Abstract
Developing an effective vaccine against HIV infection remains an urgent goal. We used a DNA prime/fowlpox virus boost regimen to immunize Chinese rhesus macaques. The animals were challenged intramuscularly with pathogenic molecularly cloned SHIV-KB9. Immunogenicity and protective efficacy of vaccines were investigated by measuring IFN-γ levels, monitoring HIV-specific binding antibodies, examining viral load, and analyzing CD4/CD8 ratio. Results show that, upon challenge, the vaccine group can induce a strong immune response in the body, represented by increased expression of IFN-γ, slow and steady elevated antibody production, reduced peak value of acute viral load, and increase in the average CD4/CD8 ratio. The current research suggests that rapid reaction speed, appropriate response strength, and long-lasting immune response time may be key protection factors for AIDS vaccine. The present study contributes significantly to AIDS vaccine and preclinical research.
1. Introduction
Human immunodeficiency virus type 1 (HIV-1) is the aetiological agent of acquired immune deficiency syndrome (AIDS). Since the first case of HIV-1 infection was reported in Los Angeles in 1981 [1], more than 68 million people have been infected with HIV worldwide. Nearly 25 million people have died, and approximately 33.3 million people are suffering from HIV globally at the end of 2009 according to the 2010 global report published by the Joint United Nations Programme on HIV/AIDS [2]. AIDS/HIV remains the deadliest crisis and the greatest social, economic, and public health challenge in modern times because of the absence of effective prophylaxis or therapy methods [3–9].
Undoubtedly, the best and most economical solution to eradicate or control the spread of HIV-1 is to develop safe and effective vaccine. Although considerable efforts have been devoted toward this goal, the success of available vaccines has not been demonstrated [3, 4, 10, 11]. Previous studies have determined that the HIV-specific CD8+ cytotoxic T-cell responses play key roles in controlling viral replication, which could reduce viral loads and postpone disease progression in individuals who are infected with HIV-1 [12–16]. Immunization with polyvalent antigens may likely stimulate more effective immunity than a single antigen as described in an earlier study [12, 17]. Thus, combining multi-CTL epitopes derived from different genes of HIV-1 to construct a chimeric antigen may be a better strategy to develop new vaccines. Previously, we designed and constructed a new immunogen, which includes 29 multiepitopes and the p24 protein of HIV-1 as carrier molecule. The selected epitopes covered the most dominant epitopes derived from structural, regulatory, and accessory proteins of HIV, such as Gag, Env, Pol, RT, IN, Vpr, Tat, and Nef; HLA-DR epitope, ER signal peptide, and Kozak sequence were considered as well. The antigenicity and immunogenicity were evaluated in vitro and in vivo [18–20].
To further confirm the immunogenicity and protective effect of this vaccine, we immunized Chinese rhesus macaques with a DNA prime/fowlpox virus boost regimen. These animals were challenged intravenously with pathogenic molecularly cloned SHIV-KB9. The monkeys were monitored by measuring their IFN-γ levels, HIV-specific binding antibodies, viral load, and CD4/CD8 ratio and by analyzing the immunogenicity and the protective effect of vaccine to facilitate clinical trials.
2. Materials and Methods
2.1. Animals
Chinese-origin rhesus macaques (Ch Rh), 3–5 years of age, both female and male, and with no signs of clinical diseases, were provided by the Laboratory Animal Center, Academy of Military Medical Sciences. Sixteen Chinese rhesus macaques from Guangxi, aged 3–5 years, weighing 3–5 kg, and without simian immunodeficiency virus (SIV), monkey T lymphocytes of I virus (STLV), monkey ART D-type virus (SRV/D), or B virus infection, were bred and provided by the experimental Animal Center of Military Medical Sciences. The present research project was approved by the relevant ethics review committee. Animal husbandry and sample collection were in accordance with relevant biosecurity requirements.
2.2. Vaccines
The vaccines used in the current study are recombinant DNA vaccine rDNA/pVMp24 and recombinant fowlpox virus rFPV/Mp24. Both are epitope-based vaccines containing the same immunogens, which includes a Kozak translation initiation sequence, ER signal peptide, 29 HIV dominant epitopes (24 CTL or CD8 T-cell epitopes and 5 B-cell epitopes), and HIV-1 p24 protein. The immunogens were provided by professor Ningyi Jin of the Institute of Military Veterinary Medicine, Academy of Military Medical Sciences. The schematic representation of the rDNA and rFPV vaccine constructs is shown in Figure 1.
Figure 1.
