Abstract
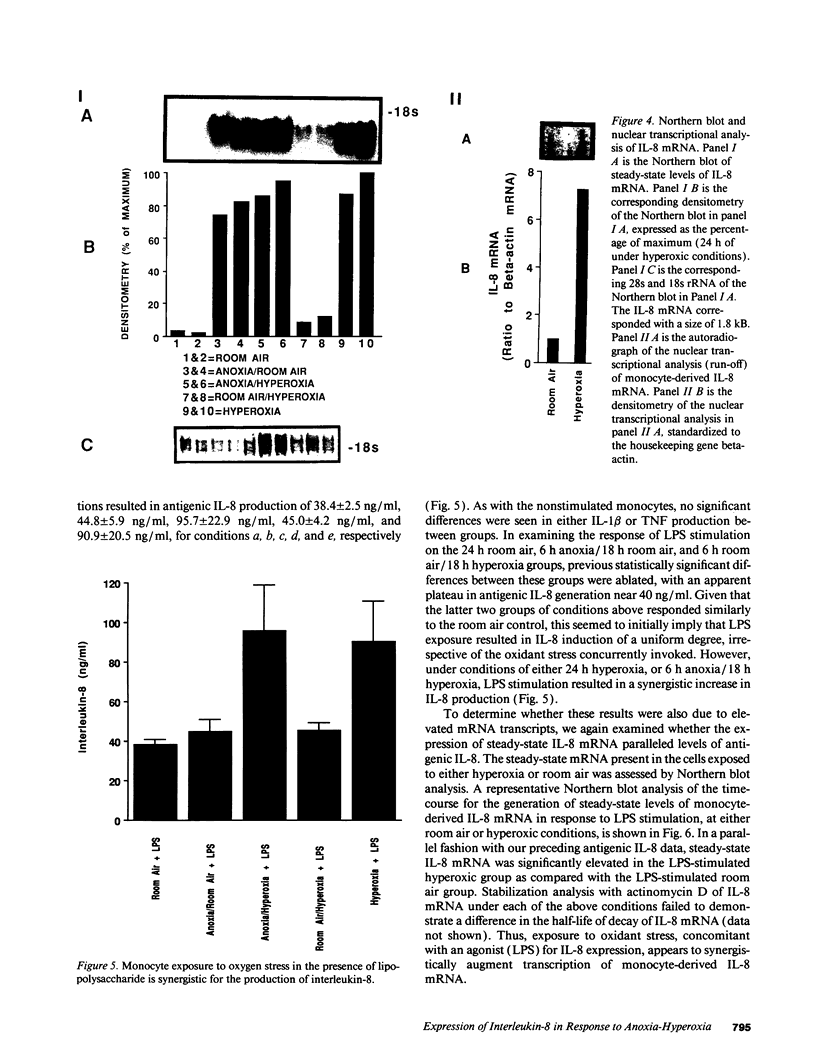
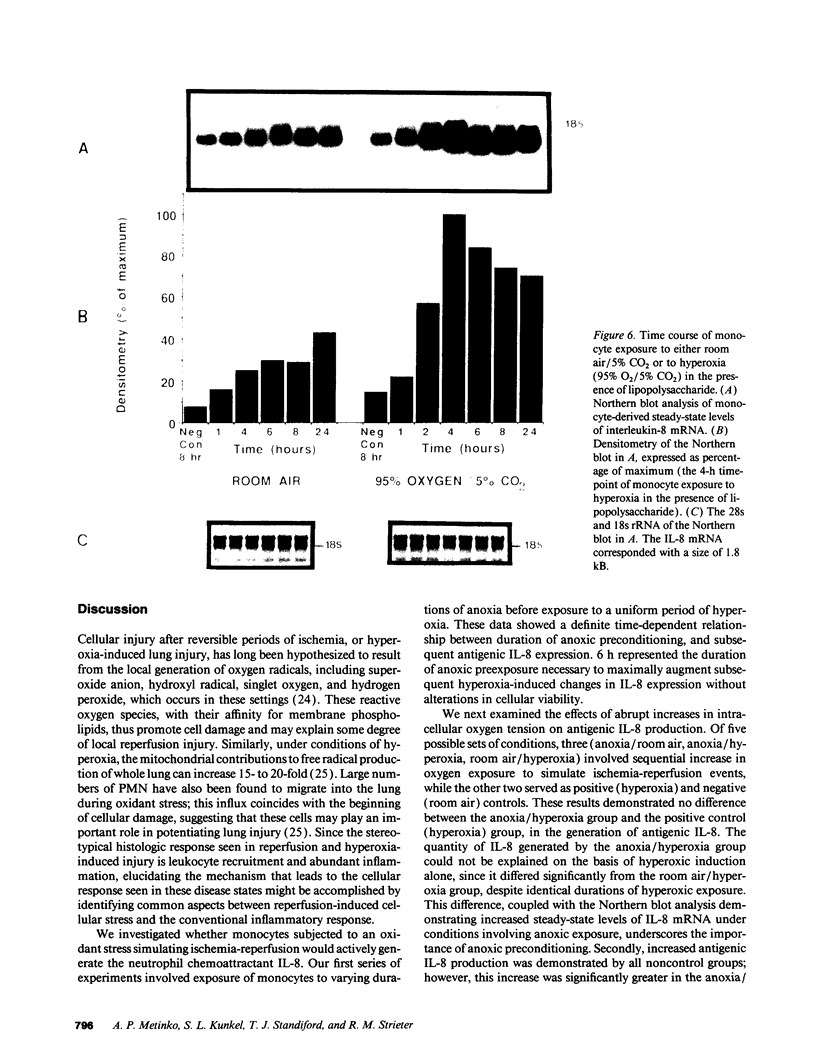
Ischemia-reperfusion and hyperoxia-induced pulmonary injury are associated with the presence of activated neutrophils (PMN) and cellular injury. Although the signals orchestrating the directed migration of these PMN during the pathogenesis of these disease states remain to be fully elucidated, it appears they may be dependent upon the production of certain neutrophil activating/chemotactic factors such as C5a, leukotriene B4, platelet-activating factor, and IL-8. The production of the latter chemotaxin by mononuclear phagocytes is especially intriguing as these cells can mediate inflammatory cell migration by either directly generating IL-8, or by inducing its production from surrounding nonimmune cells. In light of these observations, we propose that ischemia-reperfusion and oxidant stress, in vivo, may be simulated by anoxia-hyperoxia induced stress in vitro, and that this stress may act as a stimulus for the production of IL-8. We now show that isolated human blood monocytes respond to such an oxygen stress with augmented production of IL-8. In initial studies, monocytes demonstrated an increase in the production of IL-8 under anoxic preconditioning. Subsequently, monocytes were cultured under one of the following conditions for 24 h: (a) room air/5% CO2; (b) 95% N2/5% CO2 for 6 h, followed by room air/5% CO2 for 18 h; (c) 95% N2/5% CO2 for 6 h, followed by 95% O2/5% CO2 for 18 h; (d) room air/5% CO2 for 6 h, followed by 95% O2/5% CO2 for 18 h; or (e) 95% O2/5% CO2. Supernatants were isolated and analyzed for IL-8 antigen by specific IL-8 ELISA, demonstrating the production of monocyte-derived IL-8: 5.9 +/- 0.9, 11.4 +/- 1.7, 21.1 +/- 2.3, 14.6 +/- 2.4, and 26.3 +/- 4.7, ng/ml by designated conditions a, b, c, d, and e listed above, respectively. This variance in IL-8 production reflects altered rates of transcription as shown by Northern blot analysis and nuclear run-off assay. Furthermore, when monocytes were concomitantly treated with LPS (100 ng/ml) under in vitro hyperoxic conditions, both IL-8 steady-state mRNA and antigenic activity were two- to threefold greater than under room air conditions. The association of anoxic preconditioning and oxygen stress with augmented production of monocyte-derived IL-8 support the potential role for ischemia-reperfusion and hyperoxia-induced IL-8 production in vivo, providing a possible mechanism for PMN migration/activation in disease states characterized by