Abstract
Due to its crucial role for memory processes and its relevance in neurological and psychiatric disorders, the hippocampus has been the focus of neuroimaging research for several decades. In vivo measurement of human hippocampal volume and shape with magnetic resonance imaging has become an important element of neuroimaging research. Nevertheless, volumetric findings are still inconsistent and controversial for many psychiatric conditions including affective disorders. Here we review the wealth of anatomical protocols for the delineation of the hippocampus in MR images, taking into consideration 71 different published protocols from the neuroimaging literature, with an emphasis on studies of affective disorders. We identified large variations between protocols in five major areas. 1) The inclusion/exclusion of hippocampal white matter (alveus and fimbria), 2) the definition of the anterior hippocampal–amygdala border, 3) the definition of the posterior border and the extent to which the hippocampal tail is included, 4) the definition of the inferior medial border of the hippocampus, and 5) the use of varying arbitrary lines. These are major sources of variance between different protocols. In contrast, the definitions of the lateral, superior, and inferior borders are less disputed. Directing resources to replication studies that incorporate characteristics of the segmentation protocols presented herein may help resolve seemingly contradictory volumetric results between prior neuroimaging studies and facilitate the appropriate selection of protocols for manual or automated delineation of the hippocampus for future research purposes.
Introduction
The hippocampus is a brain region that has been the focus of much prior research in neuropsychiatric populations. It is crucially involved in cognition, particularly in episodic, semantic, and spatial memory processes (Moscovitch et al., 2005). It also plays a role in novelty processing (Chong et al., 2008), and endocrinologic stress-regulation (Herman et al., 2005), and is one of the two brain regions supporting adult neurogenesis (Bruel-Jungerman et al., 2007). The hippocampus has been implicated in the pathophysiology of many neurological and psychiatric diseases (Geuze et al., 2005b; Petrella et al., 2003; Sahay and Hen, 2007). These functional characteristics make the hippocampus one of the most fascinating, but also one of the more complex brain regions for study using neuroimaging methods.
Modern neuroimaging techniques have enabled us to measure hippocampal volume and shape in vivo under various conditions. Physiologically, human hippocampal volume decreases with age (Liu et al., 2003; Scahill et al., 2003), although aging effects may be less pronounced than for other brain structures (Grieve et al., 2005; Sullivan et al., 2005). Individual variability of hippocampal volume is as large in younger adults as in older people (Lupien et al., 2007). Further, hippocampal volume loss is a characteristic feature of many brain diseases, foremost Alzheimer's disease and temporal lobe epilepsy (Apostolova et al., 2006; Thompson et al., 2004; Van Paesschen et al., 1997). For many other neurological and psychiatric conditions, findings remain more ambiguous. Particularly for affective disorders, prior results appear heterogeneous. In major depression, some observations point towards bilateral reduction of hippocampal volume (Bremner et al., 2000; MacMaster et al., 2008; Sheline et al., 1999; Sheline et al., 1996). Other findings emphasize an asymmetric reduction of the right (Bell-McGinty et al., 2002) or left hippocampus (de Geus et al., 2007; MacMaster and Kusumakar, 2004; Saylam et al., 2006; Zhao et al., 2008), with an average of 10% and 8% reduction reported for the right and left hippocampus respectively (for review see Sheline et al., 2002; Videbech and Ravnkilde, 2004). However, significant volume differences in major depression have not been detected by several other volumetric studies (Coffey et al., 1993; Posener et al., 2003; Vakili et al., 2000; von Gunten et al., 2000). Further, heterogeneous observations appear present for bipolar disorder, with some authors reporting volume decrease, others volume increase with lithium treatment, and others not finding any significant volume changes (Campbell and MacQueen, 2006; Foland et al., 2008; Geuze et al., 2005b; Strakowski et al., 2002; Yucel et al., 2007). Hippocampal volume changes have also been reported for schizophrenia (Gur et al., 2007; Steen et al., 2006), post-traumatic stress disorder (Karl et al., 2006), autism (Nicolson et al., 2006), obsessive–compulsive disorder (Hong et al., 2007), panic disorder, and many other psychiatric diseases, but controversies continue (for overview see Geuze et al., 2005a).
Heterogeneous findings regarding hippocampal volumetry may be partly attributable to the use of different MRI techniques. Geuze et al. have pointed out how technical MRI parameters may contribute to inconsistencies of volumetric results (Geuze et al., 2005a). They suggested that image acquisition parameters such as MR sequences, signal-to-noise ratios, field strength, number of slices, brain coverage, and especially image resolution contribute to the heterogeneity of findings in this research field. Differences between patients and control groups were more frequently reported in studies with high than with low resolution. Further, image-processing procedures including reformatting for alignment with the longitudinal hippocampal axis, software packages for delineation, head size correction, or whole brain volume correction, and reliability measures vary considerably between studies. Finally, a large number of different anatomical protocols for delineating the hippocampus represent an important source of variance between studies (Geuze et al., 2005a). While manual delineation of the hippocampus is still considered to be the gold standard, semi-automated and automated methods for delineation have been developed. In semi-automated methods, prior knowledge is introduced by a human operator who identifies landmarks, seedpoints, or bounding boxes (Chupin et al., 2007; Ghanei et al., 1998; Perez de Alejo et al., 2003; Shen et al., 2002). Fully automated methods might be based on statistical shape-models, on affine or non-linear registration to an atlas (Barnes et al., 2007; Carmichael et al., 2005; Svarer et al., 2005; Vemuri et al., 2003) or to multiple atlases (Heckemann et al., 2006). Atlas registration might also be combined with other methods such as intensity-based voxel-classification or learning-based optimization (Hammers et al., 2007; Pitiot et al., 2004; Pohl et al., 2007; Zhou and Rajapakse, 2005). Algorithms based on learning and optimization have certain advantages over static algorithms (Powell et al., 2008; Tu et al., 2008; (van der Lijn et al., 2008). However, semi-automated and automated algorithms also require a proper definition of the hippocampus; and some of them necessitate a manually delineated training set, therefore this review is relevant to both semi-automated and automated methods as well.
With this contribution we intend to review the wealth of anatomical protocols for delineating the hippocampus in MR images, taking into consideration current anatomical evidence of hippocampal borders. Presenting these details in an ordered and structured way should enable neuroimaging researchers to appraise the weaknesses and strengths, and the value and particularities of different anatomical protocols for imaging research. This review should aid the selection of an appropriate protocol for specific research purposes, and improve the interpretation of diverging neuroimaging results.
Definition and anatomy of the hippocampus
The dilemma of defining hippocampal borders in MR images begins with the anatomical complexity of this allocortical structure and is complicated by a long history of terminological inconsistencies, which is beyond the scope of this review (for a more detailed discussion on terminology please refer to Duvernoy (2005), El-Falougy and Benuska (2006), Suzuki and Amaral (2003)). The Italian anatomist Julius Caesar Aranzius (1530–1589) first described a region in the medial temporal lobe and named it according to its form of a seahorse, lat. “hippocampus”. However, the widely used brain atlases of Brodmann (Brodmann, 1909; Brodmann and Garey, 1994) and Talairach and Tournoux (Talairach and Tournoux, 1988) do not clearly define hippocampal subregions and borders.
The hippocampus, in the anatomical sense of the word, consists of the cornu ammonis and the gyrus dentatus, but some definitions additionally include the functionally connected subiculum (also see page 9 of Duvernoy (2005)). As the subiculum is indistinguishable from the cornu ammonis in MR images, neuroimaging publications almost always include the subiculum when using the term hippocampus. In this review article, the term hippocampus therefore refers to the structures cornu ammonis, the gyrus dentatus, and the subiculum. In coronal sections of the hippocampal body, the C1 region of the cornu ammonis continues medially into the subiculum which can be subdivided into the prosubiculum (adjacent to C1), subiculum proper, presubiculum, and parasubiculum, and then continues into the entorhinal cortex (Burwell, 2000; Witter and Moser, 2006; (Zilles, 1987). Macroanatomically, the subiculum and the entorhinal cortex are located on the parahippocampal gyrus.
According to anatomical criteria, the hippocampus can be divided into three parts along its longitudinal axis, the hippocampal head, body, and tail (Duvernoy, 2005; Kiefer et al., 2004).
The head of the hippocampus (caput hippocampi) is curved, including the anterior thickened foot of the hippocampus (pes hippocampi) which is characterized by transverse folding of the cornu ammonis, the internal and external digitations (Duvernoy, 2005). Here the uncus curls back medially to rest on the parahippocampal gyrus. The head of the hippocampus is anteriorly limited by the uncal recessus of the temporal horn of the lateral ventricle and the amygdala.