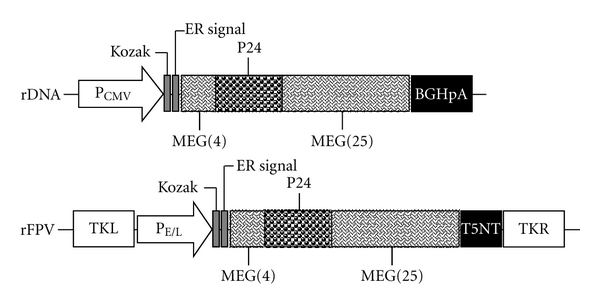
Schematic representation of the rDNA and rFPV vaccine constructs. The functional elements of the expression vector are the following. PCMV: human cytomegalovirus (CMV) immediate-early promoter/enhancer; Kozak: a Kozak translation-initiation sequence and an initiation codon (ATG) for proper initiation of translation; ER signal: endoplasmic reticulum signal peptide; MEG(4): multi-epitope gene (including 4 epitopes); P24: HIV-1 capsid protein; MEG(25): multi-epitope gene (including 25 epitopes); BGHpA: Bovine growth hormone (BGH) polyadenylation signal; TKL: the left recombinant fowlpox virus; PE/L: early and late promoter of fowlpox virus; T5NT: terminal signal of fowlpox virus; TKR: the right recombinant fowlpox virus.
2.3. Immunization and Challenge Experiments
The Chinese rhesus macaques were randomly divided into 2 groups (4 macaques per group). Each group was primed intramuscularly (i.m.) with rDNA/pVMp24 (500 μg/per animal) vaccine and empty vector pVAX1 control at weeks 0, 4, and 10 and subsequently boosted with 109 plaque-forming unit (PFU) rFPV/Mp24 vaccine and wild-type FPV at week 18. At week 28, the macaques were challenged intravenously with 20 MID50 of SHIV-KB9 provided by professor Yiming Shao of the Chinese Center for Disease Control and Prevention.
The immunization schedule and routes of administration are outlined in Figure 2.
Figure 2.
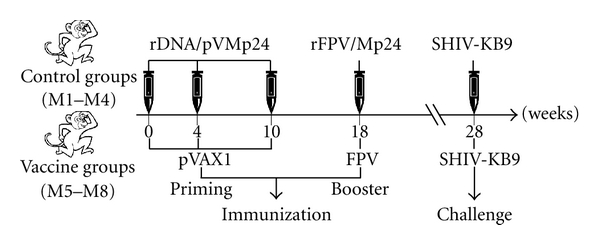
Immunization and challenge schedule. rDNA: recombinant DNA; rFPV: recombinant FPV.
2.4. Sample Collection and Processing
In the presence of EDTA anticoagulant, hind-limb venous blood was collected at 7, 13, 21, 28, and 35 d postinfection. The samples were sent to the laboratory within 6 h. Part of the unclotted blood was processed for blood routine examination and flow analysis. The remaining samples were centrifuged at 1700 rpm and kept at room temperature for 10 min. Plasma was analyzed for virus RNA load and antibodies. PBMC was separated from the blood cell for enzyme-linked immunospot (ELISPOT) and other analyses. Plasma was stored at −20°C, and the PBMC was frozen in liquid nitrogen.
2.5. IFN-γ ELISPOT Detection
ELISPOT assays were conducted to evaluate the gamma interferon-(IFN-γ-)secreting cells. PBMCs were isolated by Ficoll gradient centrifugation as previously described [21]. ELISPOT responses were detected using the monkey IFN-γ ELISPOT kit (U-CyTech Biosciences, Utrecht, the Netherlands) according to the instructions of the manufacturer. Each sample was stimulated in triplicate by adding a single pool of p24 peptides (15-mer HIV-1 consensus p24 peptides with an 11-amino-acid overlap, synthesized by HD Biosciences Co., Ltd., Shanghai, China) with a final concentration of 4 μg/mL for each peptide. PMA (50 ng/mL) and ionomycin (1 μg/mL) were used as positive controls, whereas RPMI 1640 medium was used as negative control. The results were indicated as spot-forming cells (SFC)/million PBMC. A positive response was defined as 4 times ELISPOT points higher than the negative control points and greater than 50 SFC/106 cells at each time point.
2.6. Detection of HIV-1-Specific Binding Antibodies in the Serum
Sequence alignment indicates that HIV-1 p24 and SHIV p24 proteins have strong homology. Thus, the antibodies induced by the SHIV virus have a strong cross-immune response to the HIV antigen. In the present study, the HIV-1-specific antibody responses were measured by the third-generation total HIV-binding antibody diagnostic kit (Vironostika HIV Uni-Form II Plus O, BioMérieux Corporate, France). The experiment was conducted by employing the enzyme-linked immunosorbent assay according to the protocols of the manufacturer and the literature.
2.7. Measurement of Plasma Viral RNA Load
The plasma viral RNA was extracted by QIAGEN Viral RNA minikit (QIAGEN company) and analyzed by real-time PCR using TaqMan EZ RT-PCR Core Reagents kit (ABI Company) and ABI Prism 7700 apparatus. SHIV viral RNA in the samples was quantified by the standard curve derived from RNA standards.