altered tissue oxygenation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman J. W., Rinaldo J. E., Henson J. E., Moore S. A., Dauber J. H. Modification by hyperoxia in vivo of endotoxin-induced neutrophil alveolitis in rats. Production of chemotactic factors by alveolar macrophages and ultrastructure. Am Rev Respir Dis. 1985 Jul;132(1):152–158. doi: 10.1164/arrd.1985.132.1.152. [DOI] [PubMed] [Google Scholar]
- Fox R. B., Hoidal J. R., Brown D. M., Repine J. E. Pulmonary inflammation due to oxygen toxicity: involvement of chemotactic factors and polymorphonuclear leukocytes. Am Rev Respir Dis. 1981 May;123(5):521–523. doi: 10.1164/arrd.1981.123.5.521. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Hernandez L. A., Granger D. N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986 Oct;251(4 Pt 1):G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- HARMAN J. W., GWINN R. P. The recovery of skeletal muscle fibers from acute ischemia as determined by histologic and chemical methods. Am J Pathol. 1949 Jul;25(4):741–755. [PMC free article] [PubMed] [Google Scholar]
- Jenkinson S. G. Free radical effects on lung metabolism. Clin Chest Med. 1989 Mar;10(1):37–47. [PubMed] [Google Scholar]
- Kasahara K., Strieter R. M., Chensue S. W., Standiford T. J., Kunkel S. L. Mononuclear cell adherence induces neutrophil chemotactic factor/interleukin-8 gene expression. J Leukoc Biol. 1991 Sep;50(3):287–295. doi: 10.1002/jlb.50.3.287. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Spengler M., May M. A., Spengler R., Larrick J., Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem. 1988 Apr 15;263(11):5380–5384. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lucchesi B. R., Werns S. W., Fantone J. C. The role of the neutrophil and free radicals in ischemic myocardial injury. J Mol Cell Cardiol. 1989 Dec;21(12):1241–1251. doi: 10.1016/0022-2828(89)90670-6. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Oppenheim J. J. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989 Nov;1(1):2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986 Jun;250(6 Pt 1):G749–G753. doi: 10.1152/ajpgi.1986.250.6.G749. [DOI] [PubMed] [Google Scholar]
