Abstract
It has been reported that HCV can infect not only hepatocytes but also various kinds of lymphoid cells. Although many reports have described the biological significance of lymphotropic HCV, the issue remains controversial since the target lymphoid cells might have various kinds of functions in the immune system. One of the important roles of lymphoid cells in HCV replication is being a reservoir of HCV. Several groups described the detection of HCV-RNA in lymphoid cells after HCV eradication in plasma. Another important role of lymphotropic HCV is that it acts as a carcinogenic agent and induces immune dysfunction. In this paper, we summarize the reports regarding the biological significance of lymphotropic HCV in representative lymphoid cells.
1. Introduction
Hepatitis C virus (HCV) infects about 170 million people worldwide causing chronic hepatitis, liver cirrhosis, hepatocellular carcinoma, B-cell lymphoma, cryoglobulin-related disease, and various kinds of autoimmune diseases [1–3]. HCV is basically a hepatotropic virus that causes liver disease. However, HCV replication is detected not only in hepatocytes but also in B cells, T cells, monocytes, dendritic cells (DCs), and peripheral blood mononuclear cells [4–13]. The existence of HCV strains with preferential lymphocyte tropism suggests the potential role of B cells and T cells as an HCV reservoir [14–17]. Moreover, the existence of HCV in lymphoid cells could contribute to various B-cell proliferative disorders including B-cell lymphoma and mixed cryoglobulinemia and dysregulation of the cellular and humoral immune responses that have major roles in the immunopathogenesis of HCV persistent infection [18–28]. HCV infects hepatocytes, lymphoid cells, and probably other cells through CD81 and several receptor candidates. The expression of CD81 could be detected in various types of cells, including hepatocytes, B cells, T cells, and monocytes, indicating that these types of cells are potential targets of HCV infection [13]. Previously, Sung et al. reported that HCV persistently produced from a particular B-cell line (SB), which was established from an HCV-positive B-cell lymphoma, can infect and replicate in established B-cell lines (Raji, Daudi) and primary B lymphocytes [23]. Moreover, we reported that two T-cell lines (Molt-4 and Jurkat) and primary naïve T lymphocytes were infected with SB culture supernatant [13, 27, 28]. Machida et al. reported that HCV replication in B lymphocytes could contribute to the carcinogenesis of B cells based on this useful in vitro system [29, 30]. Not only Machida's group but also many groups reported the evidence of HCV replication in lymphoid cells [18, 31–38]. Various studies about the biological significance of HCV infection in lymphoid cells have been reported, although there were some negative reports regarding HCV replication in lymphoid cells [39–41]. Therefore, understanding the various effects of lymphotropic HCV is needed to treat the extrahepatic manifestations of HCV infection. In this paper, we summarize the various studies showing evidence of HCV replication and discuss the biological significance of HCV replication in lymphoid cells according to the various subsets of lymphoid cells.
2. Biological Significance of HCV Infection in B Lymphocytes
Many reports indicating the existence of HCV in B lymphocytes and B-cell lymphoma have been published, and many of these focused on the relevance of HCV infection to B-cell proliferative diseases including B-cell lymphoma and cryoglobulinemia [18, 23, 42, 43]. Evidence of HCV infection in B lymphocytes could be detected using PCR-based methods. The detection of negative-strand HCV-RNA was widely used to prove the replication of HCV in the cells. However, the amount of negative-strand HCV-RNA is usually quite low in comparison to positive-strand HCV-RNA [28]. Therefore, many groups including us used nested PCR with rtTh polymerase that could reduce the risk of false-positive detection [13, 27, 28, 31, 44]. In addition to nested PCR for the detection of negative-strand HCV-RNA, immunostaining of HCV individual proteins was used to detect lymphocytes with HCV replication [28, 45]. False positive and negative amplification of HCV RNA frequently occurred due to the very little amount of HCV-RNA as compared with the large excess of total cellular RNA, resulting in underestimation of viral RNA copies. Underestimation of RNA copies and wide standard deviation were observed in intracellular HCV RNA quantification by conventional real-time PCR, particularly with very low amount of HCV-RNA (102 to 104 copies/ug) [28]. On the other hand, the HCV-NS3 protein could be detected over 50% of B cell line with lymphotropic HCV infection [45]. Discrepancies of these data might be due to the detection methods. Therefore, we need to consider the character of detection methods and detection limit carefully.
Sung et al. established B-cell lymphoma cell lines persistently infected with hepatitis C virus in vivo and in vitro [23]. The cell lines continuously produce infectious HCV virions in culture. The virus particles produced from the culture had a buoyant density of 1.13 to 1.15 g/mL in sucrose and could infect primary human peripheral blood mononuclear cells (PBMCs) and an established B-cell line in vitro [23]. This lymphotropic HCV strain was useful to investigate the biological significance of HCV replication in lymphoid cells.