2.8. CD3, CD4, and CD8 Lymphocyte Subset Analysis
We used three nonhuman primate antibodies, FITC-CD3, PerCP-CD4, and PE-CD8 (BD Pharmingen Inc.) in the flow analysis. Each flow tube contained 5 μL of each antibody and 100 μL of whole blood. Bland control and CD3-, CD4-, and CD8-stained controls were set up. The contents were mixed evenly by shaking and incubated under darkness at room temperature for 20 min. During shaking, 1 mL 1 x PBS dissolved in 500 μL hemolysin was added to lyse the red blood cells. The mixture was kept for 10 min until the liquid became translucent. Subsequently, the mixture was centrifuged at 1500 rpm for 5 min. The supernatant was discarded and the cells were scattered by vibration. Formaldehyde fixative (300 μL) was added and the samples were analyzed by flow cytometry (BD FACSCalibur).
2.9. Statistical Analysis
Differences between the groups were analyzed by Student's t-test. The results were expressed as mean ± SD. P value < 0.05 was considered significant.
3. Results
3.1. ELISPOT Test of IFN-γ
ELISPOT method was used to determine the IFN-γ-secreting T-cell immune responses stimulated by HIV-1 p24 peptide library. Compared with the control animals, the vaccine group produced strong ELISPOT positive responses after immunization, as shown in Figure 3. Two weeks after rFPV booster immunization (20 w), the ELISPOT response was significantly enhanced, with an average of 437SFC/106 cells. One empty vector also showed weak positive responses, indicating that fowlpox virus vector itself has certain nonspecific T-cell responses.
Figure 3.
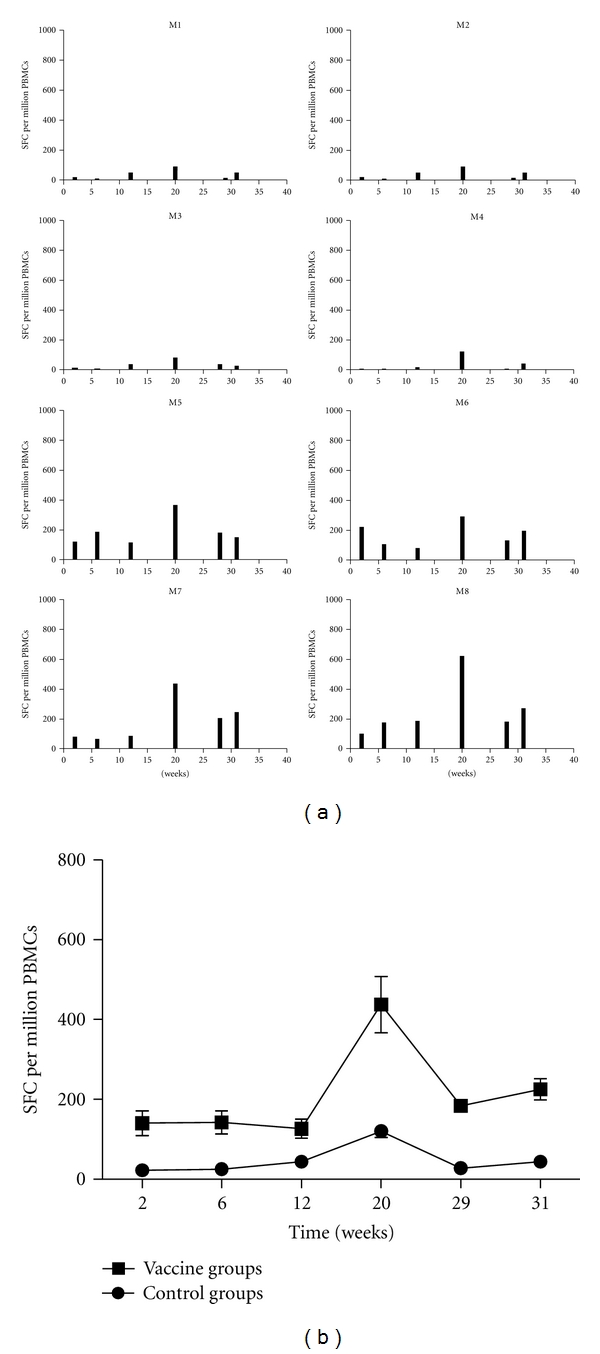
Number of IFN-γ-secreting PBMC was detected by ELISPOT after stimulation with p24 peptide pools at various times after immunization and challenge. (a) Histogram bars represent the average number of spots per million cells detected in duplicate cultures for each animal, after subtraction of the average number of spots found in duplicate control cultures of PBMC without stimulation. (b) Dynamics of the IFN-γ response was observed throughout the experiment for each group.
After the SHIV-KB9 virus attacks, all animals in the immunized group showed different degrees of ELISPOT-positive responses (peak in the range of 115–890 SFC/106 cells). At day 7 postinfection (29 w), a rapid increase in ELISPOT response was detected, and at day 21 (31 w), the ELISPOT response remained at an appropriate response level. These results suggest that the vaccine produced in the present study has good cellular memory immune response.
3.2. Measurement of Serum-Specific Binding Antibodies
The antibody analysis results after infection are shown in Table 1. The control group (A) showed weak positive response at day 35 (M1-M2). M3 showed positive response at days 28 and 35, but the antibody titers did not increase. However, antibody titers of all animals in the vaccine group showed slow, steady rise. The antibody production time was significantly earlier (M5, M7, and M8 at day 21) than the other group, indicating that the vaccine induced significant humoral immune memory response.
