Abstract
Introduction. Lactobacillus casei and Lactobacillus acidophilus were used to assess the anti-inflammatory properties in carrageenan induced acute inflammatory model. Materials and Methods. Diclofenac sodium was used as standard drug at concentration of 150 mg/kg of body weight. Culture of Lactobacillus 2 × 107 CFU/ml was given orally. Edema was induced with 1% carrageenan to all the groups after one hour of the oral treatments. Paw thickness was checked at t = 1, 2, 3, 4, 5, and 24 hours. Stair climbing score and motility score were assessed at t = 24 hours. Cytokines assay for IL-6, IL-10, and TNF-α was performed on serum samples. Results. Lactobacillus showed a statistically significant decrease in paw thickness at P < 0.001. L. acidophilus and L. casei decreased by 32% and 28% in paw thickness. They both significantly increased the stair climbing and motility score. Lactobacillus treatment significantly downregulated IL-6 and TNF-α while upregulated IL-10 at P < 0.0001. Conclusion. L. casei and L. acidophilus significantly decreased the inflammatory reactions induced by carrageenan. This study has also proposed that Lactobacillus ameliorated the inflammatory reaction by downregulating the proinflammatory cytokines pathway.
1. Introduction
Inflammation is a local response of living mammalian tissues to injury. It is a body defense reaction in order to eliminate or limit the spread of injurious agent [1]. There are various components to an inflammatory reaction that can contribute to the associated symptoms and tissue injury. Edema, leukocyte infiltration, and granuloma formation represent such components of inflammation. Though, it is a defense mechanism. The complex events and mediators involved in the inflammatory reaction can induce or aggravate many reactions [2].
As it is well known, the probiotic bacteria are nonpathogenic and consumed as/with food since a long time. The use of probiotic bacteria in dietary supplements or dietary products is widely documented in the literature. Lactobacillus and its important species like L. casei and L. acidophilus have been used against many pathological and disease conditions. Lactobacillus is known for its antimicrobial, antilipidemic, immunomodulatory, anticancerous, antidiabetic, and antiarthritic properties [3–10]. Lactobacillus has been assessed for its immunomodulatory properties in different experiments [11–15].
According to the WHO report, about 70–80% of the world's population rely on nonconventional medicine mainly from herbal sources in their primary health care [16, 17]. Especially, its demand is increasing day by day in developing countries where the cost of consulting a physician and price of medicine are beyond the limit of most people [18]. These drugs are anti-inflammatory and used to ease pain in various conditions including: arthritis, muscle, and ligament pains. Conventional drugs treatments are limited in their effectiveness in managing the incidence and outcome of many inflammatory diseases. They also present a significant number of side effects in patients [19].
With these facts taken into account, present study was planned to find out the possibility of anti-inflammatory activity of L. casei and L. acidophilus using carrageenan-induced acute inflammatory model.
2. Materials and Methods
2.1. Bacterial Cultures
Lactobacillus acidophilus (ATCC 314) and Lactobacillus casei (ATCC 334) were purchased from Hi Media, Navi Mumbai, India. Lyophilized culture was streaked over de Mann Rogosa Sharpe Agar (MRS) at 37°C in anaerobic condition.
2.2. Drugs and Chemicals Used
Carrageenan was purchased from Hi Media, Mumbai, India. Standard anti-inflammatory drug diclofenac sodium was purchased from Recon, Bangalore, India. Cytokines assay kits were purchased from Ray Biotech, Norcross GA, USA and DNA Bio, Hyderabad, Andhra Pradesh, India.
2.3. Animals
35 male Wistar rats (200 gm each) were used for the present study. They were fed with standard pellet diet and water ad libitum. All animals were acclimatized for at least one week before the experimental session. All the experimental procedures were done following the guidelines of the Institutional Animals Ethics Committee (IEAC).
2.4. Evaluation of Anti-Inflammatory Activity
For the anti-inflammatory activity against the acute inflammation, animals were divided into five groups.
Group A (carrageenan control) did not receive any oral treatment; Group B (control) received 500 μL of distilled water; Group C and Group D received 2 × 107 CFU/mL of L. casei and L. acidophilus, respectively, suspended in 500 μL of distilled water. Group E (positive control) animals were administered with diclofenac sodium (150 mg/Kg body weight).
