Abstract
A key issue in otitis media is mucous cell metaplasia which is responsible for mucous hypersecretion and persistence of the disease. However, little is known about the molecular mechanisms of mucous cell metaplasia in otitis media. Numerous studies of intestinal epithelial homeostasis have shown that Atonal homolog 1 (Atoh1), a basic helix-loop-helix (bHLH) transcription factor, is essential for the intestinal goblet cell differentiation. On the other hand, SAM-pointed domain-containing Ets transcription factor (SPDEF), a member of the “Ets” transcription factor family, has been reported to trigger the mucous cell metaplasia of pulmonary infectious diseases or athsma. Recent studies have demonstrated the relation of these factors, that is, Spdef functions downstream of Atoh1. We could take the adventages of these findings for the study of otitis media because both middle ear and pulmonary epithelia belong to the same respiratory tract. Atoh1 and SPDEF could be the therapeutic targets for otitis media associated with mucous cell metaplasia which is frequently considered “intractable” in the clinical settings.
1. Mucous Cell Metaplasia in Intractable Otitis Media
One of the most characteristic features of intractable otitis media is mucous hypersecretion. Chronic otitis media, caused by a prolonged bacterial infection, is characterized by ear discharge that is rich in mucus. Otitis media with mucoid effusion, common in young child otitis media patients and caused by a combination of bacterial infection and dysfunction of the Eustachian tube [1], is characterized by thick fluid accumulation in the middle ear cavity. Eosinophilic otitis media, which is a newly recognized intractable otitis media with bronchial asthma, is characterised by the presence of a highly viscous yellow effusion containing eosinophils [2].
The pathological basis of the mucous hypersecretion is mucous cell metaplasia, abnormal transformation of the normal respiratory tract mucosa into a thickened mucosa with a high density of the goblet cell population in response to microorganic infections or asthma. In a murine model of asthma, there was a dramatic increase in goblet cell number accompanied by a 75% decrease in Clara cells and a 25% decrease in ciliated cells after allergen challenge with a total number of epithelial cells being constant [3]. The increase of goblet cells and decrease of ciliated cells are also reported in the rat model of otitis media with effusion induced by the endotoxin plus Eustachian tube obstruction [4]. These findings suggest that the ciliated cells convert to goblet cells under the pathological conditions via a biologic process called cell transdifferentiation. It has been demonstrated that various materials such as bacteria [1], lipopolysaccharide [5], tumor necrosis factor-alpha (TNF-α) [6], and interleukin-10 (IL-10) [7] are involved in mucous cell metaplasia of the middle ear mucosa.
Mucins serve critical functions in the host defense against microorganisms. They are the principal structural components of the mucociliary transport system and the pathogens entrapped in a mucin gel layer are eliminated by constant beatings of the cilia on the mucosal surface. Under normal conditions, few mucous cells exist in the middle ear mucosa and they are mainly distributed in the orifice of the Eustachian tube, promontory area, and inferior tympanium. However, under pathological conditions inducing mucous cell metaplasia, an increased goblet cell population in the mucosa produces excessive mucins, while the loss of ciliated cells in the mucosa results in the dysfunction of the mucociliary transport system, leading to subsequent problems such as fluid accumulation and repeated infections. It is necessary to understand the disease mechanism of mucous cell metaplasia in order to cure this intractable illness.
2. The Role of Atoh1 in Mucous Cell Differentiation
Atonal homologue 1 (Atoh1), also called the mouse atonal homolog 1 (Math1) gene in mice and the human atonal homolog 1 (Hath1) gene in humans, is one of the candidate genes which play a crucial role in the mucous cell metaplasia. Atoh1 is a basic helix-loop-helix (bHLH) transcription factor that is essential for the intestinal goblet cell differentiation. It has been shown that goblet cells are completely abrogated in the intestine of Math1-null mouse [8]. Math1-null embryos die at birth due to respiratory failure and lack of many specific cell lineages, including not only intestinal secretory cells but also cerebellar granule neurons, spinal cord interneurons, and inner ear hair cells [9].