The hippocampal body (corpus hippocampi) consists of the gray matter of the cornu ammonis, rolled into the dentate gyrus including the fascia dentata. It includes the histologically defined regions CA1–4. The curled cornu ammonis rests on and more inferiorly passes over to the subiculum, which can be histologically divided into the prosubi-culum, subiculum, presubiculum, and parasubiculum, the latter fading to the entorhinal cortex (Zilles, 1987). The hippocampal body is superiorly and laterally covered by the alveus, which contains white matter fibers leading to the fimbria. It is medially bordered by the cisterna ambiens. In contrast to purely anatomical definitions (Duvernoy, 2005), the term hippocampus used in this review article includes the subiculum.
The hippocampal tail (cauda hippocampi) continues the layer structure of the body with cornu ammonis and dentate gyrus. The margo denticulatus narrows and merges into the fasciola cinera. It is medially covered by the fimbria that ascends to the crus of fornix. CA3 becomes superficial in the gyrus fasciolaris. The folded CA1 layer also becomes superficial in the Andreas-Retzius gyrus. The hippocampal tail ends with the subsplenial gyrus. The tail is medially bordered by the ambient cistern and its wing, the lateral part of the transverse fissure. The lateral border is formed by the temporal horn of the lateral ventricle.
This short overview on hippocampal anatomy illustrates that the hippocampus is a complex anatomical structure. As Amunts et al. have shown (Amunts et al., 2005), cytoarchitectonic borders of the hippocampal and entorhinal region do not precisely or reliably coincide with macroscopically visible landmarks. In particular, the borders between the hippocampus and amygdala, cornu ammonis and subiculum, and subiculum and entorhinal cortex do not reliably correspond to macroanatomical characteristics (Amunts et al., 2005). Therefore, any definition of the hippocampus based on macroanato-mical landmarks in MRI is limited by the fact that the underlying cytoarchitecture is not obvious in MR images.
Protocols for delineation of the hippocampus in MR images
Methods
To identify the relevant segmentation protocols for delineation of the hippocampus in MR images, a combination of an actual Medline search with a screening of meta-analyses and reviews was used. First, +a Medline search as of April 2008 was performed using the MESH-heading search terms “hippocampus” combined with “magnetic resonance imaging” and “shape” or “volume”, but excluding voxel based analysis studies, resulting in 1288 publications. The results of this search were combined with the terms “depression”, “major depression”, or “unipolar depression”, resulting in 93 publications. This search was then manually limited to studies using an MR scanner with at least 1 T field strength, analyzing the hippocampus as a separate structure and not in conjunction with the amygdala, and including both a patient and a control population. If authors of these studies referred to anatomical protocols from studies on different patient populations for description of their delineation method, these studies were included as well. In addition to the Medline search, a reference screening of the following meta-analyses and reviews relevant to this research field was performed, but partly overlapped with the above Medline search: Geuze et al. identified 14 segmentation protocols that were used in more than five investigations; these were also considered here (Geuze et al., 2005a). Further, 12 segmentation protocols for affective disorders identified by Campbell and Macqueen (2004) and 17 new protocols identified by the same authors 2 years later (Campbell and MacQueen, 2006) were taken into account. Eleven delineation protocols defining the hippocampus separately described in Beyer et al. were also considered (Beyer and Krishnan, 2002; MacQueen et al., 2003). After elimination of redundant findings, a total of 71 different protocols for the delineation of the hippocampus in MR images are described and discussed here. We will describe different definitions for the anterior, posterior, superior, inferior, lateral, superior medial and inferior medial borders of the hippocampus used in segmentation protocols. The term superior medial will be used to describe the medial demarcation between the dentate gyrus and the cisterna ambiens, the term inferior medial border to describe the definition of a border in the region of the subiculum on the medial surface of the parahippocampal gyrus. When describing landmarks, we will differentiate between external landmarks, i.e. anatomical structures not belonging to the hippocampus such as the superior colliculus which is sometimes used to mark the posterior border of the hippocampus, and internal landmarks, i.e. using the alveus as a demarcation between the hippocampus and amygdala. If a straight line is drawn to demarcate the hippocampus, we use the term arbitrary line to emphasize that this line does not necessarily follow the borders of biological structures.
Results
MRI acquisition parameters
The acquisition plane employed by the studies incorporated in this review was most often coronal, less often sagittal, while only a minority used axial acquisition planes. Voxel size was not isotropic in most studies. While the in-plane image resolution was about 1 mm×1 mm in most cases, slice distance was mostly around 1.5 mm. Information about head coverage or number of slices is sparse. If mentioned, the number of slices was most often 124. Some MRI acquisition parameters are not provided in the publications (Supplementary Table 1).
Tracing planes
The majority of protocols describe manual tracing of hippocampal borders in the coronal plane, in fact 64 out of 71 prefer this method (Supplementary Table 1). Tracing on sagittal slices was used by three groups (MacQueen et al., 2003; von Gunten et al., 2000; von Gunten and Ron, 2004; Yucel et al., 2007), and tracing on axial slices by one group (Hastings et al., 2004). A combination of the coronal with the sagittal plane was used by Rusch (Rusch et al., 2001), and a combination of the coronal with the two other planes according to Haller by three groups (Csernansky et al., 2002; Haller et al., 1997; Posener et al., 2003). Notably, most recent studies used all three orthogonal viewing planes to guide anatomic decisions even though tracing protocols were performed in only a single plane as noted above. This practice facilitates a more accurate definition of hippocampal boundaries than delineation in a single plane of reference (Bonilha et al., 2004).
Gray and white matter
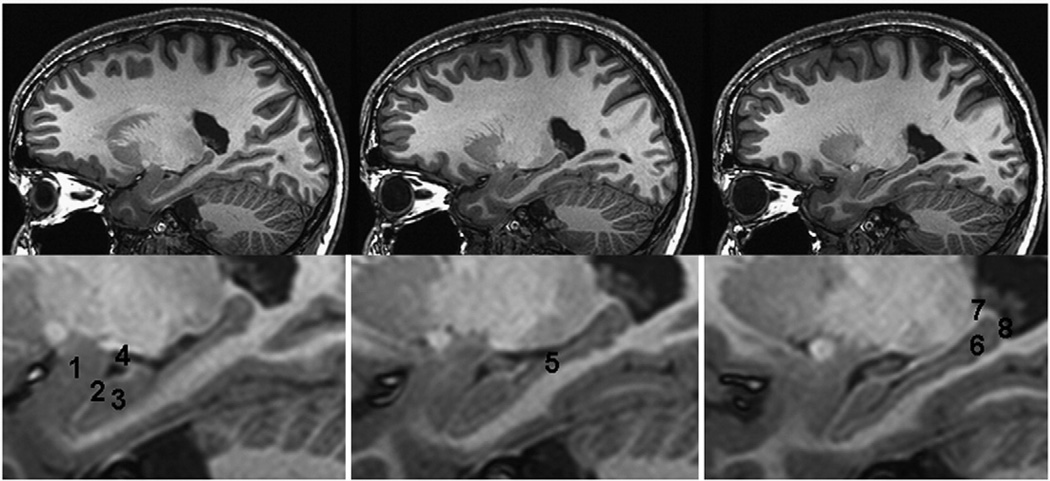
Segmentation protocols for the hippocampus can be roughly divided into those that consider only gray matter and those that consider hippocampal gray and white matter (Supplementary Table 2). The alveus and fimbria are the white matter tracts carrying axons from hippocampal, subicular and septal neurons to other limbic structures. While 41 protocols include alveus and fimbria, e.g. Pruessner and Watson (Pruessner et al., 2000; Watson et al., 1992), and 21 explicitly exclude white matter structures such as Soininen, Sheline, and Narr (Narr et al., 2004; Sheline et al.,1996; Soininen et al., 1994). Nine of the protocols do not explicitly specify if white matter is included or not, e.g. Rusch and others (Rusch et al., 2001). For details see Supplementary Table 2 and Fig. 1.
Fig. 1.