2.5. Carrageenan-Induced Acute Inflammatory Model
Anti-inflammatory activity was measured using carrageenan-induced rat paw edema assay [20, 21]. Edema was induced by subplantar injection of 100 μL of 1% freshly prepared solution of carrageenan in distilled water into the right-hind paws of each rat of all the groups except the group A. Animals of group B/C, D/E were treated with the single dose of vehicle, cultures, and drug, respectively; 30 minutes prior to carrageenan injection. Paw thickness were measured just before the carrageenan injection, that is, at “0 hour” and then at 1, 2, 3, 4, and 24th hour after carrageenan injection. Increase in paw thickness was measured as the difference in paw thickness at “0 hour” and paw thickness at respective hours.
2.6. Stair Climbing Activity Test
Overnight fasting animals were trained for one week to climb a staircase with steps at 5, 10, and 15 cm having water at the second and food at the third step. Climbing ability of the rats in above groups was scored 0 if the rats did not climb; 1, if the rats climbed onto step 1; 2, if the rats climbed onto step 2; 3, if the rat could climb all the three steps [22, 23].
2.7. Motility Test
The motility pattern of the rats was observed for a period of 5 minutes and scored 0, if the rat walked with difficulty and avoided touching the toes of the inflamed paw to the floor; 1, if the rat walked with little difficulty, but with toe touching the floor; 2, if the rat walked easily [22, 23].
2.8. Sample Collection
After 24 hours, all animals were sacrificed with cardiovascular bleeding with the help of Di ethyl ether according to the guidelines of CPSCEA committee. Blood was collected for the cytokines assay. Blood samples were left to coagulate for room temperature for 60 minutes and then centrifuged at 1500 g for 15 minutes and crude serum was kept in new tubes. Serum was harvested and stored at −20°C until use.
2.9. Cytokines Assay
IL-10 (anti-inflammatory cytokines) and IL-6, TNF-α (proinflammatory cytokines) in picogram per millilitre (pg/mL) were estimated with the help of ELISA Reader (Lisa Plus, Germany). Serum samples were used. IL-6 and TNF-α (Ray Bio ) and IL-10 (DNA bio) ELISA kits were used. Assays were performed according to the manufacturer's recommendations.
2.10. Statistical Analysis
The value for edema volume is expressed as mean ± SEM of seven observations and ANOVA followed by post hoc test. Duncans test was used to compare the groups. The stair climbing ability test and motility are expressed as median scores and the Kruskal-Wallis test was used to compare the groups.
3. Results
3.1. Carrageenan-Induced Inflammation
Injection of carrageenan into the hind paw induced a progressive edema reaching its maximum at 4 hours. In case of Group A animals paw thickness found at t = 0 was 3.023 ± 0.0408 cm and this remains constant at the end of 24 hours. Group B animals had showed an increase in paw thickness at each hour which was significant at P < 0.001. At 0 hours the thickness was 3.028 ± 0.040 cm, which increased to 3.59 ± 0.049 cm at t = 3 hours. At 24 hours the thickness was found to be 4.01 ± 0.025 cm. The paw thickness of Group C animals was 3 ± 0.028 cm which showed a mild increase at the end of 2nd hour, that is, 3.35 ± 0.0102 cm. After the 2nd hour it decreased to 3.04 ± 0.077 cm, 3.014 ± 0.0489 cm, 2.99 ± 0.0053 cm, and 3.014 ± 0.024 cm at the end of 3, 4, 5, and 24 hours, respectively. Group D animals showed an increase up to the 2nd hour. 3.03 ± 0.065 cm thickness was found at the end of third hour, which decreased to 3.0014 ± 0.0024 cm at t = 24 hours. So, Groups C and D indicated a statistically significant decrease in paw thickness (P < 0.001). Group E animals also showed an increase in paw thickness of 3.042 ± 0.077 cm (t = 0 hours), 3.26 ± 0.069 cm (t = 1 hours), 3.22 ± 0.0184 cm (t = 2 hours), and 3.074 ± 0.0762 cm (t = 3 hours). It increased after the third hour to 3.174 ± 0.057 cm (t = 24 hours). These values were found to be statistically significant at P < 0.001 (Figure 1).
