288(1):H3–H6. doi: 10.1152/ajpheart.00854.2004. . [DOI] [PubMed] [Google Scholar]
- 177.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension . 2004;44(3):248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. . [DOI] [PubMed] [Google Scholar]
- 178.Lacy F, Kailasam MT, O’Connor DT, Schmid-Schönbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension . 2000;36(5):878–884. doi: 10.1161/01.hyp.36.5.878. . [DOI] [PubMed] [Google Scholar]
- 179.Lacy F, O’Connor DT, Schmid-Schönbein GW. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. Journal of Hypertension . 1998;16(3):291–303. doi: 10.1097/00004872-199816030-00006. . [DOI] [PubMed] [Google Scholar]
- 180.Vaziri ND, Rodríguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nature Clinical Practice Nephrology . 2006;2(10):582–593. doi: 10.1038/ncpneph0283. . [DOI] [PubMed] [Google Scholar]
- 181.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? American Journal of Physiology, Regulatory, Integrative and Comparative Physiology . 2005;289(4):R913–R935. doi: 10.1152/ajpregu.00250.2005. . [DOI] [PubMed] [Google Scholar]
- 182.Welch WJ. Intrarenal oxygen and hypertension. Clinical and Experimental Pharmacology and Physiology . 2006;33(10):1002–1005. doi: 10.1111/j.1440-1681.2006.04478.x. . [DOI] [PubMed] [Google Scholar]
- 183.Kitiyakara C, Chabrashvili T, Chen Y, et al. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. Journal of the American Society of Nephrology . 2003;14(11):2775–2782. doi: 10.1097/01.asn.0000092145.90389.65. . [DOI] [PubMed] [Google Scholar]
- 184.Schreck C, O'Connor PM. NAD(P)H oxidase and renal epithelial ion transport. American Journal of Physiology, Regulatory Integrative and Comparative Physiology . 2011;300(5):R1023–R1029. doi: 10.1152/ajpregu.00618.2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Han HJ, Yun JL, Su HP, Jang HL, Taub M. High glucose-induced oxidative stress inhibits Na+/glucose cotransporter activity in renal proximal tubule cells. American Journal of Physiology . 2005;288(5):F988–F996. doi: 10.1152/ajprenal.00327.2004. . [DOI] [PubMed] [Google Scholar]
- 186.Juncos R, Hong NJ, Garvin JL. Differential effects of superoxide on luminal and basolateral Na+/H+ exchange in the thick ascending limb. American Journal of Physiology, Regulatory Integrative and Comparative Physiology . 2006;290(1):R79–R83. doi: 10.1152/ajpregu.00447.2005. . [DOI] [PubMed] [Google Scholar]
- 187.Cowley AW, Mori T, Mattson D, Zou AP. Role of renal NO production in the regulation of medullary blood flow. American Journal of Physiology, Regulatory Integrative and Comparative Physiology . 2003;284(6):R1355–R1369. doi: 10.1152/ajpregu.00701.2002. . [DOI] [PubMed] [Google Scholar]
- 188.Makino A, Skelton MM, Zou AP, Cowley AW. Increased renal medullary H2O2 leads to hypertension. Hypertension . 2003;42(1):25–30. doi: 10.1161/01.HYP.0000074903.96928.91. . [DOI] [PubMed] [Google Scholar]
- 189.Taylor NE, Cowley AW., Jr. Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. American Journal of Physiology, Regulatory Integrative and Comparative Physiology . 2005;289(6):R1573–R1579. doi: 10.1152/ajpregu.00525.2005. . [DOI] [PubMed] [Google Scholar]
- 190.Taylor NE, Glocka P, Liang M, Cowley AW. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension . 2006;47(4):692–698. doi: 10.1161/01.HYP.0000203161.02046.8d. . [DOI] [PubMed] [Google Scholar]