Gray and white matter of the hippocampus. Sagittal MR images of the hippocampus (from medial slice–left image to lateral slice–right image) and enlarged views of the hippocampal area (lower row) demonstrating gray matter of the hippocampal head, body and tail, overlaid by white matter of the alveus and fimbria and amygdala. 1=amygdala, 2=alveus, 3=hippocampal head, 4=temporal horn of the lateral ventricle and fimbria, 5=hippocampal body, 6=hippocampal tail, 7=fimbria, 8=alveus. The images displays the average image of three MR images of a young healthy male acquired using a 0.5×0.5×0.5 mm3 resolution after zero filling at 3 T field strength (Gyroscan Intera 3.0 T, Philips, Best, NL). Raw images are a courtesy of Dr. H. Schiffbauer.
Anterior border
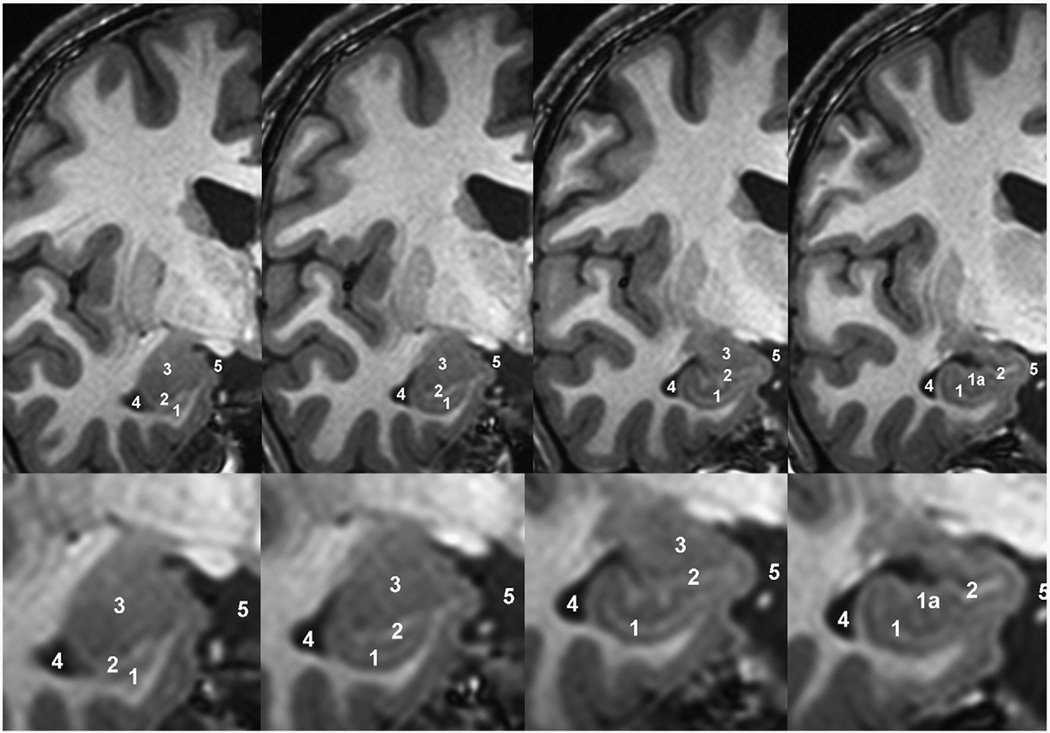
Delineating the anterior border of the hippocampus is one of the most difficult tasks in manual segmentation of the hippocampus, as the amygdala and hippocampus are hard to discern on many MR images. One prevalent internal landmark used for differentiation of the amygdala and hippocampus on coronal planes is the alveus. This method alone has been used in 15 publications, e.g. Bartzokis et al. (1998), Cook et al. (1992) and MacQueen et al., 2003). In the case that the alveus cannot be discerned, Sheline introduced an arbitrary line linking the sulcus semilunaris with the inferior horn of the lateral ventricle (Sheline et al., 1996), which was adapted by Vakili et al. (2000). Twenty-two other protocols also used the alveus for the anterior border, but combined this internal landmark with an external landmark, the appearance of cerebrospinal fluid (CSF) of the lateral ventricle, e.g. Jack (1994) and Narr et al. (2004). In the case that alveus and lateral ventricle landmarks are not discernable, Vythilingam proposed drawing an arbitrary straight line from the inferior horn of the lateral ventricle to the uncal surface (Vythilingam et al., 2002). Eight other protocols used only the appearance of the lateral ventricle as an external landmark for the anterior border, e.g. Bigler et al. (1997) and MacMaster and Kusumakar (2004). Watson and colleagues also regard the uncal recessus of the inferior horn as the most reliable external landmark, but this widely used protocol also defines alternative strategies: in the case that the uncal recessus is not visible, the authors suggest first drawing an arbitrary line from the inferior horn of the lateral ventricle to the sulcus at the inferior margin of the semilunar gyrus. If it is not possible to discern this landmark, the authors suggest using the alveus as a second landmark, or as a last resort to trace a straight line connecting the plane of the inferior horn with the surface of the uncus (Van Paesschen et al., 1997; Watson et al., 1992). Other delineation protocols based on using CSF of the lateral ventricle as an external landmark locate the anterior border “at a point where the cornu inferius of the lateral ventricle looses its slit-like appearance, widens, occupies a position lateral to the hippocampus proper and becomes triangular or boomerang-shaped in the coronal plane” (Colla et al., 2007; Niemann et al., 2000), at “the point where the third ventricle was split from the cistern by the hypothalamus” (Chen et al., 2004), “where the cornu inferius of the lateral ventricle becomes vertically oriented” in combination with the frontal cleft and alveus (Frodl et al., 2002).
Eight other protocols use the appearance of the corpora mamillaria on coronal slices as external landmarks, e.g. Brambilla and Caetano define the anterior hippocampal border on a slice posterior to the appearance of the corpora mamillaria (Brambilla et al., 2003; Caetano et al., 2004), while Bremner et al. locate the anterior border one slice anterior to the colliculus superior (Bremner et al., 1995). While the amygdala is located anterior and superior to the hippocampus, most authors describe this boundary as the anterior hippocampal border, only few also detail the superior margin. Further delineation methods and details are listed in Supplementary Table 3 (see Fig. 2).
Fig. 2.
Hippocampal head. Coronal MR images of the hippocampal head from anterior (left) to posterior (right) and enlarged views of the hippocampal area (lower row). 1=hippocampal head, 1a=internal digitations, 2=alveus, 3=amygdala, 4=temporal horn of the lateral ventricle, 5=ambient cistern.