The role of Atho1 in goblet cell differentiation has been well studied in the intestine. The epithelium of the small intestine is composed of four different cell types: absorptive enterocytes and three secretory lineages consisting of mucus-secreting goblet cells, hormone-secreting enteroendocrine cells, and antimicrobial peptide-secreting Paneth cells. The intestinal epithelium is a highly dynamic tissue and is replaced by newly differentiated cells from multipotent stem cells residing near the base of the Crypts in every 2–7 day [9]. The differentiation for an absorptive or secretory cell linage is controlled by the Notch signaling system in a manner of lateral inhibition (Figure 1). Notch is a cell surface receptor that binds to Notch ligands, Dll (Delta-like), and Jagged families. Notch ligand, secreted from a progenitor cell which has a fate to become a goblet cell, binds to the Notch receptor on the other neighboring progenitor cell. This Notch receptor activation decides the fate of the neighboring cell because the Notch target gene Hairy/enhancer-of-split 1 (Hes1) inhibits the Atoh1. In this way, the neighboring progenitor becomes an absorptive cell. Akiyama et al. [10] has shown Atoh1 is one of the downstream genes of Notch ligands and Atoh1 is involved in the mechanisms of cellular differentiation from progenitor cells to goblet cells. They showed that Dll1 and Dll4 were selectively expressed in goblet cells within the human colonic epithelium and knockdown of Dll1 abrogated the Hath1 and MUC2 gene expression under activation of the goblet cell genes by a Notch signal inhibitor, suggesting the Notch ligand triggers the goblet cell differentiation through Atoh1 activation. They have also reported forced expression of the Notch1 intracellular domain, an intracellular component of the Notch receptor, significantly suppressed Dll1, Dll4, MUC2, and Hath1 gene expression, suggesting that notch receptor activation prevents Atoh1 from triggering goblet cell differentiation by blocking the Notch ligand expression.
Figure 1.
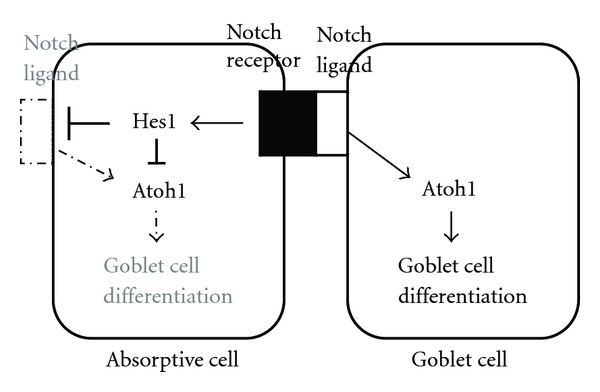
A schematic representation of lateral inhibition by a Notch ligand. A Notch ligand secreting cell releases a Notch ligand that binds to the Notch receptor of a neighboring cell. The action inhibits the activity of Atoh1 through Notch target gene Hes1 in the cell, leading to the formation of an absorptive cell. While a Notch ligand secreting cell has a high activity of Atoh1, leading to the differentiation of a goblet cell.
MUC2 is specifically expressed in the goblet cells of the intestine and thought as a goblet cell marker [11]. Park et al. found that Hath1 directly activated transcription of MUC2 gene in the human intestinal epithelial cells [12]. In the human gastric cancer cells, overexpression of Math1 strongly enhanced both the MUC6 and MUC5AC mRNA transcript levels and knockdown of the Hath1 gene significantly decreased the expression of both mucin genes [13].
The role of Atoh1 in mucous cell development is well studied. However, the role of Atoh1 in mucous cell metaplasia under diseased conditions is underinvestigated. An infection of whipworm (Trichuris muris) in the mouse intestine is reported to induce mucous cell metaplasia where Math1 is significantly upregulated in the mRNA level [14].
3. The Role of SPDEF in Mucous Cell Metaplasia
SAM Pointed Domain ETS Factor (SPDEF, also termed PDEF or PSE) is another candidate controlling mucous cell metaplasia. It is a member of the “Ets” family which regulates a number of biological processes, including cell proliferation, differentiation, and invasion. SPDEF was first described as a factor interacting with the androgen receptor to enhance expression of the prostate-specific antigen (PSA) promoter in vitro [15].