Figure 1.
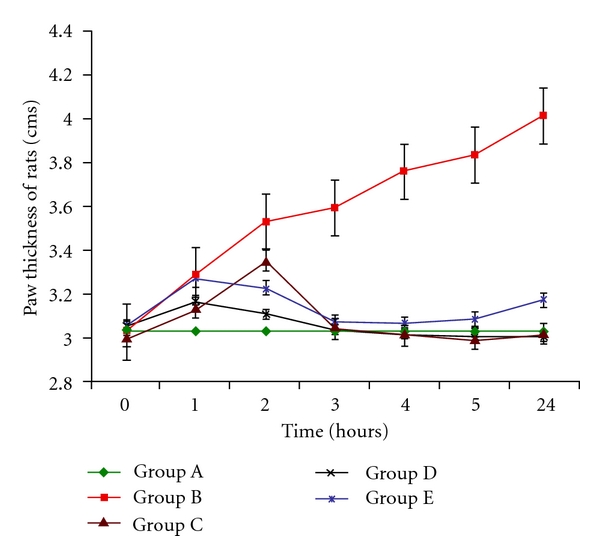
Change in paw thickness (cm) at t = 0,1, 2,3, 4,5, and 24 hours. n = 5 (significant at P < 0.001). Edema was induced by injecting 0.1 mL of 1% solution of carrageenan into the sub plantar surface of right-hind paw. Data are expressed as mean ± standard error of seven rats per group. Group A: carrageenan control; Group B: control; Group C: L. casei fed; Group D: L. acidophilus; Group E: positive control.
3.2. Stair Climbing Activity
Hyperalgesia was induced by carrageenan in Group B rats. Their stair climbing activity was 0.28 ± 0.244, while highest score was observed in Group A animals at 3 ± 0.00 (statistically significant; P < 0.001). Lactobacillus treatment significantly increased the stair climbing score of 2.57 ± 0.489 and 2.71 ± 0.408 for Group C and Group D respectively (significant at P < 0.001). Group E animals were showing a score of 1.71 ± 0.6122. Group B animal score was lowest in comparison to Groups A, C, D, and E animals (Figure 2).
Figure 2.
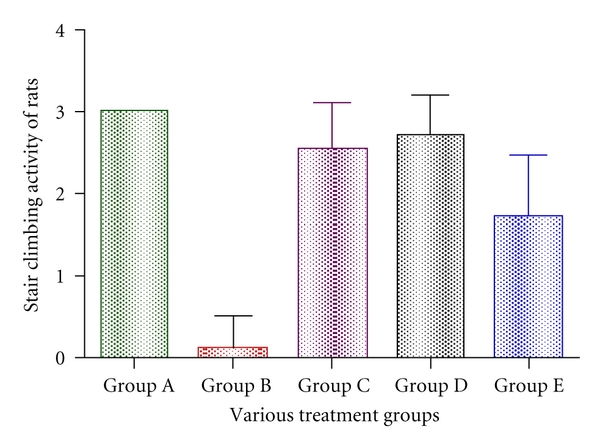
Effect of Lactobacillus on impairment in stair climbing activity score associated carrageenan-induced inflammation. Edema was induced by injecting 0.1 mL of 1% solution of carrageenan into subplantar surface of right-hind paw. The drugs were administered subcutaneously 30 minutes before injecting inflammagen. Stair climbing activity was observed at the time of peak inflammation (4 hours for carrageenan). Data are expressed as mean ± standard error of seven rats per group. Group A: carrageenan control; Group B: control; Group C: L. casei fed; Group D: L. acidophilus; Group E: positive control.
3.3. Motility
Walking ability of the rats to climb the staircase at the time of peak inflammation was checked by motility score. Group C and Group D animals were showing the highest score of 1.42 ± 0.489 (significant at P < 0.001). Score for Group A animals was 2 ± 0.00 whereas 0.14 ± 0.244 was found in case of group B animals. This was found to be the lowest when comparing Group E and Group B animals (Figure 3).
Figure 3.