Posterior border
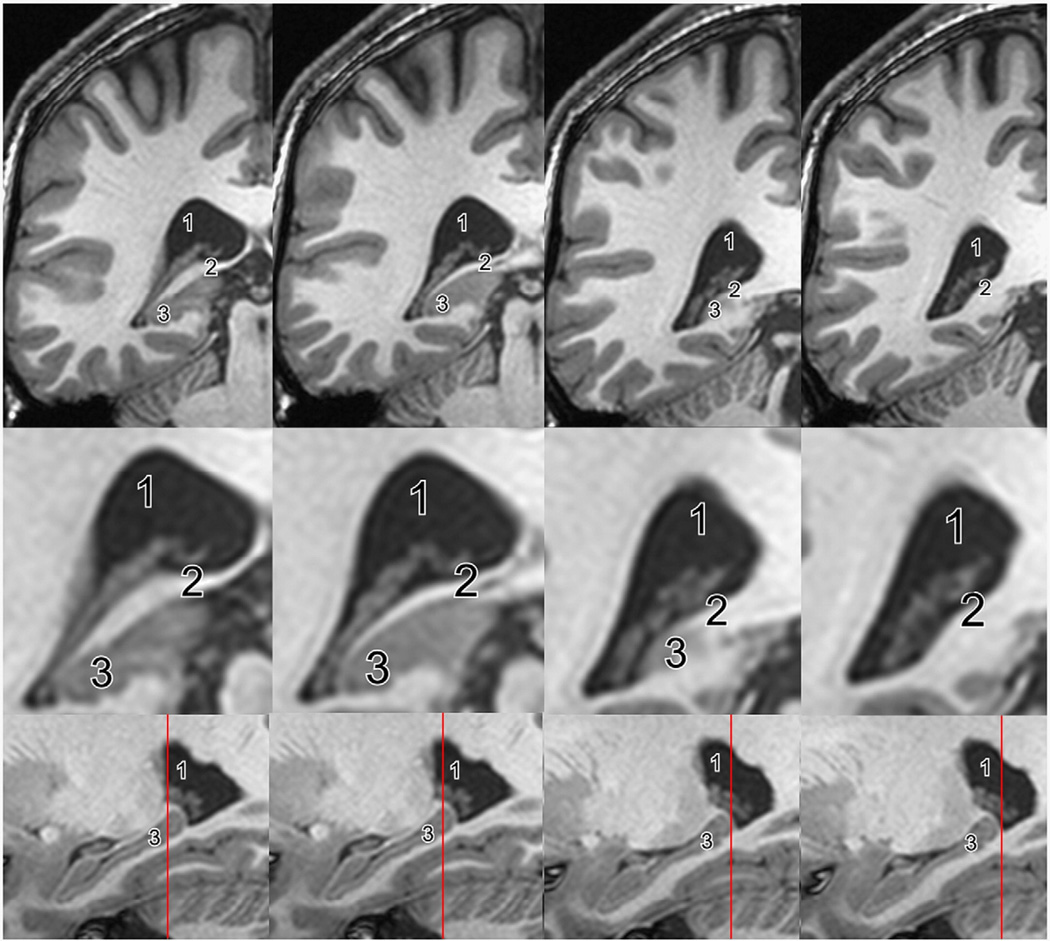
The definition of the posterior border of the hippocampus in MR images is also variable and heterogeneous. Most frequently, the lateral ventricle is used as an external landmark. Seventeen protocols localize the posterior end as the coronal slice “where an ovoid gray matter starts to appear inferiomedially to the trigone of the lateral ventricle”, e.g. Narr et al. (2004), Pruessner et al. (2000) and Sheline et al. (1996). Other protocols use the fornices as external landmarks. In ten of these, the posterior end was reached when the fornices or crura of the fornices “were still detectable in their full length” (Jack, 1994; Soininen et al., 1994; von Gunten et al., 2000). Further delineation methods using the fornices as external landmark are detailed in Supplementary Table 3. In addition, some protocols use the inferior or superior colliculi as external landmarks for the posterior border, e.g. Bartzokis et al. define the posterior end in the coronal slice “where the inferior and superior colliculi are jointly visualized” (Bartzokis et al., 1998). The thalamus also plays a role as an external landmark for the posterior border. Three protocols identify the posterior hippocampal border “where the superior colliculus is completely connected with the thalamus bilaterally” (Brambilla et al., 2003; Caetano et al., 2004; Chen et al., 2004). Further, more individual definitions of this border are described in Supplementary Table 3 (see Fig. 3).
Fig. 3.
Posterior end of the hippocampus. MR images demonstrating the posterior end of the hippocampus. Coronal slices from anterior (left) to posterior (right) and enlarged views of the hippocampal area (middle row). Sagittal slices with the position of the coronal plane indicated by a red line (lower row). While some segmentation protocols stop measuring the hippocampus when the crus of the fornix appears (image two to the left), others follow the hippocampus posteriorly until the hippocampal ovoid gray matter completely disappears (image two to the right). 1=atrium of the lateral ventricle, 2=crus of the fornix, 3=hippocampal tail.
Superior border
The hippocampus is covered by the alveus and adjoins to cerebrospinal fluid. A major difference between studies regards the inclusion or exclusion of white matter, see “Gray and white matter” above. Apart from this, the superior border is consistently defined by either the alveus as an internal landmark or cerebrospinal fluid as an external landmark (Supplementary Table 3).
Inferior border
The white matter of the parahippocampal gyrus below the subiculum is clearly discernible from the gray matter of the hippocampus (Supplementary Table 3).
Lateral border
The lateral border is not controversial either. The CSF of the lateral ventricle, which is used as an external landmark, most frequently defines this boundary (Supplementary Table 3).
Superior medial border of the hippocampus
With the term superior medial border we describe the medial demarcation of the dentate gyrus. There is a consensus concerning the superior medial border of the hippocampus between most protocols, and the superior medial border is commonly defined by the CSF of the cisterna ambiens (Supplementary Table 3).
Inferior medial border of the hippocampus
In coronal sections (see Fig. 4), the C1 region of the cornu ammonis continues medially into the prosubiculum (adjacent to C1), subiculum proper, presubiculum, and parasubiculum, and then continues into the entorhinal cortex. We use the term “inferior medial border of the hippocampus” to describe how the authors of neuroimaging articles delimit what they define as hippocampus along the inferior part of the cornu ammonis and the subiculum.
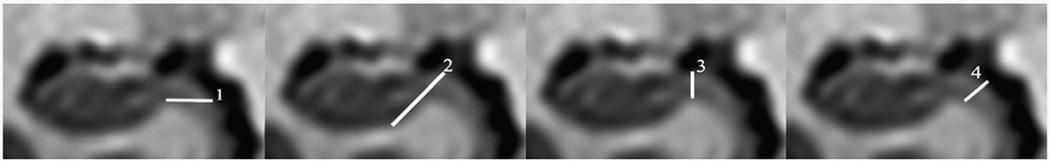
Fig. 4.
Border between subiculum and parahippocampal gyrus. Coronal MR images of the hippocampal body demonstrating different definitions of the border between the subiculum and the parahippocampal gyrus. 1=The inferior border of the hippocampus is continued medially with a straight horizontal line (Haller et al., 1997). 2=A straight line with an angle of 45° is drawn from the most inferior part of the hippocampus medially to the ambient cistern. If white matter is located between these two landmarks, this white matter is used as border (Pruessner et al., 2000). 3=A vertical arbitrary line is placed at the dorsomedial tip of the white matter of the parahippocampal gyrus (Neumeister et al., 2005). 4=A line is drawn at the angle where the hippocampus curves down into the parahippocampal gyrus (Honeycutt et al., 1998).
This border is defined in less than half of the protocols. Most protocols define arbitrary lines to approximate this border. For example, Bartzokis et al. described an arbitrary line that is “drawn medially through the brain surface” “when the most medial extent of the inferior border gray/white interface reaches the subiculum” (Bartzokis et al., 1998). This definition was later adopted by Sheline and Vakili (Sheline et al., 2003; Sheline et al., 1999; Sheline et al.,1996; Vakili et al., 2000). Honeycutt et al. defined a linear boundary that diagonally ascends from the medial “angle where the hippocampus curves down into the parahippocampal gyrus”, considering any gray matter lateral and superior to this line as hippocampal tissue (Honeycutt et al., 1998; Strasser et al., 2005). A comparable method was also used by Watson, van Paesschen, and Ashtari (Ashtari et al., 1999; Van Paesschen et al., 1997; Watson et al., 1992). Haller et al. continued “the inferior border of the cornu Ammonis […] medially with a straight horizontal line” and considered all tissue above as hippocampus, below as “parahippocampal cortex” (Haller et al., 1997) (Fig. 4.) This method was adopted by Csernansky, Posener, and Lloyd (Csernansky et al., 2002; Lloyd et al., 2004; Posener et al., 2003). Pruessner et al., 2000 suggest using the line of white matter that becomes visible if the subiculum is more detached form the entorhinal cortex, or otherwise tracing a straight line with an angle of 45° from the most inferior part of the hippocampal body to the cisterna ambiens (Pruessner et al., 2000). This method was adopted by Lange and Irle (2004). Neumeister defined a vertical arbitrary line through the dorsomedial tip of parahippocampal white matter (Neumeister et al., 2005). Niemann et al., followed by Frodl and Colla, divided the hippocampus into four subsequent shape segments along its longitudinal axis (Colla et al., 2007; Frodl et al., 2002; Niemann et al., 2000). In the first shape segment, the subiculum was included in the hippocampal measurement. In the second shape segment at the height of the vertical digitations (rabbit-like hippocampal shape), a 45° line was drawn from the lateral end of the sulcus unci in the basolateral direction through gray matter to approximate the prosubiculum. If the temporal horn was visible below, the oblique line was drawn to the ventricle instead. In the third shape segment according to Niemann et al. (binocular-like hippocampal shape), tissue density was considered to differentiate the hypointense subiculum from the hyperintense presubiculum. In the forth shape segment (hippocampal body), the hippocampal sulcus was used to delimit the inferior medial border of the hippocampus (Niemann et al., 2000).