It has been reported that the replication of HCV in B lymphocytes could induce error-prone DNA polymerase zeta, polymerase iota, and activation-induced cytidine deaminase (AID), which contribute to enhancing the mutation frequency [30]. Moreover, the cellular DNA damage and mutation were mediated by nitric oxide (NO) and reactive oxygen species (ROS) [20, 29]. However, not only HCV replication in B lymphocytes but also the direct HCV-E2 CD81 interaction could induce hypermutation of the immunoglobulin gene in B cells. E2-CD81 interaction on B cells triggered the enhanced expression of AID [46]. Recently, another group reported that persistent expression of the full genome of HCV in B cells induces the spontaneous development of B-cell lymphoma in vivo [47]. They established HCV transgenic mice that express the full HCV genome in B cells and observed a 25% incidence of diffuse, large B-cell non-Hodgkin lymphomas. Moreover, it has been reported that HCV replication could enhance the RIG-I expression mediated by interferon regulatory factor-2 (IRF-2) in human peripheral blood B lymphocytes. These reports explained the mechanism of carcinogenesis in lymphocytes. However, the relation between lymphotropic HCV and mixed cryoglobulinemia, one of the lymphoproliferative diseases, has been analyzed by many groups [3, 48–50]. Sansonno et al. reported that HCV replication could be detected especially in chronic HCV with mixed cryoglobulinemia [50]. The mechanisms of the induction of mixed cryoglobulinemia by lymphotropic HCV replication have not been clarified yet except for the induction of hypermutation of Ig-related genes and the stimulation of lymphocyte [45] (Figure 1).
Figure 1.
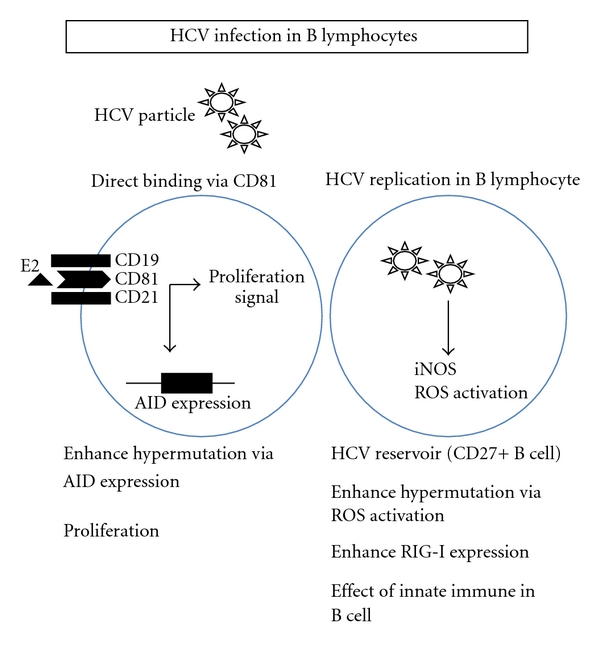
A schema of the biological significance of HCV replication in B cells is shown. The representative effects of lymphotropic HCV on B cells are shown in this figure.
It has been reported that the B lymphocyte reservoir of HCV is one of the important issues regarding the biological significance of lymphotropic HCV [17]. Recently, it was found that CD27+ memory B cells were more resistant to apoptosis than CD27− B cells. CD27+ memory B cells might be an HCV reservoir for persistent infection in chronic hepatitis C patients [51]. Moreover, a possible mechanism by which the innate immune system is blocked in B cells, which is necessary for HCV to infect B cells persistently, was reported [14] (Figure 1). TANK-binding kinase-1 (TBK1) and IκB kinase ε (IKKε) are essential for IRF-3 phosphorylation, and both kinases were markedly enhanced in B cells with hepatitis C infection. However, the reduced expression of heat shock protein of 90 kDa, a TBK1 stabilizer, and the enhanced expression of SIKE, an IKKε suppressor, were observed in B cells, and these might suppress the kinase activity of TBK1/IKKε for IRF-3 phosphorylation in CHC patients [14].