The role of SPDEF in mucous cell metaplasia is well attended in the study of lung diseases. SPDEF was markedly increased at sites of mucous cell metaplasia in bronchial tissues from patients with Cystic fibrosis or cigarette smoking [16]. In a murine model of asthma, the expression of SPDEF was also increased at sites of mucous cell metaplasia caused by IL-13 and dust mite allergen [17]. Chen et al. [16] have shown the Clara cell turns to goblet cell in the lung within 3 days after expression of SPDEF using a transgenic mouse model where the expression of Spdef gene under the Clara cell-specific promoter is controlled by doxycyline concentration (Scgb1a1-rtTA/TRE2-Spdef). This process is rapidly reversible and associated with the restoration of the Clara cells after stopping of the SPDEF expression. They have also reported some genes under the control of SPDEF. An mRNA microarray analysis study showed the expression of SPDEF influenced more than 300 genes including mucin 16 (MUC16), anterior gradient 2 (AGR2), glucosaminyl (N-acetyl) transferase 3, mucin type (Gcnt3), and chloride channel calcium activated 1 (Clca1). The involvement of these genes was confirmed by others. MUC5AC, AGR2, and Clca1 induced by IL-13 treatment were reduced in Spdef knockout human airway epithelial cells [18]. In colon cancer cells to activate the goblet cell genes by Notch signal inhibitors, knockdown of Spdef also repressed the expression of MUC2 and AGR2 [19]. Mucin is a large-molecular-weight glycoprotein and mucin production requires several steps including transcription of a MUC gene, holding, multimerization, and glycosylation. MUC genes code core mucin protein. AGR2 is a member of protein disulfide isomerase (PDI) family which is critical for efficient formation of correctly arranged disulfide bonds in the endoplasmic reticulum (ER) [20]. Gcnt3 is involved in the synthesis of a core structure in the mucin glycan chain. Clca1, whose mouse homolog is called as Gob5, is believed to have a role in secreting chloride anions into the lumen and contributing to the salt and water composition of secreted mucins [21]. All these genes contribute the production of mature mucins, which is conducted in the well-differentiated goblet cells. Inversely, blockage of SPDEF results in a failure of goblet cell maturation. The intestine of Spdef knockout mice revealed a severe loss of mature goblet cells and Paneth cells accompanied by accumulation of immature secretory progenitors. The typical aberrant goblet cells in Spdef knockout mice exhibit a clear brush border similar to adjacent enterocytes and carry poorly defined vacuoles in their cytoplasm. These immature goblet cells probably initiate the differentiation of goblet cells because they expressed trefoil factor 3 (TFF3), which is one of the goblet cell markers and thought to help in the oligomerization of mucin polysaccharides, although they did not have Alcian Blue Periodic Acid-Schiff (AB-PAS)-positive granules [22]. These data suggests SPDEF is a factor to serve for the terminal differentiation of goblet cells rather than to initiate or trigger the differentiation of goblet cells. SPDEF is thought to be a key transcription factor to start a series of sequential gene activations for the terminal differentiation and maturation of goblet cells.
4. SPDEF Functions as a Downstream Molecule of Atoh1
Accumulated pieces of evidence indicate that Spdef functions downstream of Atoh1, and this is mediated by another transcription factor, growth-factor-independent 1 (GFI1), which is normally expressed in the both Paneth and goblet cells (Figure 2). SPDEF was absent from intestine of the Math1-null mouse and was also quantitatively reduced in the GFI1-null mouse [19]. GFI1 is thought to be downstream of Atoh1 because the Math1 expression was still observed in the GFI1-null mouse [9]. Atoh1-null crypts lack all the intestinal secretory cells. On the other hand, GFI1-null crypts lack Paneth cells, have few goblet and supernumerary enteroendocrine cells. These findings indicate the selection between secretory cells (goblet cells, Paneth cells, and enteroendocrine cells) and absorptive cell is dependent on Atoh1, and the selection between goblet/Paneth and enteroendocrine cells is dependent on GFI1 [9].
Figure 2.