Discussion
This review evaluates the wealth of anatomical protocols for delineating the hippocampus in MR images. The purpose of this review is to aid the selection of an appropriate segmentation protocol for specific research purposes and to improve the interpretation of diverging neuroimaging results. This review demonstrates that anatomical protocols intended for delineating the hippocampus use a wide variety of definitions and landmarks to detect the borders of this structure in MR images. We demonstrate that discrepancies most frequently occur at specific anatomical boundaries of the hippocampus. Permuting these different definitions results in a multitude of different protocols and complicates the interpretation of neuroimaging findings. The major points of discrepancy are: 1) the inclusion/exclusion of hippocampal white matter (alveus and fimbria), 2) the definition of the anterior hippocampal–amygdala border, 3) the definition of the posterior border and the extent to which the hippocampal tail is included, 4) the definition of the inferior medial border, and 5) the use of varying arbitrary lines. While our review focuses specifically on segmentation protocols used in depression, the same discrepancies are also expected to occur in other fields of neuroscientific research.
Inclusion or exclusion of hippocampal white matter (alveus and fimbria)
Substantial discrepancies exist between segmentation protocols concerning the inclusion of white matter structures in hippocampal measurements. The alveus and fimbria represent the main efferent pathway from the hippocampus and subiculum, carrying hippocampal axons via the crus and body of the fornix to either the mamillary bodies and the mamillothalamic tract or directly to the anterior thalamic nuclei (Duvernoy, 2005). The alveus also plays an important role in demarcating the hippocampal–amygdala border. Numerous researchers include alveus and fimbria in their hippocampal volume measurements without discussing their rationale. Geuze et al. (2005a) suggest that it may be best to include the alveus in its entity to avoid confusion.
However, disease processes may affect hippocampal gray and white matter differently. For example, Frodl et al. describe differential effects of disease status in patients with a first episode of major depression. While depressed men had significantly smaller total and gray matter hippocampal volume than healthy controls, this effect was not present in depressed women. However, both sexes revealed a decrease in volume of hippocampal white matter (Frodl et al., 2002). Tupler and de Bellis found larger white, but not gray matter volume in children and adolescents with post-traumatic stress disorder (Tupler and De Bellis, 2006). Thus, some observations indicate that white and gray matter volume may be influenced differentially by gender, disease status, or their interactions.
So far, it remains unclear whether differential volume changes of hippocampal gray and white matter have any pathophysiological and clinical significance. If MR image resolution is sufficiently high to allow a separation of hippocampal gray and white matter, it seems reasonable to avoid sum values of white and gray matter volume and to avoid mixing differential effects, keeping in mind that even with high resolution, partial volume effects will occur and gray matter voxels may include smaller volumes of white matter and vice versa. While gray matter volume is always indispensable, white matter volume can be measured in addition, depending on the scientific hypothesis. However, the details of the delineation method should be included.
The definition of the anterior hippocampal–amygdala border
Detection of the anterior border is one of the most challenging tasks in hippocampal morphometry and may be largely dependant on the acquisition technique. A reliable and clear demarcation of the hippocampal–amygdala border is a quality criterion for MRI sequences that should be assured, before commencing morphometric MRI studies investigating the hippocampus. Delineating the anterior hippocampal border is commonly based on anatomical landmarks such as the CSF of the inferior horn of the lateral ventricle, the alveus, or the appearance of the corpora mamillaria. As landmarks, each of these has its advantages and disadvantages. If the definition of the anterior border is based on external landmarks such as the appearance of the corpora mamillaria in adjacent coronal slices (Brambilla et al., 2003; Bremner et al., 1995; Caetano et al., 2004), the reliability highly depends on imaging characteristics, image resolution, slice spacing, as well as position and tilt of the coronal slice. Therefore, definitions of the anterior border, based on the appearance of non-hippocampal structures, cannot necessarily be generalized to other investigations using different image acquisition protocols. Furthermore, anatomical decisions that are not based on the intrinsic anatomy of the hippocampus depend on the position of the hippocampus relative to external structures, rather than to the hippocampal anatomy. Therefore, the authors discourage the use of definitions of hippocampal borders based on the relative position of external neuroanatomical landmarks. Methods based on CSF as a landmark for the anterior border benefit from the high contrast between CSF and brain tissue and are therefore useful in MR images with lower resolution. In our experience though, CSF clefts might be extremely small or even invisible in a minority of individuals, or may be obscured by choroid plexus. Particularly in young and healthy subjects with small ventricular volume, protocols based on this landmark may not be reliable. In contrast, the alveus is an internal landmark with an anatomically fixed rapport with the hippocampus and exhibits a high contrast to gray matter tissue. The visibility of the alveus depends on MR image quality and resolution, but in our experience the alveus is commonly visible in images with 1×1×1 mm3 or higher resolution. Therefore, this internal landmark seems most reliable for those investigations with sufficient MRI resolution. In addition, this landmark is detected most reliably if the software used for delineation is capable of displaying all three viewing planes simultaneously.
Definition of the posterior border
The posterior end of the hippocampus seems to be a major source of variance between segmentation protocols (see Fig. 3). Protocols based on the appearance of non-hippocampal structures, such as inferior or superior colliculi in adjacent coronal slices, depend on image characteristics such as slice spacing and tilt, and are therefore not readily generalizable across investigations. Furthermore, using external landmarks may introduce additional variance in hippocampal measurements. Protocols using the fornices as markers for the posterior end underestimate the hippocampal volume, omitting the segment of the hippocampal tail that goes beyond the fornices. In our experience, the hippocampal volume behind the crus of the fornix measures about 250 to 400 mm3 (considering one hemisphere only). Maller et al. pointed out that the posterior hippocampal tail adds over 5 mm of length to the hippocampus, and that the proportion of the hippocampal tail is about 11% of total hippocampal volume (Maller et al., 2006). The orientation of the hippocampus also plays a role; less volume is excluded if the brain is oriented along the long axis of the hippocampus (oblique coronal plane) than if the hippocampus is traced in brains with AC-PC alignment. Assessing the gray matter of the hippocampal tail until it reaches its posterior ovoid form, corresponds best to the anatomical boundaries of this structure (Narr et al., 2004; Pruessner et al., 2000; Sheline et al., 1996), since this strategy provides the most accurate estimate of hippocampal volume.
Definition of the inferior medial border
Defining the inferior medial border is limited by the fundamental problem that prosubiculum, subiculum, presubiculum, parasubiculum, and the entorhinal cortex are cytoarchitectonically defined regions that do not correspond precisely to macroanatomical landmarks, such as the parahippocampal gyrus (Amunts et al., 2005). Therefore, most protocols define arbitrary lines approximating the border between these regions (see Fig. 4). Some researchers (Bartzokis et al., 1998; Csernansky et al., 2002; Haller et al., 1997; Sheline et al., 2003) continued “the inferior border of the cornu ammonis-subiculum […] medially with a straight horizontal line across the cortex of the parahippocampal gyrus. The cortex below this line was considered the parahippocampal gyrus, the cortex above this line was included as part of the hippocampus” (Haller et al., 1997). Others defined diagonal lines starting “mesially at the angle where the hippocampus curves down into the parahippocampal gyrus” (Honeycutt et al., 1998) and ascending into superior medial direction on coronal slices (Honeycutt et al.,1998; Watson et al., 1992). Niemann et al. accommodate for changing correspondence of macroanatomy to cytoarchitecture along the longitudinal axis of the hippocampus by using four different definitions of the medial border along four segments of the longitudinal axis. However, this creates the additional problem that a reliable segmentation along the longitudinal axis is necessary (Niemann et al., 2000). All these suggestions are, however, limited by the fact that macroanatomy does not necessarily reflect the underlying cytoarchitecture (Amunts et al., 2005). In personal correspondence with the author, Dr. O. Kedo from the Research Center Jülich, Germany, who investigated the cytoarchitecture of ten post mortem brains, reported her experience that in the rostral part of the hippocampus, a diagonal line may reflect the underlying cytoarchitecture more accurately then the methods described above, while more caudally (the exact location is difficult to assess) a horizontal line may be more appropriate. However, so far there are no systematic investigations on this topic.