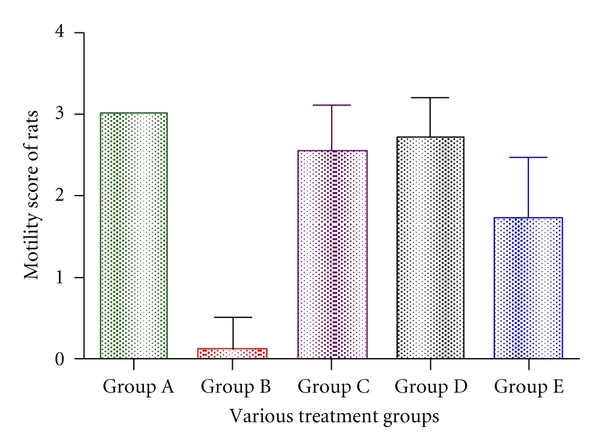
Effect of various drugs on impairment in motility associated with carrageenan-induced inflammation. Edema was induced by injecting 0.1 mL of 1% solution of carrageenan into subplantar surface of right-hind paw. The drugs were administered subcutaneously 30 minutes before injecting inflammagen. Motility score was observed at the time of peak inflammation (4 hours for carrageenan). Data are expressed as mean ± standard error of seven rats per group. Group A: carrageenan control; Group B: control; Group C: L. casei fed; Group D: L. acidophilus; Group E: positive control.
3.4. Cytokine Assay
Serum levels of IL-6 and TNF-α were highest in Group B animals, that is, 70.45 ± 0.266 pg/mL and 669.23 ± 0.050 pg/mL, respectively, while this group was showing lowest value of IL-10 (14.15 ± 0.035 pg/mL). On the contrary, Lactobacillus treatment statistically decreased the IL-6 and TNF-α in Group C and Group D at P < 0.0001. Lowest value for IL-6 was observed in case of Group D at 44.505 ± 0.198 pg/mL, while lowest value of TNF-α was observed for Group C at 420.77 ± 0.265 pg/mL. Group E animals was showing IL-6, TNF-α, and IL-10 values of 64.60 ± 0.131 pg/mL, 490.52 ± 0.228 pg/mL, and 29.675 ± 0.075 pg/mL, respectively. Only IL-6 concentration for Group E animals was not significant at P < 0.0001 while IL-10 and TNF-α concentrations were significant at P < 0.0001 (Table 1).
Table 1.
Effect of oral administration of L. casei and L. acidophilus on cytokines expression (pg/mL).
| Groups | IL-6 | TNF-α | IL-10 |
|---|---|---|---|
| Group A | 54.42 ± 0.193* | 530.75 ± 0.337* | 25.075 ± 0.047* |
| Group B | 70.45 ± 0.266 | 669.23 ± 0.050 | 14.15 ± 0.035 |
| Group C | 44.57 ± 1.809* | 420.77 ± 0.265* | 35.65 ± 0.086* |
| Group D | 44.50 ± 0.198* | 425.77 ± 0.187* | 38.725 ± 0.047* |
| Group E | 64.60 ± 0.131* | 490.52 ± 0.228* | 29.675 ± 0.075* |
Data are expressed as mean ± standard error of seven rats per group *Values along columns are statistically significant at P < 0.0001 when compared with Group B (control). Group A: carrageenan control; Group B: control; Group C: L. casei fed; Group D: L. acidophilus; Group E: positive control.
4. Discussion
Carrageenan-induced rat paw edema model is a suitable test for evaluating anti-inflammatory drugs, which has frequently been used to assess the antiedematous effect of the drug. Carrageenan is a strong chemical use for the release of inflammatory and proinflammatory mediators (prostaglandins, leukotrienes, histamine, bradykinin, TNF-α, etc.) [24].
The course of acute inflammation is biphasic. First phase starts with the release of histamine, serotonin, and kinins after the injection of phlogistic agent in the first few hours [25]. While the second phase is related to the release of prostaglandins like substances in 2-3 hours. Second phase is sensitive to both the clinically useful steroidal and nonsteroidal anti-inflammatory agent [26]. Prostaglandins are the main culprit responsible for acute inflammation. Lactobacillus sp might be containing some anti-inflammatory agent which is responsible for the blockage of prostaglandins and inflammatory pathway.
