Abstract
Background
If effective cancer screening is more common in people with a family history of cancer, the relationship between family history and cancer incidence may become distorted.
Methods
To assess the impact of screening on the association between colorectal cancer family history and risk of colorectal cancer, we developed a model to simulate screening patterns in those with and without a family history.
Results
The introduction of screening reduces the apparent risk of colorectal cancer associated with family history in subsequent generations. This reduction becomes more pronounced as the difference in the uptake of screening between those with a family history and those without becomes larger.
Conclusion
A result of effective screening is that observed family history of colorectal cancer may no longer match inherited risk, and observed family history may fail to be a strong risk factor. This may have implications for exposure-disease relationships if screening is differentially associated with the exposure.
A first-degree family history of colorectal cancer is reported by 15%–20% of colorectal cancer cases in the general population.1,2 This includes persons with rare cancer family syndromes,3,4 as well as those in familial clusters of colorectal cancer that occur in the absence of a defined genetic syndrome.5 Overall, those whose parents, siblings, or children have been diagnosed with colorectal cancer are about twice as likely to develop the disease as those without a family history.6
Regardless of family history, colorectal screening represents one of the most promising approaches to reducing cancer burden through early detection and treatment.7,8 Screening tests for colorectal cancer are effective in decreasing the incidence of new disease through the detection and removal of precancerous lesions, and in improving survival through early detection and treatment.9–18 Thus, screening utilization can have considerable impact on population-level trends in cancer incidence and mortality.
Patterns of screening can also have broader, more complicated implications on patterns of disease if screening uptake depends upon a personal risk factor such as family history. Colorectal screening utilization is almost doubled among those with a family history of colorectal cancer.19 Because screening can detect precursor lesions and may result in removal of such lesions before they become invasive, persons with a family history could experience lower incidence rates than the general population simply due to greater use of screening.20
The consequences of such an altered association between family history and colorectal cancer incidence due to differential screening by family history may extend to future generations. If a screening test is effective at removing precursor lesions from the population, a person with a high underlying genetic risk of colorectal cancer who receives screening may avoid developing the disease. As a consequence of this primary prevention, the offspring of such screened individuals, who may have the same elevated genetic risk of cancer, will no longer have a recognized family history of cancer.
METHODS
To illustrate how the relationship between cancer and an observed family history of the disease may change due to screening utilization, we developed a model that estimates the risk of colorectal cancer relative to reported parental history of the disease in three consecutive generations. Model assumptions and definitions are outlined in eAppendix 1 (http://links.lww.com). The population of generation i can be divided into three subgroups:
N11(i): Persons with an observed family history of colorectal cancer;
N01(i): Persons without family history of colorectal cancer because the screening of a parent led to removal of precancerous lesions (i.e., those who would have had a family history of the disease if not for screening); and
N00(i): Persons without an observed CRC family history that truly do not have a family history of the disease.
Model in Generation 1
In the first generation, screening is not utilized and has not been previously utilized. Therefore, no one is incorrectly classified by family history (i.e. N01(1) = 0). In this generation, the relative risk (RR) of colorectal cancer comparing those with and those without an observed family history is set to 2.0.
Model in Generation 2
Screening is introduced in generation 2. Because screening was not utilized in generation 1, there is again no misclassification by family history (i.e. N01 (2) = 0 ). Details of the model application in generation 2 are outlined in eAppendix 2 (http://links.lww.com).
As screening is introduced, some colorectal cancers will be prevented in this second generation. Because the proportion of the population receiving screening is greater in those with an observed family history than in those without, the model predicts that the relative risk observed in generation 1 (RR=2.0) will be attenuated in generation 2. Furthermore, this attenuation will increase as screening efficacy improves.
Model in Generation 3
In the third generation, screening utilization is maintained in the population. Some will have parents with occult family history because screening in generation 2 prevented colorectal cancer from occurring (i.e. N01 (3) ≠ 0). Details of the model application in generation 3 are outlined in eAppendix 3 (http://links.lww.com).
In calculating the relative risk with family history in generation 3, children of those in generation 2 who had colorectal cancer prevented by screening are now classified in the group without an observed family history (i.e. N01), despite a potentially increased underlying genetic risk for the disease. There is an attenuated relative risk in generation 3 associated with observed family history due to both the higher uptake of screening for those in generation 3 with an observed family history, and also misclassification of those with an occult family history as lacking a family history of the disease (due to the introduction of screening in generation 2).
Scenarios for Model Simulation
We applied the model to scenarios of several colorectal cancer screening prevalences in those with an observed family history (f1) and those without (f0).