Table 1.
Whole-virus HIV-specific binding antibody titers after the challenge.
| Groups | Animals | Whole-virus HIV-1 specific antibody titers | ||||
|---|---|---|---|---|---|---|
| 7 days | 14 days | 21 days | 28 days | 35 days | ||
| Control | M1 | 0 | 0 | 0 | 0 | 1 |
| M2 | 0 | 0 | 0 | 0 | 1 | |
| M3 | 0 | 0 | 0 | 1 | 1 | |
| M4 | 0 | 0 | 0 | 0 | 0 | |
| Vaccine | M5 | 0 | 0 | 1 | 1 | 3 |
| M6 | 0 | 0 | 0 | 1 | 2 | |
| M7 | 0 | 0 | 1 | 1 | 2 | |
| M8 | 0 | 0 | 1 | 2 | 3 | |
3.3. Measurement of Plasma Viral RNA Load
The results of plasma viral RNA load are shown in Figure 4. The peak value of average viral load appeared at day 17 in all the vaccine groups, which was later than that in the control group (13 d). The peak value of the average was significantly lower than that of the negative control group (P < 0.05), indicating that the vaccine has certain inhibitory effects on virus replication.
Figure 4.
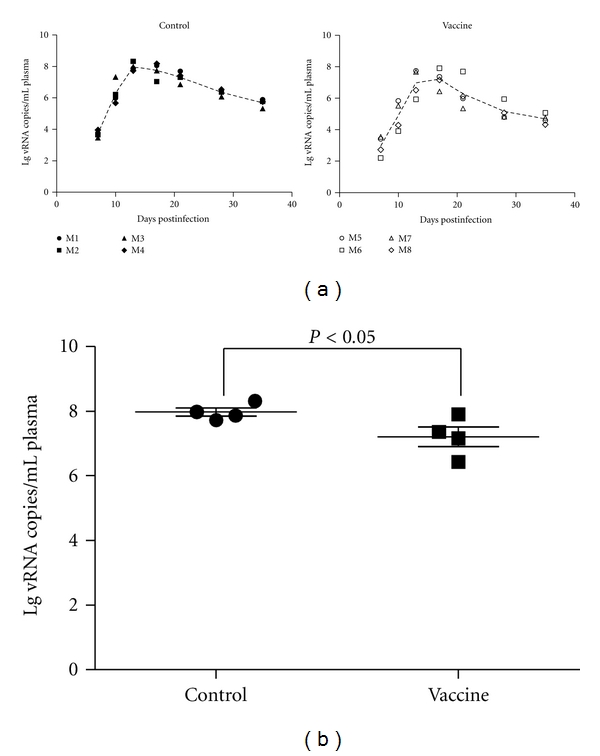
Plasma viral load analysis post-SHIV-KB9 challenge. Viremia was quantified by RT-PCR. (a) Dynamics of viral load for each group. (b) Average value of viral load for each group.
3.4. T-Lymphocyte Subset Analysis
Flow analysis of the T-lymphocyte subsets is shown in Figure 5. When the rhesus macaques were infected by the virus, all the animals in the control group exhibited continuous decline in terms of CD4/CD8 ratio, with the inversion phenomenon occurring at day 13. During the entire experimental time, no recovery of CD4/CD8 was detected. However, the overall average ratio of CD4/CD8 in the vaccine group declined at first and subsequently increased, and the average ratio of CD4/CD8 recovered to a relatively higher level at day 35.
Figure 5.
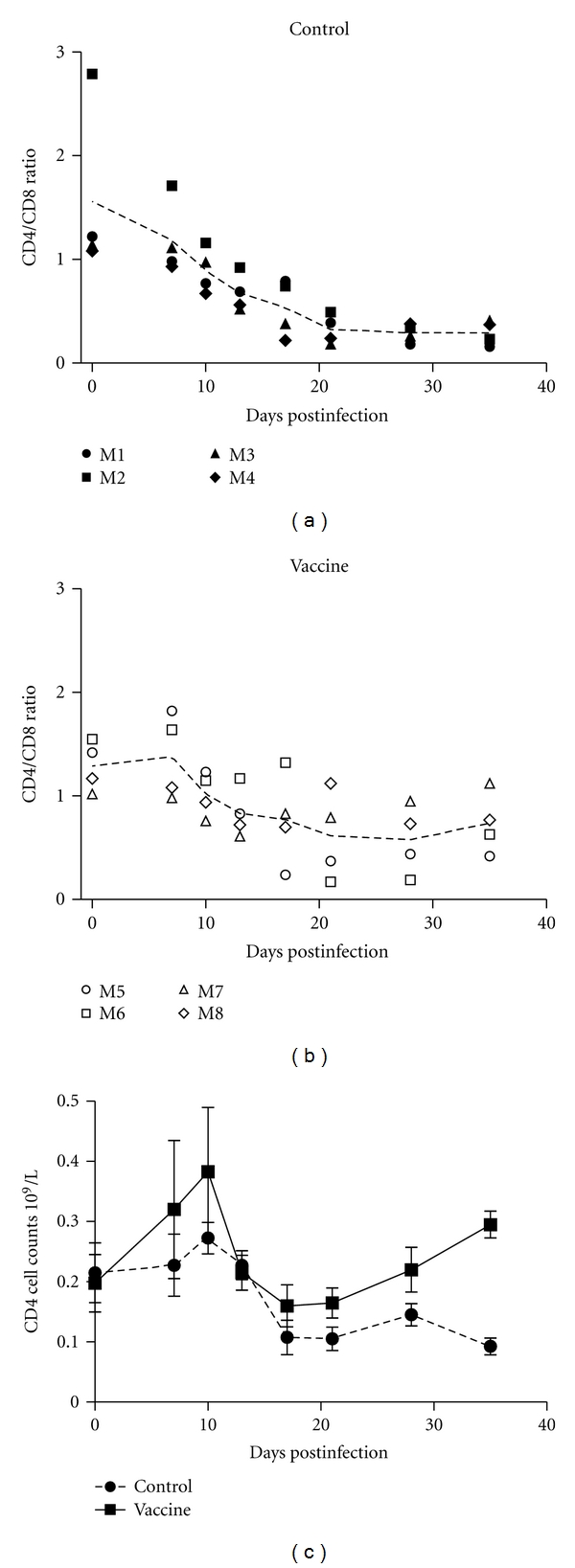
CD4/CD8 ratio analysis and total CD4 counts post-SHIV-KB9 challenge.