In this model of inflammation, L. casei and L. acidophilus had very consistent anti-inflammatory activity and thus showed significant decrease in the paw thickness of rat (Group C and Group D). Previously, some researchers have also proposed the anti-inflammatory property of Lactobacillus in gut [27–29]. Although, the cyclooxygenase and lipoxygenase pathways play a pivotal role in the inflammatory process, the inhibition of cyclooxygenase is more effective in inhibiting carrageenan-induced inflammation than lipoxygenase inhibitors [30].
Lactobacillus might have inhibited the cyclooxygenase that synthesizes prostaglandins. Oral administration of Lactobacillus significantly downregulated the proinflammatory cytokines while upregulated the anti-inflammatory cytokines. Prostaglandins play essential role in inflammation [7]. Prostaglandins synthesis is down regulated by anti-inflammatory cytokines like IL-10, IL-4 and IL-13, which also check Coxygenase-2 synthesis. Coxygenase-2 is responsible for the increased production of prostaglandins. And hence, L. casei and L. acidophilus overcome the inflammation induced by carrageenan. Our present findings are consistent with the results of some other workers [7, 31–34]. Studies have shown that IL-10 has been found as a potent macrophage deactivator, which blocked TNF-α, IL-1, IL-6, IL-8, and GM-CSF by human monocytes [35].
Knowledge on the immune-mediated mechanisms in metabolic scenario has markedly increased in the recent past, evidencing the role that dietary components may have to modulate immunity by enhancing or suppressing the immune response. For instance, certain strains of probiotics have been demonstrated to be able to modulate the immune system by stimulating release of different patterns of cytokines by different cells. It could be able to modify the number of CD4 T cells and actively interfere with anti-inflammatory and proinflammatory signalling pathways by inducing production of IL-10 and reducing INF-γ and TNF-α release [36]. Probiotics modulate both innate and acquired immunity by interacting with indigenous bacteria and/or host mucosal cells to induce or modulate the immune response. This can be another possible reason behind its anti-inflammatory property in this study.
The present results suggest that Lactobacillus suppresses the first phase of carrageenan-induced paw edema, thus, confirming an NSAID-like property. The present study showed that L. casei and L. acidophilus have both analgesic and anti-inflammatory properties.
Conflict of Interests
There are no financial competing interests (political, personal, religious, ideological, academic, intellectual, commercial, or any other) to declare in relation to this paper.
Authors' Contribution
S. Amdekar, P. Roy, and A. Kumar are equally contributing.
Acknowledgments
The author would like to thank Institute of Biomedical Sciences, Bundelkhand University, Jhansi, Uttar Pradesh for Animal House and laboratory facility and Department of Microbiology, Barkatullah University, Bhopal for laboratory facility provided to conduct the study.
References
- 1.Mahat MA, Patil BM. Evaluation of antiinflammatory activity of methanol extract of Phyllanthus amarus in experimental animal models. Indian Journal of Pharmaceutical Sciences. 2007;69(1):33–36. [Google Scholar]
- 2.Sosa S, Balick MJ, Arvigo R, et al. Screening of the topical anti-inflammatory activity of some Central American plants. Journal of Ethnopharmacology. 2002;81(2):211–215. doi: 10.1016/s0378-8741(02)00080-6. [DOI] [PubMed] [Google Scholar]
- 3.Jones SE, Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiology. 2009;9, article no. 35 doi: 10.1186/1471-2180-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Du R, Wang L, Zhang H. The antioxidative effects of probiotic Lactobacillus casei Zhang on the hyperlipidemic rats. European Food Research and Technology. 2010;231(1):151–158. [Google Scholar]