3. Biological Significance of HCV Infection in T Lymphocytes
Various groups reported that HCV could be detected not only in B lymphocytes but also in T lymphocytes, which could contribute to the humoral and cellular immune responses [8, 31]. Many research groups established HCV replication systems using T-cell lymphoma cell lines more than a decade ago [4, 5, 10, 11, 32]. One group reported that human T-lymphotropic virus type I infected cell line MT-2 was susceptible to HCV infection [4, 5]. Not only HTLV-I but also Epstein-Barr (EB) virus enhanced the ability of HCV to replicate in T-cell lines [35]. These reports suggested that the existence of other viruses might change the sensitivity of HCV infection in T lymphocytes. It is important to analyze the possibility that the susceptibility of HCV can be changed by other viruses, since HCV/HIV coinfection is an important issue [52, 53]. However, the biological significance of HCV replication in T lymphocytes has not been clarified yet. We previously reported that lymphotropic HCV strain (SB-HCV) could be replicated in two T-cell lines (Molt-4 and Jurkat) and primary T lymphocytes, especially in naïve CD4+ T lymphocytes with proliferative stimulation [13, 27, 28]. The negative strand HCV-RNA that is the evidence of viral replication could be detected temporarily after inoculation. The amount of HCV-RNA detected in T lymphocytes was lower than in B lymphocytes in this infection system. The amounts of negative strand RNA and positive-strand RNA in B cell lines were at least 4 times higher than those of T-cell lines [28]. It has been reported that depletion of CD8+ cells could increase the positive rate of HCV-RNA in PBMC. CD8+ T cells have a strong ability to produce the IFN-g that could suppress the HCV replication [54]. In our study, the negative-strand HCV-RNA could not be detected in CD8+ T cells. We have reported that HCV replication could affect IFN-g/STAT-1/T-bet signaling by reducing the amount of phospho-STAT-1 in Molt-4 and human primary naïve T lymphocytes [13, 28]. Moreover, HCV-replication could inhibit proliferation and enhance Fas-mediated apoptosis by downregulating the expression of CD44 splicing variant 6 [27] (Figure 2). In addition to lymphotropic SB-HCV strain, it has been reported that the wild-type HCV could infect human T lymphocytes with T-cell-stimulating mitogens [34]. Another group reported that HCV core protein upregulated anergy-related genes using a Jurkat T cell line stably expressing HCV core protein [26]. This cell line showed increased activation of NFAT transcription factor and impaired IL2 promoter activity [24] (Figure 2). The expression of HCV core in T lymphocytes might contribute to the establishment of persistent infections by inducing Ca2+ oscillations that regulate both the efficacy and information content of Ca2+ signals and are ultimately responsible for the induction of gene expression and functional differentiation [25]. Coinfection with HCV and HIV is associated with increased HCV replication and a more rapid progression to severe liver disease, including the development of cirrhosis and hepatocellular carcinoma. One group reported that HCV, HIV-1, and human herpes virus 6 were coinfected in single T cells. Coinfection of a T cell by all three viruses was confirmed by transmission electron microscopy [55].
Figure 2.
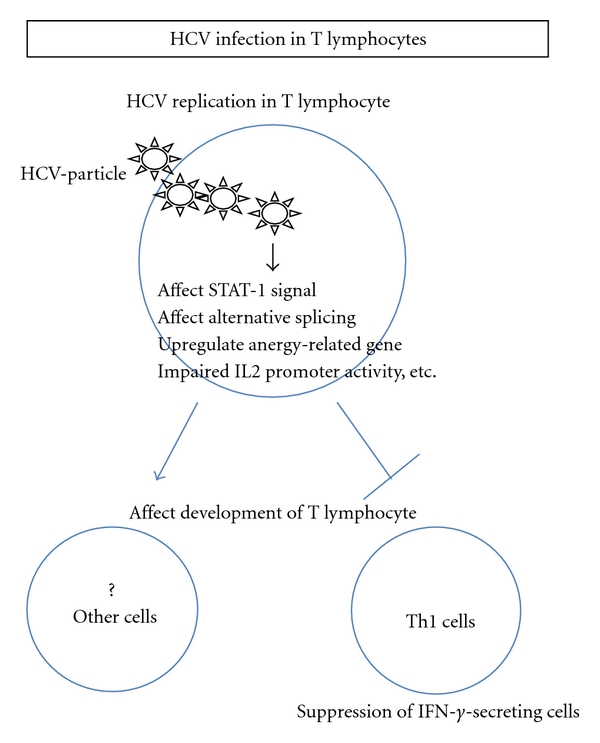
The schema of the biological significance of HCV replication in T cells is shown. The representative effects of lymphotropic HCV on T cells are shown in this figure.
4. Biological Significance of HCV Infection in Monocyte and DCs
It has been reported that HCV could infect not only B and T lymphocytes, but also monocytes and DCs [56]. Recently, a group reported that HCV could infect CD14+CD16+ cells but not CD14+CD16− cells. They found that one of the important HCV receptors, CD81, is highly expressed on CD14+CD16+ cells but not on CD14+CD16− cells [57] (Figure 3). A group reported that negative strand HCV-RNA was most commonly present in monocyte/macrophages followed by T cells and B cells in 10 HCV/HIV coinfected patients [58]. Although the samples size of this study was not so large, we need to consider the significance of HCV replication in monocytes, especially in HCV/HIV co-infected patients. The interaction between monocytes and B and T lymphocytes might have an important role in the immuno-pathogenesis of HCV infection. In addition to the effects on cell function, the existence of HCV in monocytes might serve as an HCV reservoir as seen in B and T lymphocytes [15].
Figure 3.