4. Discussion and Conclusion
SIV/rhesus macaques are the most effective models for the investigation of the mechanisms for HIV pathogenesis and prevention [22, 23]. However, the antigenic differences among SIV, HIV-1, and HIV-2 cause significant limitations to this model [24–26]. In recent years, the use of chimeric simian/human immunodeficiency virus (SHIV) instead of SIV as an infection model has increased [27]. Previously, most HIV vaccine trials in monkeys involved Indian rhesus macaques [28, 29]. The SHIV-KB9/Indian rhesus model is widely used in numerous research institutions and has become a reference model for the evaluation of the immune protective effects of various vaccines [30, 31]. However, due to the shortage of Indian rhesus animal resources, the Chinese rhesus population (comprising approximately 30 million Chinese wild rhesus macaques) has become an important alternative source [32]. Previous studies have suggested that Chinese rhesus macaques (ChR) are better models for AIDS vaccine research [33–35]. The current study made use of the SHIV/Chinese rhesus model to evaluate vaccine immunogenicity and protective efficacy.
Previous studies have shown that natural viral antigen may contain components with negative effects on protective responses including several immune suppression and immune pathological sequences. These components can interfere with the immune response and block the cell signaling pathway, leading to loss of balance for Th1/Th2 type immune response in the body [4, 36–39]. Consequently, this can result in immune response deviation or defect. Therefore, screening, alteration, or modification of the natural antigen at the level of epitope to remove negative factors on immune response while ensuring response specificity is extremely important for vaccine designing. In addition, vaccines with only single antigen gene have no significant immune effects. Thus, the vaccine design needs to incorporate multiple and different immunogens as well as structural variety to obtain broad virus-specific immune response [17, 40, 41]. Thus, the current research presents a multi-epitope gene based on the advantageous HIV epitope using macromolecular particle protein p24 as carrier molecule. The multi-epitope gene contains HIV-specific T-cell epitope, HIV-specific B-cell epitope, universal Th epitope, B-cell epitope, and one B-cell epitope from tetanus toxin (TT). In addition to the function of structural proteins such as Gag, Env, and Pol, the vaccine also strengthens the important role of nonstructural proteins (vpr, nef, tat, etc.) in immune response and viral replication control. For chronic infections, such as HIV, the cellular immune response, particularly CTL, is of considerable significance to clear virus-infected cells [42–44]. Thus, we emphasized the epitopes of nonstructural proteins of HIV, and these epitopes were selected mainly from the classic, advantageous kind with conservation capacity, broad cross-reactivity, and have been proven in both patients and animal experiments. This will allow focusing on the nonstructural protein CTL of HIV. Considering the new features of domestic and Asian HIV epidemic, the epitope was adjusted based on the recently published HIV subtype at GenBank (Genbank Accession Number AX149898). During the vaccine construction, Kozak rules were considered. Peptide signal sequence, codon preference, and other factors that can increase antigen transcription and translation were targeted, with the goal of achieving an effective candidate vaccine with induction-specific immune response to break the immune resistance to HIV antigens in the host. Essentially, the aims are preventing and controlling HIV infection as well as providing remedy to the disease after vaccination.
“Prime-boost” immunization strategy is a sensational topic in current vaccine study [45]. The first reported use of this strategy was in the immunization of influenza virus [46]. Currently, this immunization strategy is widely used in the research of a variety of pathogen vaccines, especially in AIDS vaccine study [17, 41, 47].