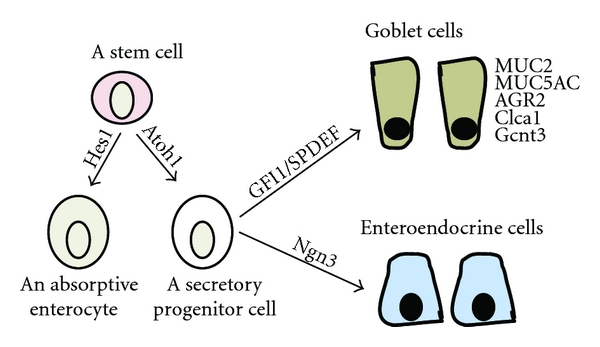
The differentiation path of a goblet cell starting from a progenitor and the genes involved in this process. An intestine stem cell becomes an absorptive cell in the absence of the Atoh1 protein but activation of Hes1 or becomes a secretory progenitor cell under the influence of Atoh1 protein with the potential to become either enteroendocrine cells under the direction of neurogenin 3 (Ngn3) or goblet cells under the direction of GFI1 and SPDEF.
5. The Role of Atoh1 in Otitis Media
There is no report to discuss the involvement of Atoh1, GFI1, or Spdef in otitis media. However, the similarity of the middle ear epithelium to the pulmonary epithelium, Atoh1, the upstream gene of Spdef which is involved in several pulmonary diseases, may also have a role in the mucous cell metaplasia of otitis media. To test this hypothesis, we performed the transfection of Math1 in the mouse middle ear. It was found that mucous cell numbers were significantly increased in Math1 transfected middle ears compared with empty vector transfection (Figure 3). We also evaluated the effects of administration of TNFα, which is a common proinflammatory cytokine induced by bacterial or virus infection, and retinoic acid (RA) on mouse middle ear epithelial cells [23]. The administration of these factors for two weeks synergistically promoted the expression of MUC2 and TFF3 with an activation of Math1 in vitro (Figure 4). These data suggest that Atoh1 activated by proinflammatory cytokines triggers the mucous cell metaplasia in the middle ear epithelium as well.
Figure 3.
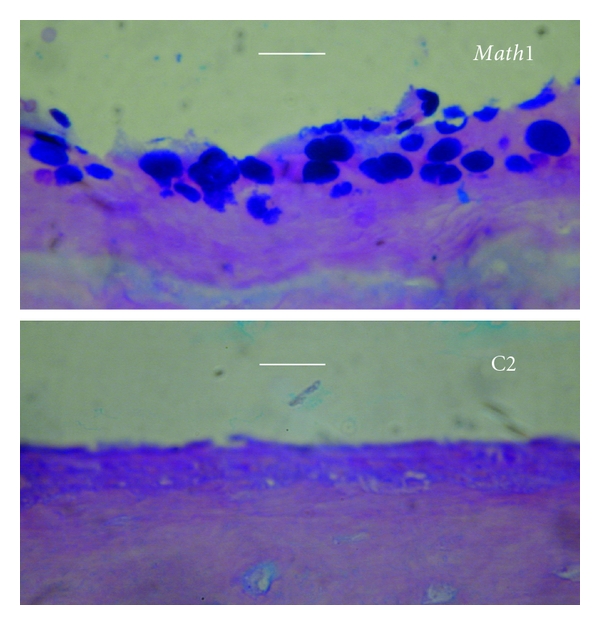
Math1 transfection increases the mucous cells in the middle ear mucosa of mice. Full-length Math1 cDNA was cloned into a protein-expressing vector (C2, pEGFP, Clontech). To study the role of Math1 in the middle ear mucosa, bilateral bullae of 5 mice were transfected with 10 μL of Math1 and empty vectors, respectively, at 1.4 μg/mL in Opti-MEM containing Lipofectin at 6 μg/mL via the tympanic membrane approach. Transfected animals were sacrified 7 days after Math1 transfection for harvest of the bullae. Math1 transfection had more AB-PAS positive cells in the mouse middle ear mucosa (upper) than empty vector (C2) transfection (lower). Bar = 10 μm.