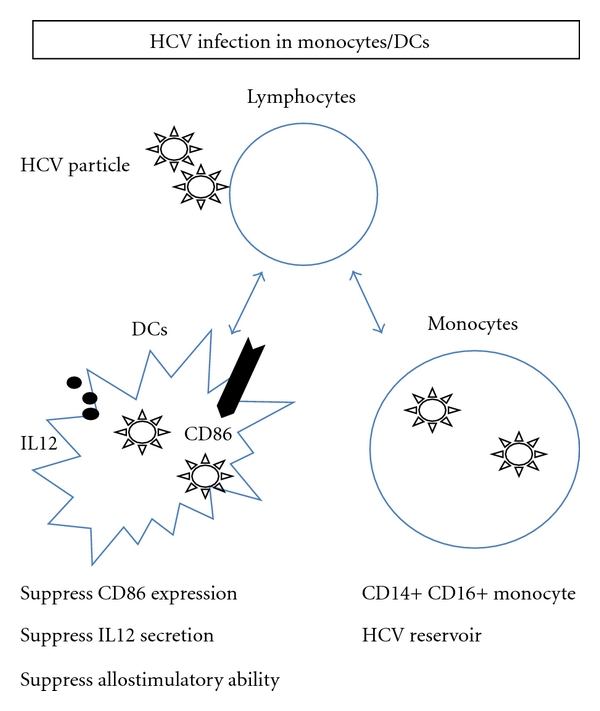
The schema of the biological significance of HCV replication in DCs and monocytes is shown. The representative effects of lymphotropic HCV on DCs and monocytes are shown in this figure.
Dendritic cells (DCs), which play a central role in coordinating the immune response in HCV infection, have been studied by many research groups. DCs are the most potent activators of CD4 T cells for supporting Th1 differentiation, which is important for the cellular immune response. It has been reported that DCs from chronic hepatitis C patients expressed lower amounts of CD86 and IL12 than those from healthy subjects [59]. Moreover, other groups have reported that the existence of HCV in DCs might suppress the allostimulatory function, although the detailed mechanism of suppression was not clear [60–62] (Figure 3). In vitro infection of human monocyte-derived DCs was carried out, and strand-specific rTth reverse transcription polymerase chain reaction was used to prove the HCV replication in DCs [63]. Replicative-strand RNA could be detected in 3 of 24 peripheral DCs purifications. Moreover, the analysis of the HCV quasispecies distribution in the peripheral DC population of 1 patient showed the presence of a dominant variant different from that found in plasma with respect to the primary amino acid sequence and physiological profile of the hypervariable region 1 of glycoprotein E2 [64]. However, the relationship between the dysfunction of DCs and HCV replication has been still controversial [65].
5. HCV Infection in Nonparenchymal Liver Cells and Other Lymphoid Cells
Nonparenchymal liver cells include lymphocytes, kupffer, polymorphonuclear, pit, endothelial, stellate and fibroblast-like cells. One of the curious subsets of nonparenchymal liver cells is hepatic stellate cells (HSCs) that play an important role in the control of extracellular matrix synthesis and degradation in fibrotic livers. One report described that HCV-core antigen could colocalize with large lipid droplets present in HSC and with collagen fibers in the extracellular matrix. These data indicated that HCV-core antigen in the stellate cells might modulate the immune function and fibrosis [66]. However, the amount of HCV-RNA and the level of HCV replication could not be mentioned in this report. More recently, it has been reported that HCV-RNA replication could affect the gene expression of extracellular matrix-related molecules in HSC [67]. In that report, they used subgenomic HCV replicon. The average amount of HCV RNA was 3.9 copies × 105/ug total RNA.
Pluripotent hematopoietic CD34+ cells were another curious target of HCV replication since these cells have the ability to develop various kinds of cells. It has been reported that positive- and negative-strand HCV-RNA and HCV individual proteins could be detected in CD34+ hematopoietic progenitor cells [68]. The amount of negative-strand HCV-RNA was 1 × 103 HCV-RNA Eq. Moreover, the existence of HCV not only in peripheral blood mononuclear cells but also in bone marrow mononuclear cells has been reported [69]. These observations are important since transplantation of allogenic CD34+-selected peripheral stem cells can result in the transmission of hepatitis C virus from an infected donor [70].
6. Concluding Remarks
Although various reports described the biological significance of HCV replication in lymphoid cells, these reports were not conclusive due to the lack of efficient in vitro culture systems. However, we should not underestimate the effect of HCV replication in lymphoid cells. In this paper, we focused on the effect of HCV replication in lymphoid cells. In addition to the role as an HCV reservoir, various reports described that HCV replication in lymphoid cells could induce dysregulation of the immune system. We need to focus not only on suppression of the immune system but also on stimulation of the immune system, since the prevalence of autoimmune diseases is much higher than in healthy subjects. A study regarding the relationship between lymphotropic HCV and autoimmune diseases is ongoing in our laboratory. As for the understanding of autoimmune diseases, HCV persistent infection might be one of the representative models of viral-induced autoimmune diseases. Recently, the technologies of deep sequencing, immunoassays with increased numbers of multicolor flow cytometry analyses, and chimera mice with human lymphocytes have been developed. These technologies, together with previous data, might be able to clarify the biological significance of lymphotropic HCV.
Acknowledgments
The authors are grateful to Professor Lai MM (Keck School of Medicine, USC, and Academia Sinica), who was the previous mentor of the first author and kindly provided SB-HCV strain, and Dr. K. Machida (Keck School of Medicine, USC) who was the previous coworker of the first author and gave them valuable insights regarding lymphotropic HCV. The first author was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sport, Science, and Technology of Japan (no. 23790761 to Y. Kondo).