The use of varying arbitrary lines
As outlined in the sections above, some scientists introduced arbitrary lines to delineate the borders of the hippocampus. These definitions are mainly used if borders are barely visible and difficult to detect, which also depends on scanning parameters such as slicing and resolution (and motion artifacts). Although arbitrary lines do not necessarily reflect anatomic boundaries exactly, they may be useful for ensuring reproducibility and reliability of a segmentation protocol. The arbitrary lines defined in the studies reviewed here are listed in Supplementary Table 3. Thirty-five authors (35/71 or 49%) used arbitrary lines to delimit the inferior medial border of the hippocampus, 18 authors (18/71 or 25%) for the anterior hippocampal border, four authors (4/71 or 6%) for the superior medial, and four (4/71 or 6%) for the superior border. For the inferior medial border of the hippocampus, the definition of an arbitrary line is necessary, because there is no macroanatomical correspondence to cytoarchitectonical borders (Amunts et al., 2005). It is surprising that 36 protocols do not define an arbitrary line for the inferior medial border, leaving the exact demarcation rule unclear. As described in the section above, horizontal and diagonal lines were used. For the anterior hippocampal border, all authors use arbitrary lines as a secondary rule, only if their primary landmarks such as the ventricular CSF or alveus are not visible (Kates et al., 1997; Kates et al., 2006; MacMillan et al., 2003; Saylam et al., 2006; Sheline et al., 1996; Vermetten et al., 2006; Watson et al., 1992). These second-line rules are justified to avoid random decisions and to enhance reliability. For the superior medial border, only two definitions for arbitrary lines can be found in the literature. Pruessner provides a detailed protocol that deals with the delineation of the hippocampal tail, the fasciolar gyrus and the gyrus of Andreas-Retzius, a problem that is not considered in most other protocols (Pruessner et al., 2000). He defined an arbitrary vertical line from the medial end of the temporal horn of the lateral ventricle down to the parahippocampal gyrus. Neumeister et al. used a vertical line starting at the dorsomedial tip of the white matter below the subiculum, in order to define the superior medial hippocampal border (Neumeister et al., 2005). For the superior border, Pruessner again defined a horizontal line from the superior border of the ambient cistern to the temporal horn of the lateral ventricle (Pruessner et al., 2000). Jack et al. suggest an arbitrary line for the superior hippocampal border as a secondary rule if their primary landmark, the uncal recess of the temporal horn or the alveus is not visible (Jack, 1994).
In addition to the anatomical rules outlined above, some more general guidelines should be considered for delineation of these borders in MR images. Geuze et al. have thoroughly reviewed the technical sources of variance between studies on hippocampal volume, such as image acquisition and pre-processing parameters (Geuze et al., 2005a). Some of these parameters are also relevant for the detection of anatomical borders: high MR field strength, enabling high MRI resolution, improves the visibility and detection of anatomical borders, e.g. at the difficult anterior border that is marked by the alveus (Bonilha et al., 2004; Geuze et al., 2005a). In the authors' experience, a resolution of 1×1×1 mm3 or less is desirable. A comparison of images acquired with our Gyroscan Intera 3 T (Philips, Best, Netherlands) showed that the detection of the anterior border was still obscured in some individuals at the resolution of 1×1×1 mm3 compared to images with a resolution of 0.5×0.5×0.5 mm3 after zero filling (Fig. 4). Furthermore, the authors do not recommend delineating the hippocampus in automatically gray–white matter segmented images, as segmentation algorithms are prone to misclassify signal intensities in the hippocampal area. Misclassification of tissue classes by the rater can be reduced by including a bias correction step in the pre-processing algorithm. The precision reached by acquiring high resolution images, employing bias field correction, and applying trained anatomical knowledge should not be compromised by the software used for delineating anatomy. Some software tools are only able to include or exclude whole voxels, endangering precise manual delineation by this technical limitation. Other software tools allow the tracing of continuous borders through the image volume with preferred subvoxel spatial resolution (Woods, 2003). The tracing software should also enable simultaneous viewing of all three orthogonal planes to guide anatomical decisions (Bonilha et al., 2004). Another important point to reduce variance not related to intrinsic hippocampal anatomy is image tilt and alignment, in particular if external landmarks are used for delineation of borders (which we do not recommend). Aligning the brain according to the long axis of the hippocampus rather than to the AC-PC plane has the advantage of increasing the surface area outlined on the oblique coronal slices, allowing for more detailed surface rendering. Observing these general recommendations in manual and most automated procedures will ensure that delineation of the hippocampus primarily depends on the anatomical definitions.
Conclusion
In sum, discrepancies between different published hippocampal delineation protocols appear to occur as a result of some specific hippocampal “problem areas”. Striving for consensus in defining these borders could reduce variability in hippocampal volume measurements considerably. As a first step for quality control, we suggest that the five points mentioned above should be detailed in any methods section for studies investigating hippocampal morphometry with MRI. This is particularly relevant for the definition of the inferior medial border of the hippocampus, which is not mentioned in the majority of articles published to date. Furthermore, we suggest that further resources should be allocated to replication studies, which are common in other areas of research such as genetic analysis. If two research groups reach different results in groups with similar demographic and clinical characteristics using their specific segmentation protocol, this should motivate a second look at the datasets. Considering the anatomical details discussed above should further enhance the quality, the validity, and the inter- and intra rater reliability of segmentation protocols for the human hippocampus in MR images.
Supplementary Material
Acknowledgments
This work was supported by a grant to C.K. by the Interdisciplinary Center for Clinical Research (IZKF FG4) of the University of Münster, Germany. A NIMH funded Career Development Award (KO1 MH073990) supported K.LN's contribution to this project. We thank Dr. H. Schiffbauer, Department of Clinical Radiology, University of Münster, Germany, for acquisition of high resolution MR images and the permission to publish them in this review. We thank the members of the Laboratory of Neuro Imaging at the University of California at Los Angeles, USA, in particular Liberty Hamilton, Cornelius Hojatka-shani, and Craig Schwartz, for their support. We are grateful for helpful discussions with Dr. J. Pruessner, McGill University, Montreal, and Dr. O. Kedo, Research Center Jülich, Germany.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2009.05.019.
References
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probabilitymaps. Anat. Embryol. (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Dutton RA, Dinov D, Hayashi KM, Toga AW, Cummings JL, Thompson PM. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch. Neurol. 2006;63:693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Greenwald BS, Kramer-Ginsberg E, Hu J, Wu H, Patel M, Aupperle P, Pollack S. Hippocampal/amygdala volumes in geriatric depression. Psychol. Med. 1999;29:629–638. doi: 10.1017/s0033291799008405. [DOI] [PubMed] [Google Scholar]
- Barnes J, Boyes RG, Lewis EB, Schott JM, Frost C, Scahill RI, Fox NC. Automatic calculation of hippocampal atrophy rates using a hippocampal template and the boundary shift integral. Neurobiol. Aging. 2007;28:1657–1663. doi: 10.1016/j.neurobiolaging.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Altshuler LL, Greider T, Curran J, Keen B, Dixon WJ. Reliability of medial temporal lobe volume measurements using reformatted 3D images. Psychiatry Res. 1998;82:11–24. doi: 10.1016/s0925-4927(98)00007-9. [DOI] [PubMed] [Google Scholar]
- Bell-McGinty S, Butters MA, Meltzer CC, Greer PJ, Reynolds 3rd CF, Becker JT. Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am. J. Psychiatry. 2002;159:1424–1427. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- Beyer JL, Krishnan KR. Volumetric brain imaging findings in mood disorders. Bipolar. Disord. 2002;4:89–104. doi: 10.1034/j.1399-5618.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Blatter DD, Anderson CV, Johnson SC, Gale SD, Hopkins RO, Burnett B. Hippocampal volume in normal aging and traumatic brain injury. AJNR Am. J. Neuroradiol. 1997;18:11–23. [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Kobayashi E, Cendes F, Min Li L. Protocol for volumetric segmentation of medial temporal structures using high-resolution 3-D magnetic resonance imaging. Hum. Brain. Mapp. 2004;22:145–154. doi: 10.1002/hbm.20023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. MRI investigation of temporal lobe structures in bipolar patients. J. Psychiatr. Res. 2003;37:287–295. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am. J. Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress. Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: J. A. Barth; 1909. [Google Scholar]