- 5.Singh V, Singh K, Amdekar S, et al. Innate and specific gut-associated immunity and microbial interference. FEMS Immunology and Medical Microbiology. 2009;55(1):6–12. doi: 10.1111/j.1574-695X.2008.00497.x. [DOI] [PubMed] [Google Scholar]
- 6.Amdekar S, Dwivedi D, Roy P, Kushwah S, Singh V. Probiotics: multifarious oral vaccine against infectious traumas. FEMS Immunology and Medical Microbiology. 2010;58(3):299–306. doi: 10.1111/j.1574-695X.2009.00630.x. [DOI] [PubMed] [Google Scholar]
- 7.Amdekar S, Singh V, Singh R, Sharma P, Keshav P, Kumar A. Lactobacillus casei reduces the inflammatory joint damage associated with collagen induced arthritis by reducing the Pro-inflammatory cytokines. Journal of Clinical Immunology. 2011;31(2):147–154. doi: 10.1007/s10875-010-9457-7. [DOI] [PubMed] [Google Scholar]
- 8.Rafter J. Probiotics and colon cancer. Best Practice and Research in Clinical Gastroenterology. 2003;17(5):849–859. doi: 10.1016/s1521-6918(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 9.Yadav H, Jain S, Sinha PR. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23(1):62–68. doi: 10.1016/j.nut.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Yadav H, Jain S, Sinha PR. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. Journal of Dairy Research. 2008;75(2):189–195. doi: 10.1017/S0022029908003129. [DOI] [PubMed] [Google Scholar]
- 11.Mileti E, Matteoli G, Iliev ID, Rescigno M. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS One. 2009;4(9) doi: 10.1371/journal.pone.0007056. Article ID e7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peran L, Camuesco D, Comalada M, et al. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. Journal of Applied Microbiology. 2007;103(4):836–844. doi: 10.1111/j.1365-2672.2007.03302.x. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez EM, Valenti V, Rockel C, et al. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut. 2011;60(8):1050–1059. doi: 10.1136/gut.2010.232918. [DOI] [PubMed] [Google Scholar]
- 14.Han W, Fioramonti J. Anti-inflammatory properties of lactic acid bacteria producing superoxide dismutase. American Journal of Physiology. 2007;294(1):p. G353. doi: 10.1152/ajpgi.00517.2007. [DOI] [PubMed] [Google Scholar]
- 15.Carroll IM, Andrus JM, Bruno-Bárcena JM, Klaenhammer TR, Hassan HM, Threadgill DS. Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. American Journal of Physiology. 2007;293(4):G729–G738. doi: 10.1152/ajpgi.00132.2007. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Traditional Medicine Strategy 2002–2005. Geneva, Switzerland: WHO; 2002. [Google Scholar]
- 17.Roy P, Amdekar S, Kumar A, Singh V. Preliminary study of the antioxidant properties of flowers and roots of Pyrostegia venusta (Ker Gawl) Miers. BMC Complementary and Alternative Medicine. 2011;11, article 69 doi: 10.1186/1472-6882-11-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barua CC, Talukdar A, Begum SA, et al. Antinociceptive activity of methanolic extract of leaves of Achyranthes aspera Linn. (Amaranthaceae) in animal models of nociception. Indian Journal of Experimental Biology. 2010;48(8):817–821. [PubMed] [Google Scholar]
- 19.Ayoola GA, Alpanika GA, Awabajo FO, et al. Anti-inflammatory properties of the fruits of Allanblanckia floribunda olive (guttiferae) Botany Research International. 2009;2(2):21–26. [Google Scholar]
- 20.Winter CA, Risley EA, Nuss GW. Carrageenan-induced edema in the hind paw of rat as an assay for anti-inflammatory activity. Proceedings of the Society for Experimental Biology and Medicine. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 21.Adeyemi OO, Okpo SO, Ogunti OO. Analgesic and anti-inflammatory effects of the aqueous extract of leaves of Persea americana Mill (Lauraceae) Fitoterapia. 2002;73(5):375–380. doi: 10.1016/s0367-326x(02)00118-1. [DOI] [PubMed] [Google Scholar]