In the study, we used an rDNA/rFPV “prime-boost” coimmunization strategy to immunize the Chinese rhesus macaques. Moreover, we used the SHIV-KB9 infection to analyze the immune protective effect of the vaccine. The results show that, during the primary immunization of rDNA/pVMp24 vaccine, the vaccine only induced a relatively low level of IFN-γ-secreting T-cell immune response in the immunized group, whereas after rFPV booster immunization, the immune response significantly increased. This is consistent with the related literature [41, 48]. The primary immunization of DNA vaccines can induce high-affinity T cells, but with low levels of immunity. However, after fowlpox virus booster immunization, the fowlpox enhances the immune effect of primary immunization through proinflammatory immune response of the body.
IFN-γ-secreting T-cell immune response analysis performed one week and three weeks after the SHIV-KB9 virus attack shows that when exposed to the virus, all the vaccinated groups quickly activated antigen-specific CD8+ T-cell immunity. Rapid increase in ELISPOT response was detected 7 days after infection and was maintained at an appropriate response level at day 21. This indicates that the vaccine effectively extends the memory CD8+ T-cell survival and maintains the capability of T-cell immune responses.
With regard to humoral immunity, we focused on the production rate of p24 antibodies, antibody titers, and antibody duration. The M1 and M2 in the negative control group showed weak HIV-1 binding antibody response after 35 days of virus attack. M3 showed positive response at days 28 and 35. However, the antibody titers did not rise. M4 remained negative during the experiment. Three animals in the immunized group (M5, M7, M8) showed positive antibody response at day 21, and the antibody titers exhibited a smooth, steady rise over time, indicating that the immunized group effectively induced the production of specific antibodies.
Viral load is a major parameter which can be used to evaluate whether HIV vaccine can induce immune protection, and the viral load change after the viral attack predicts the progress of the disease. In the present study, the positive response of viral load appeared in the control animals after 7 days of viral attack, and the peak value of average load occurred at day 13. In contrast, the peak value of the average load occurred at day 17 in the immunized group, and the peak value was lower than that in the control group. These results suggest that the vaccine delays the production of virus peak point at the early-infection stage and reduces the viral load at the peak point.
After virus infection, all the rhesus macaques in the negative control group exhibited the inversion phenomenon in CD4/CD8 ratio at day 13, and the ratio continued to decline rapidly. No recovery of CD4/CD8 ratio was observed during the experiment. However, in the immunized animals, the average CD4/CD8 ratio decreased at first, subsequently increased, and finally was restored to a relatively higher level at day 35. This indicates that the vaccine effectively induced T-cell proliferation.
The fowlpox virus expression system in the study is a newly developed poxvirus vector based on vaccinia virus vector [48, 49]. The vector inherits the same advantages of vaccinia virus vectors. Furthermore, the fowlpox virus has a narrow host range and more safe [50, 51]. Except for consideration on vectors, the immunogen design is also a crucial factor for good vaccine. In the present study, we selected and designed an optimal antigen combination containing HIV-1 advantageous epitope by scanning and analyzing the entire HIV-1 genome, searching the authoritative database, and referring to the new features of Asian and domestic HIV epidemic strains. In addition, we utilized p24 protein as a molecular scaffold. Based on the secure plasmid DNA and the new FPV transfer vector, we constructed the AIDS vaccine containing the above-mentioned antigens. The preliminary monkey immune experiments and infection study show that this vaccine can decrease the peak value of viral load at acute phase, delay the peak time, and make the viral load rapidly decline. The results indicated that the vaccine constructed in the current study has certain immune protection effects. And this provides new ideas and methods for AIDS vaccine development. We are currently conducting the safety, quality control, and other preclinical researches for this vaccine so as to move forward to the next phase of the clinical study.
Acknowledgments
This work was supported by Grants from the National 863 Project of China (no. 2006AA02Z447) and National Natural Science Foundation of China (no. 81001342).
References
- 1.Sinoussi FB, Chermann JC, Rey F. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2. http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf.