- Brodmann K, Garey LJ. Brodmann’s “Localisation in the cerebral cortex”. London: Smith-Gordon; 1994. [Google Scholar]
- Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev. Neurosci. 2007;18:93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Ann. N. Y. Acad. Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Caetano SC, Hatch JP, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res. 2004;132:141–147. doi: 10.1016/j.pscychresns.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Campbell S, MacQueen G. An update on regional brain volume differences associated with mood disorders. Curr. Opin. Psychiatry. 2006;19:25–33. doi: 10.1097/01.yco.0000194371.47685.f2. [DOI] [PubMed] [Google Scholar]
- Carmichael OT, Aizenstein HA, Davis SW, Becker JT, Thompson PM, Meltzer CC, Liu Y. Atlas-based hippocampus segmentation in Alzheimer’s disease and mild cognitive impairment. Neuroimage. 2005;27:979–990. doi: 10.1016/j.neuroimage.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BK, Sassi R, Axelson D, Hatch JP, Sanches M, Nicoletti M, Brambilla P, Keshavan MS, Ryan ND, Birmaher B, Soares JC. Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biol. Psychiatry. 2004;56:399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Chong H, Riis JL, McGinnis SM, Williams DM, Holcomb PJ, Daffner KR. To ignore or explore: top-down modulation of novelty processing. J. Cogn. Neurosci. 2008;20:120–134. doi: 10.1162/jocn.2008.20003. [DOI] [PubMed] [Google Scholar]
- Chupin M, Hammers A, Bardinet E, Colliot O, Liu RS, Duncan JS, Garnero L, Lemieux L. Fully automatic segmentation of the hippocampus and the amygdala from MRI using hybrid prior knowledge. Med. Image. Comput. Comput Assist Interv. Int Conf. Med. Image. Comput. Comput. Assist. Interv. 2007;10:875–882. doi: 10.1007/978-3-540-75757-3_106. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Wilkinson WE, Weiner RD, Parashos A, Djang WT, Webb MC, Figiel GS, Spritzer CE. Quantitative cerebral anatomy in depression. A controlled magnetic resonance imaging study. Arch. Gen. Psychiatry. 1993;50:7–16. doi: 10.1001/archpsyc.1993.01820130009002. [DOI] [PubMed] [Google Scholar]
- Colla M, Kronenberg G, Deuschle M, Meichel K, Hagen T, Bohrer M, Heuser I. Hippocampal volume reduction and HPA-system activity in major depression. J. Psychiatr. Res. 2007;41:553–560. doi: 10.1016/j.jpsychires.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Cook MJ, Fish DR, Shorvon SD, Straughan K, Stevens JM. Hippocampal volumetric and morphometric studies in frontal and temporal lobe epilepsy. Brain. 1992;115(Pt 4):1001–1015. doi: 10.1093/brain/115.4.1001. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Jones D, Rastogi-Cruz D, Posener JA, Heydebrand G, Miller JP, Miller MI. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am. J. Psychiatry. 2002;159:2000–2006. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- de Geus EJ, van t Ent D, Wolfensberger SP, Heutink P, Hoogendijk WJ, Boomsma DI, Veltman DJ. Intrapair differences in hippocampal volume in monozygotic twins discordant for the risk for anxiety and depression. Biol. Psychiatry. 2007;61:1062–1071. doi: 10.1016/j.biopsych.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. Berlin Heidelberg: Springer Verlag; 2005. [Google Scholar]
- El-Falougy H, Benuska J. History, anatomical nomenclature, comparative anatomy and functions of the hippocampal formation. Bratisl. Lek. Listy. 2006;107:103–106. [PubMed] [Google Scholar]
- Foland LC, Altshuler LL, Sugar CA, Lee AD, Leow AD, Townsend J, Narr KL, Asuncion DM, Toga AW, Thompson PM. Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport. 2008;19:221–224. doi: 10.1097/WNR.0b013e3282f48108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, Leinsinger G, Bottlender R, Hahn K, Moller HJ. Hippocampal changes in patients with a first episode of major depression. Am. J. Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 1. Review of methodologies currently employed. Mol. Psychiatry. 2005a;10:147–159. doi: 10.1038/sj.mp.4001580. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol. Psychiatry. 2005b;10:160–184. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- Ghanei A, Soltanian-Zadeh H, Windham JP. Segmentation of the hippocampus from brain MRI using deformable contours. Comput. Med. Imaging Graph. 1998;22:203–216. doi: 10.1016/s0895-6111(98)00026-3. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum. Brain. Mapp. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Keshavan MS, Lawrie SM. Deconstructing psychosis with human brain imaging. Schizophr. Bull. 2007;33:921–931. doi: 10.1093/schbul/sbm045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller JW, Banerjee A, Christensen GE, Gado M, Joshi S, Miller MI, Sheline Y, Vannier MW, Csernansky JG. Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology. 1997;202:504–510. doi: 10.1148/radiology.202.2.9015081. [DOI] [PubMed] [Google Scholar]
- Hammers A, Heckemann R, Koepp MJ, Duncan JS, Hajnal JV, Rueckert D, Aljabar P. Automatic detection and quantification of hippocampal atrophy on MRI in temporal lobe epilepsy: a proof-of-principle study. Neuroimage. 2007;36:38–47. doi: 10.1016/j.neuroimage.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–959. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- Heckemann RA, Hajnal JV, Aljabar P, Rueckert D, Hammers A. Automatic anatomical brain MRI segmentation combining label propagation and decision fusion. Neuroimage. 2006;33:115–126. doi: 10.1016/j.neuroimage.2006.05.061. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Honeycutt NA, Smith PD, Aylward E, Li Q, Chan M, Barta PE, Pearlson GD. Mesial temporal lobe measurements on magnetic resonance imaging scans. Psychiatry Res. 1998;83:85–94. doi: 10.1016/s0925-4927(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Hong SB, Shin YW, Kim SH, Yoo SY, Lee JM, Kim Y, Kim SI, Kwon JS. Hippocampal shape deformity analysis in obsessive-compulsive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2007;257:185–190. doi: 10.1007/s00406-006-0655-5. [DOI] [PubMed] [Google Scholar]
- Jack CR., Jr MRI-based hippocampal volume measurements in epilepsy. Epilepsia. 1994;35 Suppl 6:S21–29. doi: 10.1111/j.1528-1157.1994.tb05986.x. [DOI] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci. Biobehav. Rev. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Kates WR, Miller AM, Abdulsabur N, Antshel KM, Conchelos J, Fremont W, Roizen N. Temporal lobe anatomy and psychiatric symptoms in velocardio-facial syndrome(22q11.2 deletion syndrome) J. Am. Acad. Child. Adolesc. Psychiatry. 2006;45:587–595. doi: 10.1097/01.chi.0000205704.33077.4a. [DOI] [PubMed] [Google Scholar]
- Kiefer C, Slotboom J, Buri C, Gralla J, Remonda L, Dierks T, Strik WK, Schroth G, Kalus P. Differentiating hippocampal subregions by means of quantitative magnetization transfer and relaxometry: preliminary results. Neuroimage. 2004;23:1093–1099. doi: 10.1016/j.neuroimage.2004.07.066. [DOI] [PubMed] [Google Scholar]
- Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol. Med. 2004;34:1059–1064. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- Liu RS, Lemieux L, Bell GS, Sisodiya SM, Shorvon SD, Sander JW, Duncan JS. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. Neuroimage. 2003;20:22–33. doi: 10.1016/s1053-8119(03)00219-2. [DOI] [PubMed] [Google Scholar]
- Lloyd AJ, Ferrier IN, Barber R, Gholkar A, Young AH, O Brien JT. Hippocampal volume change in depression: late- and early-onset illness compared. Br. J. Psychiatry. 2004;184:488–495. doi: 10.1192/bjp.184.6.488. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Evans A, Lord C, Miles J, Pruessner M, Pike B, Pruessner JC. Hippocampal volume is as variable in young as in older adults: implications for the notion of hippocampal atrophy in humans. Neuroimage. 2007;34:479–485. doi: 10.1016/j.neuroimage.2006.09.041. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Kusumakar V. Hippocampal volume in early onset depression. BMC. Med. 2004;2:2. doi: 10.1186/1741-7015-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster FP, Mirza Y, Szeszko PR, Kmiecik LE, Easter PC, Taormina SP, Lynch M, Rose M, Moore GJ, Rosenberg DR. Amygdala and hippocampal volumes in familial early onset major depressive disorder. Biol. Psychiatry. 2008;63:385–390. doi: 10.1016/j.biopsych.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan S, Szeszko PR, Moore GJ, Madden R, Lorch E, Ivey J, Banerjee SP, Rosenberg DR. Increased amygdala: hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J. Child. Adolesc. Psychopharmacol. 2003;13:65–73. doi: 10.1089/104454603321666207. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JJ, Reglade-Meslin C, Anstey KJ, Sachdev P. Sex and symmetry differences in hippocampal volumetrics: before and beyond the opening of the crus of the fornix. Hippocampus. 2006;16:80–90. doi: 10.1002/hipo.20133. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. J. Anat. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW, Bilder RM. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, Bain EE, Charney DS, Drevets WC. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol. Psychiatry. 2005;57:935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Nicolson R, DeVito TJ, Vidal CN, Sui Y, Hayashi KM, Drost DJ, Williamson PC, Rajakumar N, Toga AW, Thompson PM. Detection and mapping of hippocampal abnormalities in autism. Psychiatry Res. 2006;148:11–21. doi: 10.1016/j.pscychresns.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Niemann K, Hammers A, Coenen VA, Thron A, Klosterkotter J. Evidence of a smaller left hippocampus and left temporal horn in both patients with first episode schizophrenia and normal control subjects. Psychiatry Res. 2000;99:93–110. doi: 10.1016/s0925-4927(00)00059-7. [DOI] [PubMed] [Google Scholar]