References
- 1.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. The New England Journal of Medicine. 1999;341(8):556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 2.Chuang SS, Liao YL, Chang ST, et al. Hepatitis C virus infection is significantly associated with malignant lymphoma in Taiwan, particularly with nodal and splenic marginal zone lymphomas. Journal of Clinical Pathology. 2010;63(7):595–598. doi: 10.1136/jcp.2010.076810. [DOI] [PubMed] [Google Scholar]
- 3.Lunel F, Musset L. Mixed cryoglobulinemia and hepatitis C virus infection. Minerva Medica. 2001;92(1):35–42. [PubMed] [Google Scholar]
- 4.Mizutani T, Kato N, Hirota M, Sugiyama K, Murakami A, Shimotohno K. Inhibition of hepatitis C virus replication by antisense oligonucleotide in culture cells. Biochemical and Biophysical Research Communications. 1995;212(3):906–911. doi: 10.1006/bbrc.1995.2055. [DOI] [PubMed] [Google Scholar]
- 5.Mizutani T, Kato N, Saito S, Ikeda M, Sugiyama K, Shimotohno K. Characterization of hepatitis C virus replication in cloned cells obtained from a human T-cell leukemia virus type 1-infected cell line, MT-2. Journal of Virology. 1996;70(10):7219–7223. doi: 10.1128/jvi.70.10.7219-7223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakajima N, Hijikata M, Yoshikura H, Shimizu YK. Characterization of long-term cultures of hepatitis C virus. Journal of Virology. 1996;70(5):3325–3329. doi: 10.1128/jvi.70.5.3325-3329.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nissen E, Hohne M, Schreier E. In vitro replication of hepatitis C virus in a human lymphoid cell line (H9) Journal of Hepatology. 1994;20(3):p. 437. doi: 10.1016/s0168-8278(94)80023-5. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu YK, Feinstone SM, Kohara M, Purcell RH, Yoshikura H. Hepatitis C virus: detection of intracellular virus particles by electron microscopy. Hepatology. 1996;23(2):205–209. doi: 10.1002/hep.510230202. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu YK, Igarashi H, Kanematu T, et al. Sequence analysis of the hepatitis C virus genome recovered from serum, liver, and peripheral blood mononuclear cells of infected chimpanzees. Journal of Virology. 1997;71(8):5769–5773. doi: 10.1128/jvi.71.8.5769-5773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu YK, Iwamoto A, Hijikata M, Purcell RH, Yoshikura H. Evidence for in vitro replication of hepatitis C virus genome in a human T-cell line. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(12):5477–5481. doi: 10.1073/pnas.89.12.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu YK, Purcell RH, Yoshikura H. Correlation between the infectivity of hepatitis C virus in vivo and its infectivity in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(13):6037–6041. doi: 10.1073/pnas.90.13.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu YK, Yoshikura H. Multicycle infection of hepatitis C virus in cell culture and inhibition by alpha and beta interferons. Journal of Virology. 1994;68(12):8406–8408. doi: 10.1128/jvi.68.12.8406-8408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondo Y, Ueno Y, Kakazu E, et al. Lymphotropic HCV strain can infect human primary naïve CD4+ cells and affect their proliferation and IFN-γ secretion activity. Journal of Gastroenterology. 2011;46(2):232–241. doi: 10.1007/s00535-010-0297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito M, Masumi A, Mochida K, et al. Peripheral B cells may serve as a reservoir for persistent hepatitis C virus infection. Journal of Innate Immunity. 2010;2(6):607–617. doi: 10.1159/000317690. [DOI] [PubMed] [Google Scholar]
- 15.Pham TNQ, Michalak TI. Occult persistence and lymphotropism of hepatitis C virus infection. World Journal of Gastroenterology. 2008;14(18):2789–2793. doi: 10.3748/wjg.14.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zignego AL, Giannini C, Monti M, Gragnani L. Hepatitis C virus lymphotropism: lessons from a decade of studies. Digestive and Liver Disease. 2007;39(1):S38–S45. doi: 10.1016/s1590-8658(07)80009-0. [DOI] [PubMed] [Google Scholar]
- 17.Michalak TI. Immune cell reservoirs of persisting hepatitis C virus. Gut. 2010;59(7, supplement):867–868. doi: 10.1136/gut.2010.210054. [DOI] [PubMed] [Google Scholar]
- 18.Karavattathayyil SJ, Kalkeri G, Liu HJ, et al. Detection of hepatitis C virus RNA sequences in B-cell non-Hodgkin lymphoma. American Journal of Clinical Pathology. 2000;113(3):391–398. doi: 10.1309/REV9-FDTM-5NGC-HBWY. [DOI] [PubMed] [Google Scholar]
- 19.Luppi M, Ferrari MG, Bonaccorsi G, et al. Hepatitis C virus infection in subsets of neoplastic lymphoproliferations not associated with cryoglobulinemia. Leukemia. 1996;10(2):351–355. [PubMed] [Google Scholar]