- 3.Girard MP, Osmanov SK, Kieny MP. A review of vaccine research and development: the human immunodeficiency virus (HIV) Vaccine. 2006;24(19):4062–4081. doi: 10.1016/j.vaccine.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Barouch DH. Challenges in the development of an HIV-1 vaccine. Nature. 2008;455(7213):613–619. doi: 10.1038/nature07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS, Hellmann N, Levy JA, Decock K, Lange J. The spread, treatment, and prevention of HIV-1: evolution of a global pandemic. Journal of Clinical Investigation. 2008;118(4):1244–1254. doi: 10.1172/JCI34706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas C. Roadblocks in HIV research: five questions. Nature Medicine. 2009;15(8):855–859. doi: 10.1038/nm0809-855. [DOI] [PubMed] [Google Scholar]
- 7.Fauci AS. 25 years of HIV. Nature. 2008;453(7193):289–290. doi: 10.1038/453289a. [DOI] [PubMed] [Google Scholar]
- 8.Wainberg MA, Jeang KT. 25 years of HIV-1 research—progress and perspectives. BMC Medicine. 2008;6:p. 31. doi: 10.1186/1741-7015-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haigwood NL, Hirsch VM. Blocking and tackling HIV. Nature Medicine. 2009;15(8):841–842. doi: 10.1038/nm0809-841. [DOI] [PubMed] [Google Scholar]
- 10.Andersson J. HIV after 25 years: how to induce a vaccine? Journal of Internal Medicine. 2008;263(3):215–217. doi: 10.1111/j.1365-2796.2007.01919.x. [DOI] [PubMed] [Google Scholar]
- 11.Brinckmann S, da Costa K, van Gils MJ, et al. Rational design of HIV vaccines and microbicides: report of the EUROPRISE network annual conference 2010. Journal of Translational Medicine. 2011;9:p. 40. doi: 10.1186/1479-5876-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen TM, Altfeld M, Geer SC, et al. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. Journal of Virology. 2005;79(21):13239–13249. doi: 10.1128/JVI.79.21.13239-13249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiepiela P, Ngumbela K, Thobakgale C, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nature Medicine. 2007;13(1):46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 14.Watkins DI. The hope for an HIV vaccine based on induction of CD8+ T lymphocytes—a review. Memorias do Instituto Oswaldo Cruz. 2008;103(2):119–129. doi: 10.1590/s0074-02762008000200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Piechocka-Trocha A, Miura T, et al. Differential neutralization of human immunodeficiency virus (HIV) replication in autologous CD4 T cells by HIV-specific cytotoxic T lymphocytes. Journal of Virology. 2009;83(7):3138–3149. doi: 10.1128/JVI.02073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blish CA, Sather DN, Sellhorn G, et al. Comparative immunogenicity of subtype A human immunodeficiency virus type 1 envelope exhibiting differential exposure of conserved neutralization epitopes. Journal of Virology. 2010;84(5):2573–2584. doi: 10.1128/JVI.01687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shu Y, Winfrey S, Yang ZY, et al. Efficient protein boosting after plasmid DNA or recombinant adenovirus immunization with HIV-1 vaccine constructs. Vaccine. 2007;25(8):1398–1408. doi: 10.1016/j.vaccine.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Jin NY, Jin HT, et al. Construction and immunogenicity of multiple-epitope DNA vaccine against HIV. Chinese Journal of Biologicals. 2007;20(3):178–180. [Google Scholar]
- 19.Shen ZW, Jin HT, Li C, et al. Adjuvant effects of dual co-stimulatory molecules on cellular responses to HIV multiple-epitope DNA vaccination. Chemical Research in Chinese Universities. 2009;25(3):347–352. [Google Scholar]
- 20.Zhang L, Jin N, Song Y, et al. Construction and characterization of a recombinant fowlpox virus containing HIV-1 multi-epitope-p24 chimeric gene in mice. Science in China Series C. 2007;50(2):212–220. doi: 10.1007/s11427-007-0017-1. [DOI] [PubMed] [Google Scholar]
- 21.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scandinavian Journal of Clinical and Laboratory Investigation, Supplement. 1968;97:77–89. [PubMed] [Google Scholar]
- 22.Desrosiers RC, Ringler DJ. Use of simian immunodeficiency viruses for AIDS research. Intervirology. 1989;30(6):301–312. doi: 10.1159/000150108. [DOI] [PubMed] [Google Scholar]
- 23.Desrosiers RC, Wyand MS, Kodama T, et al. Vaccine protection against simian immunodeficiency virus infection. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(16):6353–6357. doi: 10.1073/pnas.86.16.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li JT, Halloran M, Lord CI, et al. Persistent infection of macaques with simian-human immunodeficiency viruses. Journal of Virology. 1995;69(11):7061–7071. doi: 10.1128/jvi.69.11.7061-7067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joag SV, Li Z, Foresman L, et al. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. Journal of Virology. 1996;70(5):3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn CS, Beyer C, Kieny MP, et al. High viral load and CD4 lymphopenia in rhesus and cynomolgus macaques infected by a chimeric primate lentivirus constructed using the env, rev, tat, and vpu genes from HIV-1 Lai. Virology. 1996;223(2):351–361. doi: 10.1006/viro.1996.0486. [DOI] [PubMed] [Google Scholar]
- 27.Narayan SV, Mukherjee S, Jia F, et al. Characterization of a neutralization-escape variant of SHIV(KU-1), a virus that causes acquired immune deficiency syndrome in pig-tailed macaques. Virology. 1999;256(1):54–63. doi: 10.1006/viro.1999.9605. [DOI] [PubMed] [Google Scholar]