- 22.De Castro Costa M, De Sutter P, Gybels J, Van Hees J. Adjuvant-induced arthritis in rats: a possible animal model of chronic pain. Pain. 1981;10(2):173–185. doi: 10.1016/0304-3959(81)90193-7. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Huang C, Cao Y, Han JS. Repeated administration of low dose ketamine for the treatment of monoarthritic pain in the rat. Life Sciences. 2000;67(3):261–267. doi: 10.1016/s0024-3205(00)00625-1. [DOI] [PubMed] [Google Scholar]
- 24.Wills AL. Release of histamin,kinin and prostaglandins during carrageenin induced inflammation of the rats. In: Montagazza P, Horton EW, editors. Prostaglandins, Peptides and Amins. London, UK: Academic Press; 1969. pp. 31–48. [Google Scholar]
- 25.Bhukya B, Anreddy RNR, William CM, Gottumukkala KM. Analgesic and anti-inflammatory activities of leaf extract of Kydia calycina Roxb. Bangladesh Journal of Pharmacology. 2009;4(2):101–104. [Google Scholar]
- 26.Brooks PM, Day RO. Non steroidal anti-inflammatory drugs differecnce and similarities. New England Journal of Medicine. 1991;324:1716–1725. doi: 10.1056/NEJM199106133242407. [DOI] [PubMed] [Google Scholar]
- 27.Holma R, Salmenperä P, Lohi J, Vapaatalo H, Korpela R. Effects of Lactobacillus rhamnosus GG and Lactobacillus reuteri R2LC on acetic acid-induced colitis in rats. Scandinavian Journal of Gastroenterology. 2001;36(6):630–635. doi: 10.1080/003655201750163114. [DOI] [PubMed] [Google Scholar]
- 28.Møller PL, Pærregaard A, Gad M, Kristensen NN, Claesson MH. Colitic scid mice fed Lactobacillus spp. show an ameliorated gut histopathology and an altered cytokine profile by local T cells. Inflammatory Bowel Diseases. 2005;11(9):814–819. doi: 10.1097/01.mib.0000175906.77340.15. [DOI] [PubMed] [Google Scholar]
- 29.Peran L, Camuesco D, Comalada M, et al. Lactobacillus fermentum, a probiotic capable to release glutathione, prevents colonic inflammation in the TNBS model of rat colitis. International Journal of Colorectal Disease. 2006;21(8):737–746. doi: 10.1007/s00384-005-0773-y. [DOI] [PubMed] [Google Scholar]
- 30.Giuliano F, Warner TD. Origins of prostaglandin E2: involvements of cyclooxygenase (COX)-1 and COX-2 in human and rat systems. Journal of Pharmacology and Experimental Therapeutics. 2002;303(3):1001–1006. doi: 10.1124/jpet.102.041244. [DOI] [PubMed] [Google Scholar]
- 31.Kirjavainen PV, Elnezami HS, Salminen SJ, Ahokas JT, Wright PFA. Effects of orally administered viable Lactobacillus rhamnosus GG and Propionibacterium freudenreichii subsp. shermanii JS on mouse lymphocyte proliferation. Clinical and Diagnostic Laboratory Immunology. 1999;6(6):799–802. doi: 10.1128/cdli.6.6.799-802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldenberg MM. Celecoxib, a selective cyclooxygenase-2 inhibitor for the treatment of rheumatoid arthritis and osteoarthritis. Clinical Therapeutics. 1999;21(9):1497–1513. doi: 10.1016/s0149-2918(00)80005-3. [DOI] [PubMed] [Google Scholar]
- 33.Johnson-Henry KC, Mitchell DJ, Avitzur Y, Galindo-Mata E, Jones NL, Sherman PM. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Digestive Diseases and Sciences. 2004;49(7-8):1095–1102. doi: 10.1023/b:ddas.0000037794.02040.c2. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Lee B, Lee HS, et al. Lactobacillus suntoryeus inhibits pro-inflammatory cytokine expression and TLR-4-linked NF-κB activation in experimental colitis. International Journal of Colorectal Disease. 2009;24(2):231–237. doi: 10.1007/s00384-008-0618-6. [DOI] [PubMed] [Google Scholar]
- 35.Trushin SA, Pennington KN, Carmona EM, et al. Protein kinase Cα (PKCα) acts upstream of PKCθ to activate IκB kinase and NF-κB in T lymphocytes. Molecular and Cellular Biology. 2003;23(19):7068–7081. doi: 10.1128/MCB.23.19.7068-7081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon HK, Lee CG, So JS, et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(5):2159–2164. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]