- 20.Machida K, Cheng KT-H, Lai CK, Jeng KS, Sung VM-H, Lai MMC. Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STATS activation. Journal of Virology. 2006;80(14):7199–7207. doi: 10.1128/JVI.00321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machida K, Cheng KTH, Sung VMH, Levine AM, Foung S, Lai MMC. Hepatitis C virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. Journal of Virology. 2006;80(2):866–874. doi: 10.1128/JVI.80.2.866-874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roboz GJ. Hepatitis C and B-cell lymphoma. AIDS Patient Care and STDs. 1998;12(8):605–609. doi: 10.1089/apc.1998.12.605. [DOI] [PubMed] [Google Scholar]
- 23.Sung MHV, Shimodaira S, Doughty AL, et al. Establishment of B-cell lymphoma cell lines persistently infected with hepatitis C virus in vivo and in vitro: the apoptotic effects of virus infection. Journal of Virology. 2003;77(3):2134–2146. doi: 10.1128/JVI.77.3.2134-2146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergqvist A, Rice CM. Transcriptional activation of the interleukin-2 promoter by hepatitis C virus core protein. Journal of Virology. 2001;75(2):772–781. doi: 10.1128/JVI.75.2.772-781.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergqvist A, Sundström S, Dimberg LY, Gylfe E, Masucci MG. The hepatitis C virus core protein modulates T cell responses by inducing spontaneous and altering T-cell receptor-triggered Ca2+ oscillations. The Journal of Biological Chemistry. 2003;278(21):18877–18883. doi: 10.1074/jbc.M300185200. [DOI] [PubMed] [Google Scholar]
- 26.Domínguez-Villar M, Muñoz-Suano A, Anaya-Baz B, et al. Hepatitis C virus core protein up-regulates anergy-related genes and a new set of genes, which affects T cell homeostasis. Journal of Leukocyte Biology. 2007;82(5):1301–1310. doi: 10.1189/jlb.0507335. [DOI] [PubMed] [Google Scholar]
- 27.Kondo Y, Machida K, Liu HM, et al. Hepatitis C virus infection of T cells inhibits proliferation and enhances Fas-mediated apoptosis by down-regulating the expression of CD44 splicing variant 6. Journal of Infectious Diseases. 2009;199(5):726–736. doi: 10.1086/596739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondo Y, Sung VMH, Machida K, Liu M, Lai MMC. Hepatitis C virus infects T cells and affects interferon-γ signaling in T cell lines. Virology. 2007;361(1):161–173. doi: 10.1016/j.virol.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Machida K, Cheng KTH, Sung VMH, Lee KJ, Levine AM, Lai MMC. Hepatitis C virus infection activates the immunologic (type II) isoform of nitric oxide synthase and thereby enhances DNA damage and mutations of cellular genes. Journal of Virology. 2004;78(16):8835–8843. doi: 10.1128/JVI.78.16.8835-8843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machida K, Cheng KTN, Sung VMH, et al. Hepatitis C virus induces a mutator phenotype: enhanced mutations of immunoglobulin and protooncogenes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(12):4262–4267. doi: 10.1073/pnas.0303971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y, Shahidi A, Park S, Guilfoyle D, Hirshfield I. Detection of extrahepatic hepatitis C virus replication by a novel, highly sensitive, single-tube nested polymerase chain reaction. American Journal of Clinical Pathology. 2003;119(1):95–100. doi: 10.1309/33TA-JLB7-48KL-MXVG. [DOI] [PubMed] [Google Scholar]
- 32.Kato N, Ikeda M, Sugiyama K, Mizutani T, Tanaka T, Shimotohno K. Hepatitis C virus population dynamics in human lymphocytes and hepatocytes infected in vitro. Journal of General Virology. 1998;79(8):1859–1869. doi: 10.1099/0022-1317-79-8-1859. [DOI] [PubMed] [Google Scholar]
- 33.Kato N, Nakazawa T, Mizutani T, Shimotohno K. Susceptibility of human T-lymphotropic virus type I infected cell line MT-2 to hepatitis C virus infection. Biochemical and Biophysical Research Communications. 1995;206(3):863–869. doi: 10.1006/bbrc.1995.1123. [DOI] [PubMed] [Google Scholar]
- 34.MacParland SA, Pham TNO, Gujar SA, Michalak TI. De novo infection and propagation of wild-type Hepatitis C virus in human T lymphocytes in vitro. Journal of General Virology. 2006;87(12):3577–3586. doi: 10.1099/vir.0.81868-0. [DOI] [PubMed] [Google Scholar]
- 35.Sugawara Y, Makuuchi M, Kato N, Shimotohno K, Takada K. Enhancement of hepatitis C virus replication by Epstein-Barr virus-encoded nuclear antigen 1. EMBO Journal. 1999;18(20):5755–5760. doi: 10.1093/emboj/18.20.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baré P, Massud I, Parodi C, et al. Continuous release of hepatitis C virus (HCV) by peripheral blood mononuclear cells and B-lymphoblastoid cell-line cultures derived from HCV-infected patients. Journal of General Virology. 2005;86(6):1717–1727. doi: 10.1099/vir.0.80882-0. [DOI] [PubMed] [Google Scholar]