RESULTS
The Figure shows the attenuation of the relative risk with observed family history of colorectal cancer, given screening in the parent’s generation. With an increasing difference in screening fraction between those with and those without family history (i.e., moving from left to right along the X-axis, relative to a given point on the Y-axis), the attenuation of relative risk for family history becomes more pronounced. Attenuation is also more marked with higher population levels of screening coverage, where the relative difference in screening for those with and those without a family history remains constant. Estimated relative risks with family history in generation 2 and 3 are provided in the Table for a select series of possible screening scenarios. For example, with screening coverage of 70% in those with an observed family history and 30% in those without an observed family history, the relative risk for family history will fall from 2.00 in generation 1, to 1.23 in generation 2, to 1.17 in generation 3.
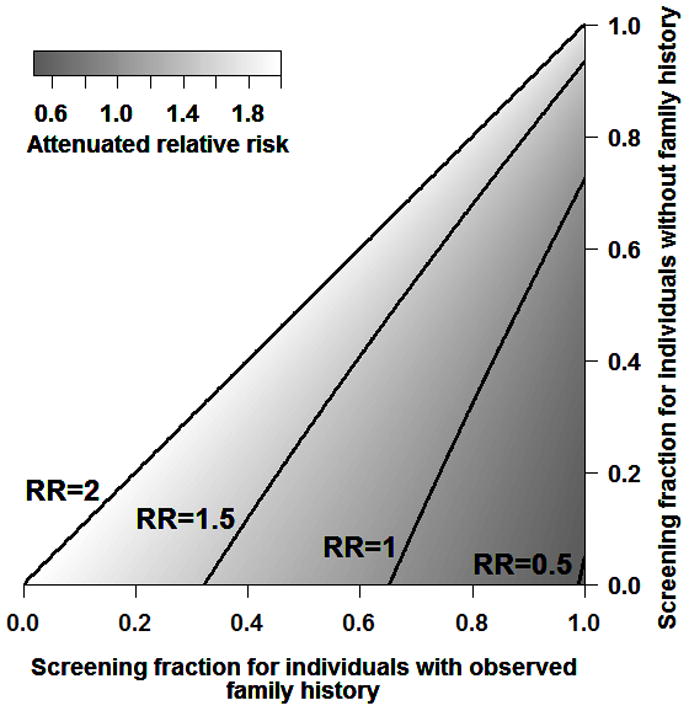
Table.
Colorectal Cancer Screening and Familial Risk of Colorectal Cancer in Three Generations under Different Scenarios of Screening Prevalence and Screening Efficacy*
| γ | f1 | f0 | Relative Risk with Family History, by Generation
|
||
|---|---|---|---|---|---|
| RR(1) a | RR(2) | RR(3) | |||
| 0.75 | 0.5 | 0.3 | 2.0 | 1.61 | 1.54 |
| 0.7 | 0.3 | 2.0 | 1.23 | 1.17 | |
| 0.7 | 0.5 | 2.0 | 1.52 | 1.42 | |
| 0.9 | 0.7 | 2.0 | 1.37 | 1.26 | |
|
| |||||
| 0.90 | 0.5 | 0.3 | 2.0 | 1.51 | 1.43 |
| 0.7 | 0.3 | 2.0 | 1.01 | 0.96 | |
| 0.7 | 0.5 | 2.0 | 1.35 | 1.25 | |
| 0.9 | 0.7 | 2.0 | 1.03 | 0.93 | |
γ indicates the proportion of colorectal cancers that can be prevented by screening; f1, the prevalence of screening in individuals with an observed colorectal cancer family history; f0, the prevalence of screening in individuals without an observed colorectal cancer family history; RR(i), relative risk in generation i.
RR(1) is assumed to be the true relative risk between observed colorectal cancer family history and colorectal cancer incidence.
DISCUSSION
As colorectal cancer screening is more widely performed and precursor lesions are more often removed, more cases of colorectal cancer will be prevented. Because those with a family history of the disease are screened at a higher rate, their risk will be reduced even further.
As previously described, attenuation of the relative risk in generation 2 reflects the benefits of screening: persons with family history are more likely to receive screening and thus more likely to avoid cancer diagnosis through the detection and treatment of precancerous lesions. The attenuation of the relative risk in generation 3 similarly reflects differential screening by observed family history, but also reflects misclassification of occult family history in persons whose parents would have been diagnosed with colorectal cancer in the absence of screening in generation 2 (i.e., N01(3)). As demonstrated in this simulation, many parents who would have developed colorectal cancer in the absence of screening remain cancer-free because of screening. Because screening prevented the disease in parents, the offspring no longer have an observed family history of disease to report, despite any underlying inherited genetic risk or shared environmental risk factors for the disease that remain unchanged. Thus, with increased screening uptake, observed family history may no longer match true, inherited family risk (i.e., what would have happened in the absence of screening), and observed family history will fail to appear as a strong and consistent risk factor for developing colorectal cancer.