- 191.Cao C, Edwards A, Sendeski M, et al. Intrinsic nitric oxide and superoxide production regulates descending vasa recta contraction. American Journal of Physiology . 2010;299(5):F1056–F1064. doi: 10.1152/ajprenal.00070.2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Edwards A, Cao C, Pallone TL. Cellular mechanisms underlying nitric oxide-induced vasodilation of descending vasa recta. American Journal of Physiology, Renal Physiology . 2011;300(2):F441–F456. doi: 10.1152/ajprenal.00499.2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang Z, Asico LD, Yu P, et al. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. American Journal of Physiology, Regulatory Integrative and Comparative Physiology . 2006;290(1):R96–R104. doi: 10.1152/ajpregu.00434.2005. . [DOI] [PubMed] [Google Scholar]
- 194.Kopkan L, Majid DS. Superoxide contributes to development of salt sensitivity and hypertension induced by nitric oxide deficiency. Hypertension. . 2005;46(4):1026–1031. doi: 10.1161/01.HYP.0000174989.39003.58. . [DOI] [PubMed] [Google Scholar]
- 195.Kopkan L, Hess A, Husková Z, Červenka L, Navar LG, Majid DSA. High-salt intake enhances superoxide activity in enos knockout mice leading to the development of salt sensitivity. American Journal of Physiology . 2010;299(3):F656–F663. doi: 10.1152/ajprenal.00047.2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang R, Hording P, Garvin JL, et al. Isoforms and functions of NAD(P)H oxidase at the macula densa. Hypertension . 2009;53(3):556–563. doi: 10.1161/HYPERTENSIONAHA.108.124594. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. American Journal of Physiology . 2002;283(5):F957–F962. doi: 10.1152/ajprenal.00102.2002. . [DOI] [PubMed] [Google Scholar]
- 198.Mori T, Cowley AW. Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension . 2003;42(4):588–593. doi: 10.1161/01.HYP.0000091821.39824.09. . [DOI] [PubMed] [Google Scholar]
- 199.Johns EJ, O’Shaughnessy B, O’Neill S, Lane B, Healy V. Impact of elevated dietary sodium intake on NAD(P)H oxidase and SOD in the cortex and medulla of the rat kidney. American Journal of Physiology, Regulatory Integrative and Comparative Physiology . 2010;299(1):R234–R240. doi: 10.1152/ajpregu.00541.2009. . [DOI] [PubMed] [Google Scholar]
- 200.Wang T. Nitric oxide regulates HCO3 and Na+ transport by a cGMP-mediated mechanism in the kidney proximal tubule. American Journal of Physiology, Renal Physiology . 1997;272(2):F242–F248. doi: 10.1152/ajprenal.1997.272.2.F242. . [DOI] [PubMed] [Google Scholar]
- 201.Li H, Han W, Van Villar AM, et al. D1-like receptors regulate nadph oxidase activity and subunit expression in lipid raft microdomains of renal proximal tubule cells. Hypertension . 2009;53(6):1054–1061. doi: 10.1161/HYPERTENSIONAHA.108.120642. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Banday AA, Lokhandwala MF. Loss of biphasic effect on Na/K-ATPase activity by angiotensin II involves defective angiotensin type 1 receptor-nitric oxide signaling. Hypertension . 2008;52(6):1099–1105. doi: 10.1161/HYPERTENSIONAHA.108.117911. . [DOI] [PubMed] [Google Scholar]
- 203.Moe OW, Tejedor A, Levi M, Seldin DW, Preisig PA, Alpern RJ. Dietary NaCl modulates Na+-H+ antiporter activity in renal cortical apical membrane vesicles. American Journal of Physiology - Renal Fluid and Electrolyte Physiology . 1991;260(1):F130–F137. doi: 10.1152/ajprenal.1991.260.1.F130. . [DOI] [PubMed] [Google Scholar]
- 204.Yang LE, Sandberg MB, Can AD, Pihakaski-Maunsbach K, McDonough AA. Effects of dietary salt on renal Na+ transporter subcellular distribution, abundance, and phosphorylation status. American Journal of Physiology . 2008;295(4):F1003–F1016. doi: 10.1152/ajprenal.90235.2008. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liu J, Yan Y, Liu L, et al. Impairment of Na/K-ATPase signaling in renal proximal tubule contributes to Dahl salt-sensitive hypertension. Journal of Biological Chemistry . 2011;286(26):22806–22813. doi: 10.1074/jbc.M111.246249. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fisher KA, Lee SH, Walker J, Dileto-Fang C, Ginsberg L, Stapleton SR. Regulation of proximal tubule sodium/hydrogen antiporter with chronic volume contraction. American Journal of Physiology . 2001;280(5):F922–F926. doi: 10.1152/ajprenal.2001.280.5.F922. . [DOI] [PubMed] [Google Scholar]