Figure 4.
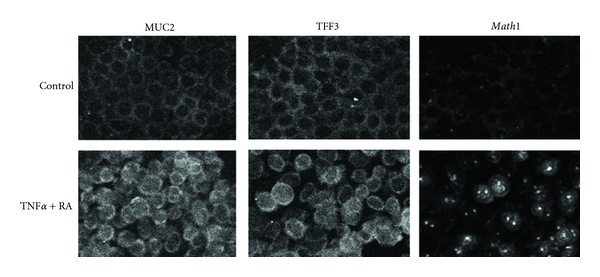
Mouse middle ear epithelial cells were incubated with 20 ng/mL of TNFα and 10−9 M of RA for two weeks on 8-well chamber slides (media and factors were supplied every two days). Immunohistochemistry showed that these factors increased the expression of goblet cell markers, MUC2 and TFF3, suggesting that this could be a model of mucous cell metaplasia in infectious otitis media. Math1 was located only in the cytoplasm in the control culture, however, the administration of TNFα + RA made Math1 translocate into nuclei. This Math1 activation (translocation to nuclei) probably triggers the expression of MUC2 and TFF3.
6. Conclusions
Over the past decade there have been significant advances in the studies of intestinal epithelial homeostasis and pulmonary mucous cell metaplasia. The former studies found Atoh1 as a factor to trigger the differentiation of goblet cells, and the latter studies found SPDEF as a factor to be responsible for mucous cell metaplasia, respectively. Now we can take advantages of these molecular understanding about mucous cells for the therapy of otitis media. Mucous cell metaplasia is a very primitive response to pathogens. Originally, this response is to protect the mucociliary system by secreting more mucins and their chaperones. These secreted substances are supposed to lubricate the lumen of the respiratory tract and discharge hazardous materials. However, mucous cell metaplasia also disturbs the well-organized mucociliary transport system at the expense of ciliated cells. Excessive mucus severely impairs the function of the mucociliary transport system and leads to subsequent infections. To make matter worse, bacterial cell wall components, or inflammatory cytokines trapped in accumulated mucus can be a driving force again for mucous cell metaplasia and thus form a vicious cycle. This vicious circle could explain why mucous cell metaplasia prolongs infectious diseases on a long-term basis. The effective blockage of the Atoh1, SPDEF, or GFI1 expression in the early phase of disease may be a new strategy for treating intractable otitis media. It could break the vicious circle of mucous cell metaplasia and lessen the burden of otitis media patients with mucoid effusion.
References
- 1.Tsuboi Y, Kim Y, Giebink GS, et al. Induction of mucous cell metaplasia in the middle ear of rats using a three-step method: an improved model for otitis media with mucoid effusion. Acta Oto-Laryngologica. 2002;122(2):153–160. doi: 10.1080/00016480252814153. [DOI] [PubMed] [Google Scholar]
- 2.Iino Y. Role of IgE in eosinophilic otitis media. Allergology International. 2010;59(3):233–238. doi: 10.2332/allergolint.10-RAI-0223. [DOI] [PubMed] [Google Scholar]
- 3.Reader JR, Tepper JS, Schelegle ES, et al. Pathogenesis of mucous cell metaplasia in a murine asthma model. American Journal of Pathology. 2003;162(6):2069–2078. doi: 10.1016/S0002-9440(10)64338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nell MJ, Grote JJ. Structural changes in the rat middle ear mucosa due to endotoxin and eustachian tube obstruction. European Archives of Oto-Rhino-Laryngology. 1999;256(4):167–172. doi: 10.1007/s004050050134. [DOI] [PubMed] [Google Scholar]
- 5.Hunter SE, Singla AK, Prazma J, Jewett BS, Randell SH, Pillsbury HC. Mucin production in the middle ear in response to lipopolysaccharides. Otolaryngology—Head and Neck Surgery. 1999;120(6):884–888. doi: 10.1016/S0194-5998(99)70331-1. [DOI] [PubMed] [Google Scholar]