Our model is simplified in that it considers only cancer history in a person’s parents. Although parental history is most often collected in epidemiologic studies, history among other relatives are also likely to be important. However, the overall principle would remain valid; as colorectal cancer is avoided due to screening, a person’s true risk profile may no longer match the observed family history of cancer. Our model also does not address a potential exaggeration of the association between observed family history and colorectal cancer: in the situation where screening is very effective and prevents most disease from occurring, cancers that occur despite screening may be more aggressive and associated with a strong, inherited family history genotype.
Ultimately, as screening utilization increases, the prevalence of risk factors for cancer and their association with disease may change over time. We have illustrated this potential effect using the example of differential colorectal screening uptake by observed family history of the disease. Similarly, if other exposures are associated with greater screening, the relative risk for that exposure disease relationship may change over time. This may also apply to other cancers, although with possibly different effects depending on the capacity of screening to identify and treat precancerous lesions versus lesions with limited malignant potential.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Cancer Institute at the National Institutes of Health (grant numbers U24CA074794, T32CA009168, and R25CA94880), and by the Canadian Institutes of Health Research Operating Grant entitled “Statistical Methods for Epidemiological Investigations”.
We thank Emily White and Yingye Zheng for their helpful reviews and thoughtful comments on this manuscript.
Footnotes
SDC: Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com). This content is not peer-reviewed or copy-edited; it is the sole responsibility of the author.
References
- 1.Newcomb PA, Taylor JO, Trentham-Dietz A. Interactions of familial and hormonal risk factors for large bowel cancer in women. Int J Epidemiol. 1999;28:603–608. doi: 10.1093/ije/28.4.603. [DOI] [PubMed] [Google Scholar]
- 2.Potter JD. Colorectal cancer: molecules and populations. J Natl Cancer Inst. 1999;91:916–32. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- 3.Lindor NM, Greene MH. The concise handbook of family cancer syndromes. Mayo Familial Cancer Program. J Natl Cancer Inst. 1998;90:1039–71. doi: 10.1093/jnci/90.14.1039. [DOI] [PubMed] [Google Scholar]
- 4.Potter JD, Lindor NM. Genetics of colorectal cancer. New York ; London: Springer; 2009. [Google Scholar]
- 5.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 6.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003. doi: 10.1111/j.1572-0241.2001.04677.x. [DOI] [PubMed] [Google Scholar]
- 7.Chu KC, Kramer BS, Smart CR. Analysis of the role of cancer prevention and control measures in reducing cancer mortality. J Natl Cancer Inst. 1991;83:1636–43. doi: 10.1093/jnci/83.22.1636. [DOI] [PubMed] [Google Scholar]
- 8.Etzioni R, Urban N, Ramsey S, et al. The case for early detection. Nat Rev Cancer. 2003;3:243–52. doi: 10.1038/nrc1041. [DOI] [PubMed] [Google Scholar]
- 9.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103:1541–9. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 10.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 11.Hoff G, Grotmol T, Skovlund E, Bretthauer M. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ. 2009;338:b1846. doi: 10.1136/bmj.b1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selby JV. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. [see comments.] New England Journal of Medicine. 1992;326:653–7. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 13.Newcomb PA, Norfleet RG, Storer BE, Surawicz TS, Marcus PM. Screening sigmoidoscopy and colorectal cancer mortality. J Natl Cancer Inst. 1992;84:1572–1575. doi: 10.1093/jnci/84.20.1572. [DOI] [PubMed] [Google Scholar]
- 14.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 15.Singh H, Nugent Z, Mahmud SM, Demers AA, Bernstein CN. Predictors of colorectal cancer after negative colonoscopy: a population-based study. Am J Gastroenterol. 2010;105:663–73. doi: 10.1038/ajg.2009.650. quiz 674. [DOI] [PubMed] [Google Scholar]
- 16.Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 17.Mandel JS. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. [see comments.] Journal of the National Cancer Institute. 1999;91:434–7. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 18.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. [letter; comment]. [see comments] New England Journal of Medicine. 2000;343:1603–7. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 19.Shah M, Zhu K, Palmer RC, Wu H. Family history of cancer and utilization of prostate, colorectal and skin cancer screening tests in U.S. men. Prev Med. 2007;44:459–64. doi: 10.1016/j.ypmed.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Bloom JR, Stewart SL, Oakley-Girvans I, Banks PJ, Chang S. Family history, perceived risk, and prostate cancer screening among African American men. Cancer Epidemiol Biomarkers Prev. 2006;15:2167–73. doi: 10.1158/1055-9965.EPI-05-0738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