- 207.Silva E, Soares-da-Silva P. Reactive oxygen species and the regulation of renal Na+-K+-ATPase in opossum kidney cells. American Journal of Physiology, Regulatory Integrative and Comparative Physiology . 2007;293(4):R1764–R1770. doi: 10.1152/ajpregu.00425.2007. . [DOI] [PubMed] [Google Scholar]
- 208.McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. American Journal of Physiology, Regulatory Integrative and Comparative Physiology . 2010;298(4):R851–R861. doi: 10.1152/ajpregu.00002.2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yang LE, Leong PKK, McDonough AA. Reducing blood pressure in SHR with enalapril provokes redistribution of NHE3, NaPi2, and NCC and decreases NaPi2 and ACE abundance. American Journal of Physiology . 2007;293(4):F1197–F1208. doi: 10.1152/ajprenal.00040.2007. . [DOI] [PubMed] [Google Scholar]
- 210.Pallone TL. Control of renal Na+ excretion by heme oxygenase. Hypertension . 2007;49(1):23–24. doi: 10.1161/01.HYP.0000250089.99513.f6. . [DOI] [PubMed] [Google Scholar]
- 211.Zou AP, Billington H, Su N, Cowley AW. Expression and actions of heme oxygenase in the renal medulla of rats. Hypertension . 2000;35(1):342–347. doi: 10.1161/01.hyp.35.1.342. . [DOI] [PubMed] [Google Scholar]
- 212.Rodriguez F, Zhang F, Dinocca S, Nasjletti A. Nitric oxide synthesis influences the renal vascular response to heme oxygenase inhibition. American Journal of Physiology . 2003;284(6):F1255–F1262. doi: 10.1152/ajprenal.00435.2002. . [DOI] [PubMed] [Google Scholar]
- 213.Arregui B, López B, Salom MG, Valero F, Navarro C, Fenoy FJ. Acute renal hemodynamic effects of dimanganese decacarbonyl and cobalt protoporphyrin. Kidney International . 2004;65(2):564–574. doi: 10.1111/j.1523-1755.2004.00409.x. . [DOI] [PubMed] [Google Scholar]
- 214.Johnson RA, Lavesa M, Askari B, Abraham NG, Nasjletti A. A heme oxygenase product, presumably carbon monoxide, mediates a vasodepressor function in rats. Hypertension . 1995;25(2):166–169. doi: 10.1161/01.hyp.25.2.166. . [DOI] [PubMed] [Google Scholar]
- 215.Hirakawa H, Hayashida Y. Autonomic cardiovascular responses to heme oxygenase inhibition in conscious rats. Hypertension . 2006;48(6):1124–1129. doi: 10.1161/01.HYP.0000245678.56354.21. . [DOI] [PubMed] [Google Scholar]
- 216.Johnson RA, Lavesa M, Deseyn K, Scholer MJ, Nasjletti A. Heme oxygenase substrates acutely lower blood pressure in hypertensive rats. American Journal of Physiology . 1996;271(3):H1132–H1138. doi: 10.1152/ajpheart.1996.271.3.H1132. . [DOI] [PubMed] [Google Scholar]
- 217.Levere RD, Martasek P, Escalante B, Schwartzman ML, Abraham NG. Effect of heme arginate administration on blood pressure in spontaneously hypertensive rats. Journal of Clinical Investigation . 1990;86(1):213–219. doi: 10.1172/JCI114686. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sacerdoti D, Escalante B, Abraham NG, McGiff JC, Levere RD, Schwartzman ML. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science . 1989;243(4889):388–390. doi: 10.1126/science.2492116. . [DOI] [PubMed] [Google Scholar]
- 219.Sabaawy HE, Zhang F, Nguyen X, et al. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension . 2001;38(2):210–215. doi: 10.1161/01.hyp.38.2.210. . [DOI] [PubMed] [Google Scholar]