- 28.Joag SV. Primate models of AIDS. Microbes and Infection. 2000;2(2):223–229. doi: 10.1016/s1286-4579(00)00266-5. [DOI] [PubMed] [Google Scholar]
- 29.Johnston MI. The role of nonhuman primate models in AIDS vaccine development. Molecular Medicine Today. 2000;6(7):267–270. doi: 10.1016/s1357-4310(00)01724-x. [DOI] [PubMed] [Google Scholar]
- 30.Heeney JL. Primate models for AIDS vaccine development. AIDS. 1996;10(supplement A):S115–122. doi: 10.1097/00002030-199601001-00016. [DOI] [PubMed] [Google Scholar]
- 31.Karlsson GB, Halloran M, Li J, et al. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. Journal of Virology. 1997;71(6):4218–4225. doi: 10.1128/jvi.71.6.4218-4225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen J. AIDS research. Vaccine studies stymied by shortage of animals. Science. 2000;287(5455):959–960. doi: 10.1126/science.287.5455.959. [DOI] [PubMed] [Google Scholar]
- 33.Ling B, Veazey RS, Luckay A, et al. SIV(mac) pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. AIDS. 2002;16(11):1489–1496. doi: 10.1097/00002030-200207260-00005. [DOI] [PubMed] [Google Scholar]
- 34.Crowe S. SIVmac pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans, by Stellbrink et al. AIDS. 2003;17(supplement 4):S107–S108. [PubMed] [Google Scholar]
- 35.Monceaux V, Viollet L, Petit F, et al. CD4+ CCR5+ T-cell dynamics during simian immunodeficiency virus infection of Chinese rhesus macaques. Journal of Virology. 2007;81(24):13865–13875. doi: 10.1128/JVI.00452-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balter M. Can immune systems be trained to fight HIV? Science. 1999;286(5444):1470–1471. doi: 10.1126/science.286.5444.1470. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J. AIDS research. Novel protein delivers HIV to target cells. Science. 2000;287(5458):p. 1567. doi: 10.1126/science.287.5458.1567a. [DOI] [PubMed] [Google Scholar]
- 38.Rappuoli R, Aderem A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature. 2011;473(7348):463–469. doi: 10.1038/nature10124. [DOI] [PubMed] [Google Scholar]
- 39.Virgin HW, Walker BD. Immunology and the elusive AIDS vaccine. Nature. 2010;464(7286):224–231. doi: 10.1038/nature08898. [DOI] [PubMed] [Google Scholar]
- 40.Lu J, Wang C, Zhou Z, et al. Immunogenicity and protective efficacy against murine tuberculosis of a prime-boost regimen with BCG and a DNA vaccine expressing ESAT-6 and Ag85A fusion protein. Clinical and Developmental Immunology. 2011;2011:10 pages. doi: 10.1155/2011/617892. Article ID 617892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paris RM, Kim JH, Robb ML, Michael NL. Prime-boost immunization with poxvirus or adenovirus vectors as a strategy to develop a protective vaccine for HIV-1. Expert Review of Vaccines. 2010;9(9):1055–1069. doi: 10.1586/erv.10.106. [DOI] [PubMed] [Google Scholar]
- 42.Letvin NL. Progress in the development of an HIV-1 vaccine. Science. 1998;280(5371):1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 43.Bazhan SI, Belavin PA, Seregin SV, et al. Designing and engineering of DNA-vaccine construction encoding multiple CTL-epitopes of major HIV-1 antigens. Vaccine. 2004;22(13-14):1672–1682. doi: 10.1016/j.vaccine.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 44.Su J, Luscher MA, Xiong Y, et al. Novel simian immunodeficiency virus CTL epitopes restricted by MHC class I molecule Mamu-B∗01 are highly conserved for long term in DNA/MVA-vaccinated, SHIV-challenged rhesus macaques. International Immunology. 2005;17(5):637–648. doi: 10.1093/intimm/dxh245. [DOI] [PubMed] [Google Scholar]
- 45.Brown SA, Surman SL, Sealy R, et al. Heterologous prime-boost HIV-1 vaccination regimens in pre-clinical and clinical trials. Viruses. 2010;2(2):435–467. doi: 10.3390/v2020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsay AJ, Leong KH, Ramshaw IA. DNA vaccination against virus infection and enhancement of antiviral immunity following consecutive immunization with DNA and viral vectors. Immunology and Cell Biology. 1997;75(4):382–388. doi: 10.1038/icb.1997.60. [DOI] [PubMed] [Google Scholar]
- 47.McShane H. Prime-boost immunization strategies for infectious diseases. Current Opinion in Molecular Therapeutics. 2002;4(1):23–27. [PubMed] [Google Scholar]
- 48.Kent SJ, Zhao A, Best SJ, Chandler JD, Boyle DB, Ramshaw IA. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. Journal of Virology. 1998;72(12):10180–10188. doi: 10.1128/jvi.72.12.10180-10188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Jin N, Mi Z, et al. Antitumor effects of a recombinant fowlpox virus expressing Apoptin in vivo and in vitro. International Journal of Cancer. 2006;119(12):2948–2957. doi: 10.1002/ijc.22215. [DOI] [PubMed] [Google Scholar]
- 50.Ma M, Jin N, Shen G, et al. Immune responses of swine inoculated with a recombinant fowlpox virus co-expressing P12A and 3C of FMDV and swine IL-18. Veterinary Immunology and Immunopathology. 2008;121(1-2):1–7. doi: 10.1016/j.vetimm.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 51.Qiao C, Jiang Y, Tian G, et al. Recombinant fowlpox virus vector-based vaccine completely protects chickens from H5N1 avian influenza virus. Antiviral Research. 2009;81(3):234–238. doi: 10.1016/j.antiviral.2008.12.002. [DOI] [PubMed] [Google Scholar]