- Perez de, Alejo R, Ruiz-Cabello J, Cortijo M, Rodriguez I, Echave I, Regadera J, Arrazola J, Aviles P, Barreiro P, Gargallo D, Grana M. Computer-assisted enhanced volumetric segmentation magnetic resonance imaging data using a mixture of artificial neural networks. Magn. Reson. Imaging. 2003;21:901–912. doi: 10.1016/s0730-725x(03)00193-0. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Coleman RE, Doraiswamy PM. Neuroimaging and early diagnosis of Alzheimer disease: a look to the future. Radiology. 2003;226:315–336. doi: 10.1148/radiol.2262011600. [DOI] [PubMed] [Google Scholar]
- Pitiot A, Delingette H, Thompson PM, Ayache N. Expert knowledge-guided segmentation system for brain MRI. Neuroimage. 2004;23 Suppl 1:S85–S96. doi: 10.1016/j.neuroimage.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Pohl KM, Bouix S, Nakamura M, Rohlfing T, McCarley RW, Kikinis R, Grimson WE, Shenton ME, Wells WM. A hierarchical algorithm for MR brain image parcellation. IEEE. Trans. Med. Imaging. 2007;26:1201–1212. doi: 10.1109/TMI.2007.901433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posener JA, Wang L, Price JL, Gado MH, Province MA, Miller MI, Babb CM, Csernansky JG. High-dimensional mapping of the hippocampus in depression. Am. J. Psychiatry. 2003;160:83–89. doi: 10.1176/appi.ajp.160.1.83. [DOI] [PubMed] [Google Scholar]
- Powell S, Magnotta VA, Johnson H, Jammalamadaka K, Pierson R, Andreasen NC. Registration and machine learning-based automated segmentation of subcortical and cerebellar brain structures. Neuroimage. 2008;39:238–247. doi: 10.1016/j.neuroimage.2007.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb. Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Rusch BD, Abercrombie HC, Oakes TR, Schaefer SM, Davidson RJ. Hippocampal morphometry in depressed patients and control subjects: relations to anxiety symptoms. Biol. Psychiatry. 2001;50:960–964. doi: 10.1016/s0006-3223(01)01248-3. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat. Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Saylam C, Ucerler H, Kitis O, Ozand E, Gonul AS. Reduced hippocampal volume in drug-free depressed patients. Surg. Radiol. Anat. 2006;28:82–87. doi: 10.1007/s00276-005-0050-3. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch. Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am. J. Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Mittler BL, Mintun MA. The hippocampus and depression. Eur. Psychiatry. 2002;3(17 Suppl):300–305. doi: 10.1016/s0924-9338(02)00655-7. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J. Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc. Natl. Acad. Sci. U. S. A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Moffat S, Resnick SM, Davatzikos C. Measuring size and shape of the hippocampusinMRimages usingadeformableshapemodel. Neuroimage. 2002;15:422–434. doi: 10.1006/nimg.2001.0987. [DOI] [PubMed] [Google Scholar]
- Soininen HS, Partanen K, Pitkanen A, Vainio P, Hanninen T, Hallikainen M, Koivisto K, Riekkinen Sr PJ. Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: correlation to visual and verbal memory. Neurology. 1994;44:1660–1668. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br. J. Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Zimmerman ME, Getz GE, Mills NP, Ret J, Shear P, Adler CM. Ventricular and periventricular structural volumes in first-versus multiple-episode bipolar disorder. Am. J. Psychiatry. 2002;159:1841–1847. doi: 10.1176/appi.ajp.159.11.1841. [DOI] [PubMed] [Google Scholar]
- Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE, Hopkins RO, Depaulo JR, Potash JB, Schweizer B, Yates KO, Kurian E, Barta PE, Pearlson GD. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and com munity control subjects: a pilot study. Biol. Psychiatry. 2005;57:633–639. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Pfefferbaum A. Preservation of hippocampal volume throughoutadulthoodinhealthy men and women. Neurobiol. Aging. 2005;26:1093–1098. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Where are the perirhinal and parahippocampal cortices? A historical overview of the nomenclature and boundaries applied to the primate medial temporal lobe. Neuroscience. 2003;120:893–906. doi: 10.1016/s0306-4522(03)00281-1. [DOI] [PubMed] [Google Scholar]
- Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbol S, Frokjaer G, Holm S, Paulson OB, Knudsen GM. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage. 2005;24:969–979. doi: 10.1016/j.neuroimage.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart: Thieme; 1988. [Google Scholar]
- Thompson PM, Hayashi KM, DeZubicaray GI, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, Toga AW. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Tu Z, Narr KL, Dollar P, Dinov I, Thompson PM, Toga AW. Brain anatomical structure segmentation by hybrid discriminative/generative models. IEEE. Trans. Med. Imaging. 2008;27:495–508. doi: 10.1109/TMI.2007.908121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupler LA, De Bellis MD. Segmented hippocampal volume in children and adolescents with posttraumatic stress disorder. Biol. Psychiatry. 2006;59:523–529. doi: 10.1016/j.biopsych.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, Yurgelun-Todd DA. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol. Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- van der Lijn F, den Heijer T, Breteler MM, Niessen WJ. Hippocampus segmentation in MR images using atlas registration, voxel classification, and graph cuts. Neuroimage. 2008;43:708–720. doi: 10.1016/j.neuroimage.2008.07.058. [DOI] [PubMed] [Google Scholar]
- Van Paesschen W, Connelly A, King MD, Jackson GD, Duncan JS. The spectrum of hippocampal sclerosis: a quantitative magnetic resonance imaging study. Ann. Neurol. 1997;41:41–51. doi: 10.1002/ana.410410109. [DOI] [PubMed] [Google Scholar]
- Vemuri BC, Ye J, Chen Y, Leonard CM. Image registration via level-set motion: applications to atlas-based segmentation. Med. Image. Anal. 2003;7:1–20. doi: 10.1016/s1361-8415(02)00063-4. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD. Hippocampal and amygdalar volumes in dissociative identity disorder. Am. J. Psychiatry. 2006;163:630–636. doi: 10.1176/appi.ajp.163.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am. J. Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- von Gunten A, Fox NC, Cipolotti L, Ron MA. A volumetric study of hippocampus and amygdala in depressed patients with subjective memory problems. J. Neuropsychiatry Clin. Neurosci. 2000;12:493–498. doi: 10.1176/jnp.12.4.493. [DOI] [PubMed] [Google Scholar]
- von Gunten A, Ron MA. Hippocampal volume and subjective memory impairment in depressed patients. Eur. Psychiatry. 2004;19:438–440. doi: 10.1016/j.eurpsy.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am. J. Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Witter MP, Moser EI. Spatial representation and the architecture of the entorhinal cortex. Trends. Neurosci. 2006;29:671–678. doi: 10.1016/j.tins.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Woods RP. Multitracer: a Java-based tool for anatomic delineation of grayscale volumetric images. Neuroimage. 2003;19:1829–1834. doi: 10.1016/s1053-8119(03)00243-x. [DOI] [PubMed] [Google Scholar]
- Yucel K, McKinnon MC, Taylor VH, Macdonald K, Alda M, Young LT, MacQueen GM. Bilateral hippocampal volume increases after long-term lithium treatment in patients with bipolar disorder: a longitudinal MRI study. Psycho-pharmacology (Berl) 2007;195:357–367. doi: 10.1007/s00213-007-0906-9. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Taylor WD, Styner M, Steffens DC, Krishnan KR, MacFall JR. Hippocampus shape analysis and late-life depression. PLoS ONE. 2008;3:e1837. doi: 10.1371/journal.pone.0001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Rajapakse JC. Segmentation of subcortical brain structures using fuzzy templates. Neuroimage. 2005;28:915–924. doi: 10.1016/j.neuroimage.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Zilles K. Graue und weiβe Substanz des Hirnmantels. In: Leonhard H, Tillmann B, Töndury G, Zilles K, editors. Anatomie des Menschen. Stuttgart: InGeorg Thieme Verlag; 1987. pp. 382–471. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.