- 220.Wang R, Shamloul R, Wang X, Meng Q, Wu L. Sustained normalization of high blood pressure in spontaneously hypertensive rats by implanted hemin pump. Hypertension . 2006;48(4):685–692. doi: 10.1161/01.HYP.0000239673.80332.2f. . [DOI] [PubMed] [Google Scholar]
- 221.Johnson FK, Durante W, Peyton KJ, Johnson RA. Heme oxygenase inhibitor restores arteriolar nitric oxide function in dahl rats. Hypertension . 2003;41(1):149–155. doi: 10.1161/01.hyp.0000046923.52222.58. . [DOI] [PubMed] [Google Scholar]
- 222.Teran FJ, Johnson RA, Stevenson BK, et al. Heme oxygenase-derived carbon monoxide promotes arteriolar endothelial dysfunction and contributes to salt-induced hypertension in Dahl salt-sensitive rats. American Journal of Physiology, Regulatory Integrative and Comparative Physiology . 2005;288(3):R615–R622. doi: 10.1152/ajpregu.00123.2004. . [DOI] [PubMed] [Google Scholar]
- 223.Johnson RA, Teran FJ, Durante W, Peyton KJ, Johnson FK. Enhanced heme oxygenase-mediated coronary vasodilation in Dahl salt-sensitive hypertension. American Journal of Hypertension . 2004;17(1):25–30. doi: 10.1016/j.amjhyper.2003.08.009. . [DOI] [PubMed] [Google Scholar]
- 224.Katavetin P, Inagi R, Miyata T, et al. Erythropoietin induces heme oxygenase-1 expression and attenuates oxidative stress. Biochemical and Biophysical Research Communications . 2007;359(4):928–934. doi: 10.1016/j.bbrc.2007.05.207. . [DOI] [PubMed] [Google Scholar]
- 225.Münzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? European Heart Journal . 2010;31(22):2741–2748. doi: 10.1093/eurheartj/ehq396. . [DOI] [PubMed] [Google Scholar]
- 226.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. New England Journal of Medicine . 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. . [DOI] [PubMed] [Google Scholar]
- 227.Miller ER, III, Appel LJ, Risby TH. Effect of dietary patterns on measures of lipid peroxidation: results from a randomized clinical trial. Circulation . 1998;98(22):2390–2395. doi: 10.1161/01.cir.98.22.2390. . [DOI] [PubMed] [Google Scholar]
- 228.John JH, Ziebland S, Yudkin P, Roe LS, Neil HAW. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: a randomised controlled trial. Lancet . 2002;359(9322):1969–1974. doi: 10.1016/s0140-6736(02)98858-6. . [DOI] [PubMed] [Google Scholar]
- 229.Collins R, Armitage J, Parish S, Sleight P, Peto R. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet . 2002;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. . [DOI] [PubMed] [Google Scholar]
- 230.Galley HF, Thornton J, Howdle PD, Walker BE, Webster NR. Combination oral antioxidant supplementation reduces blood pressure. Clinical Science . 1997;92(4):361–365. doi: 10.1042/cs0920361. . [DOI] [PubMed] [Google Scholar]
- 231.Ceriello A, Giugliano D, Quatraro A, Lefebvre PJ. Anti-oxidants show an anti-hypertensive effect in diabetic and hypertensive subjects. Clinical Science . 1991;81(6):739–742. doi: 10.1042/cs0810739. . [DOI] [PubMed] [Google Scholar]
- 232.Ceriello A, Motz E, Giugliano D. Hypertension and ascorbic acid. Lancet . 2000;355(9211):1272–1273. doi: 10.1016/S0140-6736(05)74700-0. . [DOI] [PubMed] [Google Scholar]
- 233.Huang H-Y, Caballero B, Chang S, et al. The efficacy and safety of multivitamin and mineral supplement use to prevent cancer and chronic disease in adults: a systematic review for a National Institutes of Health state-of-the-science conference. Annals of Internal Medicine . 2006;145(5):372–385. doi: 10.7326/0003-4819-145-5-200609050-00135. . [DOI] [PubMed] [Google Scholar]
- 234.Garvin JL, Ortiz PA. The role of reactive oxygen species in the regulation of tubular function. Acta Physiologica Scandinavica . 2003;179(3):225–232. doi: 10.1046/j.0001-6772.2003.01203.x. . [DOI] [PubMed] [Google Scholar]