- 37.Pham TNQ, King D, MacParland SA, et al. Hepatitis C Virus Replicates in the Same Immune Cell Subsets in Chronic Hepatitis C and Occult Infection. Gastroenterology. 2008;134(3):812–822. doi: 10.1053/j.gastro.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Rasul I, Shepherd FA, Kamel-Reid S, Krajden M, Pantalony D, Jenny Heathcote E. Detection of occult low-grade B-cell non-Hodgkin’s lymphoma in patients with chronic hepatitis C infection and mixed cryoglobulinemia. Hepatology. 1999;29(2):543–547. doi: 10.1002/hep.510290224. [DOI] [PubMed] [Google Scholar]
- 39.Marukian S, Jones CT, Andrus L, et al. Cell culture-produced hepatitis C virus does not infect peripheral blood mononuclear cells. Hepatology. 2008;48(6):1843–1850. doi: 10.1002/hep.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanford RE, Chavez D, Chisari FV, Sureau C. Lack of detection of negative-strand hepatitis C virus RNA in peripheral blood mononuclear cells and other extrahepatic tissues by the highly strand- specific rTth reverse transcriptase PCR. Journal of Virology. 1995;69(12):8079–8083. doi: 10.1128/jvi.69.12.8079-8083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGuinness PH, Bishop GA, McCaughan GW, Trowbridge R, Gowans EJ. False detection of negative-strand hepatitis C virus RNA. The Lancet. 1994;343(8896):551–552. [PubMed] [Google Scholar]
- 42.Muratori L, Gibellini D, Lenzi M, et al. Quantification of hepatitis C virus-infected peripheral blood mononuclear cells by in situ reverse transcriptase-polymerase chain reaction. Blood. 1996;88(7):2768–2774. [PubMed] [Google Scholar]
- 43.De Vita S, Sansonno D, Dolcetti R, et al. Hepatitis C virus within a malignant lymphoma lesion in the course of type II mixed cryoglobulinemia. Blood. 1995;86(5):1887–1892. [PubMed] [Google Scholar]
- 44.Pham TNQ, MacParland SA, Coffin CS, Lee SS, Bursey FR, Michalak TI. Mitogen-induced upregulation of hepatitis C virus expression in human lymphoid cells. Journal of General Virology. 2005;86(3):657–666. doi: 10.1099/vir.0.80624-0. [DOI] [PubMed] [Google Scholar]
- 45.Machida K, Kondo Y, Huang JY, et al. Hepatitis C virus (HCV)-induced immunoglobulin hypermutation reduces the affinity and neutralizing activities of antibodies against HCV envelope protein. Journal of Virology. 2008;82(13):6711–6720. doi: 10.1128/JVI.02582-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Machida K, Cheng KTH, Pavio N, Sung VMH, Lai MMC. Hepatitis C virus E2-CD81 interaction induces hypermutation of the immunoglobulin gene in B cells. Journal of Virology. 2005;79(13):8079–8089. doi: 10.1128/JVI.79.13.8079-8089.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasama Y, Sekiguchi S, Saito M, et al. Persistent expression of the full genome of hepatitis C virus in B cells induces spontaneous development of B-cell lymphomas in vivo. Blood. 2010;116(23):4926–4933. doi: 10.1182/blood-2010-05-283358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Craxì A, Laffi G, Zignego AL. Hepatitis C virus (HCV) infection: a systemic disease. Molecular Aspects of Medicine. 2008;29(1-2):85–95. doi: 10.1016/j.mam.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 49.Simula MP, Caggiari L, Gloghini A, De Re V. HCV-related immunocytoma and type II mixed cryoglobulinemia-associated autoantigens. Annals of the New York Academy of Sciences. 2007;1110:121–130. doi: 10.1196/annals.1423.014. [DOI] [PubMed] [Google Scholar]
- 50.Sansonno D, Tucci FA, Lauletta G, et al. Hepatitis C virus productive infection in mononuclear cells from patients with cryoglobulinaemia. Clinical and Experimental Immunology. 2007;147(2):241–248. doi: 10.1111/j.1365-2249.2006.03272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizuochi T, Ito M, Takai K, Yamaguchi K. Peripheral blood memory B cells are resistant to apoptosis in chronic hepatitis C patients. Virus Research. 2011;155(1):349–351. doi: 10.1016/j.virusres.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. The presence of active hepatitis C virus replication in lymphoid tissue in patients coinfected with human immunodeficiency virus type 1. Journal of Infectious Diseases. 1998;178(4):1189–1192. doi: 10.1086/515682. [DOI] [PubMed] [Google Scholar]
- 53.Thomas DL, Shih JW, Alter HJ, et al. Effect of human immunodeficiency virus on hepatitis C virus infection among injecting drug users. Journal of Infectious Diseases. 1996;174(4):690–695. doi: 10.1093/infdis/174.4.690. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Wang X, Douglas SD, et al. CD8+ T cell depletion amplifies hepatitis C virus replication in peripheral blood mononuclear cells. Journal of Infectious Diseases. 2005;192(6):1093–1101. doi: 10.1086/432957. [DOI] [PubMed] [Google Scholar]