- 6.Kawano H, Haruta A, Tsuboi Y, et al. Induction of mucous cell metaplasia by tumor necrosis factor alpha in rat middle ear: the pathological basis for mucin hyperproduction in mucoid otitis media. Annals of Otology, Rhinology and Laryngology. 2002;111(5 I):415–422. doi: 10.1177/000348940211100506. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchiya K, Komori M, Zheng QY, Ferrieri P, Lin J. Interleukin-10 is an essential modulator of mucoid metaplasia in a mouse otitis media model. Annals of Otology, Rhinology and Laryngology. 2008;117(8):630–636. doi: 10.1177/000348940811700814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294(5549):2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 9.Shroyer NF, Wallis D, Venken KJT, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes and Development. 2005;19(20):2412–2417. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akiyama J, Okamoto R, Iwasaki M, et al. Delta-like 1 expression promotes goblet cell differentiation in Notch-inactivated human colonic epithelial cells. Biochemical and Biophysical Research Communications. 2010;393(4):662–667. doi: 10.1016/j.bbrc.2010.02.048. [DOI] [PubMed] [Google Scholar]
- 11.Winterford CM, Walsh MD, Leggett BA, Jass JR. Ultrastructural localization of epithelial mucin core proteins in colorectal tissues. Journal of Histochemistry and Cytochemistry. 1999;47(8):1063–1074. doi: 10.1177/002215549904700811. [DOI] [PubMed] [Google Scholar]
- 12.Park ET, Oh HK, Gum JR, et al. HATH1 expression in mucinous cancers of the colorectum and related lesions. Clinical Cancer Research. 2006;12(18):5403–5410. doi: 10.1158/1078-0432.CCR-06-0573. [DOI] [PubMed] [Google Scholar]
- 13.Sekine A, Akiyama Y, Yanagihara K, Yuasa Y. Hath1 up-regulates gastric mucin gene expression in gastric cells. Biochemical and Biophysical Research Communications. 2006;344(4):1166–1171. doi: 10.1016/j.bbrc.2006.03.238. [DOI] [PubMed] [Google Scholar]
- 14.Hasnain SZ, Thornton DJ, Grencis RK. Changes in the mucosal barrier during acute and chronic Trichuris muris infection. Parasite Immunology. 2011;33(1):45–55. doi: 10.1111/j.1365-3024.2010.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oettgen P, Finger E, Sun Z, et al. PDEF, a novel prostate epithelium-specific Ets transcription factor, interacts with the androgen receptor and activates prostate-specific antigen gene expression. Journal of Biological Chemistry. 2000;275(2):1216–1225. doi: 10.1074/jbc.275.2.1216. [DOI] [PubMed] [Google Scholar]
- 16.Chen G, Korfhagen TR, Xu Y, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. Journal of Clinical Investigation. 2009;119(10):2914–2924. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park KS, Korfhagen TR, Bruno MD, et al. SPDEF regulates goblet cell hyperplasia in the airway epithelium. Journal of Clinical Investigation. 2007;117(4):978–988. doi: 10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu H, Li Q, Kolosov VP, Perelman JM, Zhou X. Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Communication and Adhesion. 2011;17(4-6):83–92. doi: 10.3109/15419061.2010.551682. [DOI] [PubMed] [Google Scholar]
- 19.Noah TK, Kazanjian A, Whitsett J, Shroyer NF. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Experimental Cell Research. 2010;316(3):452–465. doi: 10.1016/j.yexcr.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SW, Zhen G, Verhaeghe C, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(17):6950–6955. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thai P, Chen Y, Dolganov G, Wu R. Differential regulation of MUC5AC/Muc5ac and hCLCA-1/mGob 5 expression in airway epithelium. American Journal of Respiratory Cell and Molecular Biology. 2005;33(6):523–530. doi: 10.1165/rcmb.2004-0220RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregorieff A, Stange DE, Kujala P, et al. The Ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137(4):1333–e3. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 23.Tsuchiya K, Kim Y, Ondrey FG, Lin J. Characterization of a temperature-sensitive mouse middle ear epithelial cell line. Acta Oto-Laryngologica. 2005;125(8):823–829. doi: 10.1080/00016480510031533. [DOI] [PubMed] [Google Scholar]