- 55.Salahuddin SZ, Snyder KA, Godwin A, et al. The simultaneous presence and expression of human hepatitis C virus (HCV), human herpesvirus-6 (HHV-6), and human immunodeficiency virus-1 (HIV-1) in a single human T-cell. Virology Journal. 2007;4, article 106 doi: 10.1186/1743-422X-4-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radkowski M, Wang LFU, Vargas HE, Rakela J, Laskus T. Detection of hepatitis C virus replication in peripheral blood mononuclear cells after orthotopic liver transplantation. Transplantation. 1998;66(5):664–666. doi: 10.1097/00007890-199809150-00022. [DOI] [PubMed] [Google Scholar]
- 57.Coquillard G, Patterson BK. Determination of hepatitis C virus-infected, monocyte lineage reservoirs in individuals with or without HIV coinfection. Journal of Infectious Diseases. 2009;200(6):947–954. doi: 10.1086/605476. [DOI] [PubMed] [Google Scholar]
- 58.Laskus T, Radkowski M, Piasek A, et al. Hepatitis C virus in lymphoid cells of patients coinfected with human immunodeficiency virus type 1: evidence of active replication in monocytes/macrophages and lymphocytes. Journal of Infectious Diseases. 2000;181(2):442–448. doi: 10.1086/315283. [DOI] [PubMed] [Google Scholar]
- 59.Kanto T, Hayashi N, Takehara T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. Journal of Immunology. 1999;162(9):5584–5591. [PubMed] [Google Scholar]
- 60.Bain C, Fatmi A, Zoulim F, Zarski JP, Trépo C, Inchauspé G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120(2):512–524. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 61.Agaugué S, Perrin-Cocon L, André P, Lotteau V. Hepatitis C lipo-viro-particle from chronically infected patients interferes with TLR4 signaling in dendritic cell. PLoS ONE. 2007;2(3, article e330) doi: 10.1371/journal.pone.0000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsubouchi E, Akbar SMF, Horiike N, Onji M. Infection and dysfunction of circulating blood dendritic cells and their subsets in chronic hepatitis C virus infection. Journal of Gastroenterology. 2004;39(8):754–762. doi: 10.1007/s00535-003-1385-3. [DOI] [PubMed] [Google Scholar]
- 63.Navas MC, Fuchs A, Schvoerer E, Bohbot A, Aubertin AM, Stoll-Keller F. Dendritic cell susceptibility to hepatitis C virus genotype 1 infection. Journal of Medical Virology. 2002;67(2):152–161. doi: 10.1002/jmv.2204. [DOI] [PubMed] [Google Scholar]
- 64.Goutagny N, Fatmi A, De Ledinghen V, et al. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. Journal of Infectious Diseases. 2003;187(12):1951–1958. doi: 10.1086/375350. [DOI] [PubMed] [Google Scholar]
- 65.Pachiadakis I, Pollara G, Chain BM, Naoumov NV. Is hepatitis C virus infection of dendritic cells a mechanism facilitating viral persistence? The Lancet Infectious Diseases. 2005;5(5):296–304. doi: 10.1016/S1473-3099(05)70114-6. [DOI] [PubMed] [Google Scholar]
- 66.Falcón V, Acosta-Rivero N, Shibayama M, et al. HCV core protein localizes in the nuclei of nonparenchymal liver cells from chronically HCV-infected patients. Biochemical and Biophysical Research Communications. 2005;329(4):1320–1328. doi: 10.1016/j.bbrc.2005.02.107. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe N, Aizaki H, Matsuura T, Kojima S, Wakita T, Suzuki T. Hepatitis C virus RNA replication in human stellate cells regulates gene expression of extracellular matrix-related molecules. Biochemical and Biophysical Research Communications. 2011;407(1):135–140. doi: 10.1016/j.bbrc.2011.02.125. [DOI] [PubMed] [Google Scholar]
- 68.Sansonno D, Lotesoriere C, Cornacchiulo V, et al. Hepatitis C virus infection involves CD34+ hematopoietic progenitor cells in hepatitis C virus chronic carriers. Blood. 1998;92(9):3328–3337. [PubMed] [Google Scholar]
- 69.Sansonno D, Iacobelli AR, Cornacchiulo V, Iodice G, Dammacco F. Detection of hepatitis C virus (HCV) proteins by immunofluorescence and HCV RNA genomic sequences by non-isotopic in situ hybridization in bone marrow and peripheral blood mononuclear cells of chronically HCV-infected patients. Clinical and Experimental Immunology. 1996;103(3):414–421. doi: 10.1111/j.1365-2249.1996.tb08296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tomás JF, Rodriguez-Iñigo E, Bartolomé J, Alegre A, Fernández-Rañada JM, Carreño V. Transplantation of allogeneic CD34-selected peripheral stem cells does not prevent transmission of hepatitis C virus from an infected donor. Bone Marrow Transplantation. 1999;24(1):109–112. doi: 10.1038/sj.bmt.1701810. [DOI] [PubMed] [Google Scholar]