- Peveri P., Walz A., Dewald B., Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988 May 1;167(5):1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo J. E., Dauber J. H., Christman J., Rogers R. M. Neutrophil alveolitis following endotoxemia. Enhancement by previous exposure to hyperoxia. Am Rev Respir Dis. 1984 Dec;130(6):1065–1071. doi: 10.1164/arrd.1984.130.6.1065. [DOI] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Kunkel S. L., Abrams G. D., Schork M. A., Lucchesi B. R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983 May;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Simpson P. J., Todd R. F., 3rd, Fantone J. C., Mickelson J. K., Griffin J. D., Lucchesi B. R. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest. 1988 Feb;81(2):624–629. doi: 10.1172/JCI113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn S. A., Eierman D. F., Johnson C. E., Morris J., Martin G., Ladner M., Haskill S. Monocyte adherence results in selective induction of novel genes sharing homology with mediators of inflammation and tissue repair. J Immunol. 1990 Jun 1;144(11):4434–4441. [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Basha M. A., Chensue S. W., Lynch J. P., 3rd, Toews G. B., Westwick J., Strieter R. M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990 Dec;86(6):1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford T. J., Strieter R. M., Chensue S. W., Westwick J., Kasahara K., Kunkel S. L. IL-4 inhibits the expression of IL-8 from stimulated human monocytes. J Immunol. 1990 Sep 1;145(5):1435–1439. [PubMed] [Google Scholar]
- Strieter R. M., Chensue S. W., Basha M. A., Standiford T. J., Lynch J. P., Baggiolini M., Kunkel S. L. Human alveolar macrophage gene expression of interleukin-8 by tumor necrosis factor-alpha, lipopolysaccharide, and interleukin-1 beta. Am J Respir Cell Mol Biol. 1990 Apr;2(4):321–326. doi: 10.1165/ajrcmb/2.4.321. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Kasahara K., Allen R., Showell H. J., Standiford T. J., Kunkel S. L. Human neutrophils exhibit disparate chemotactic factor gene expression. Biochem Biophys Res Commun. 1990 Dec 14;173(2):725–730. doi: 10.1016/s0006-291x(05)80095-6. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Remick D. G., Phan S. H., Ward P. A., Marks R. M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989 Mar 17;243(4897):1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Phan S. H., Showell H. J., Remick D. G., Lynch J. P., Genord M., Raiford C., Eskandari M., Marks R. M., Kunkel S. L. Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem. 1989 Jun 25;264(18):10621–10626. [PubMed] [Google Scholar]
- Thornton A. J., Strieter R. M., Lindley I., Baggiolini M., Kunkel S. L. Cytokine-induced gene expression of a neutrophil chemotactic factor/IL-8 in human hepatocytes. J Immunol. 1990 Apr 1;144(7):2609–2613. [PubMed] [Google Scholar]
- Westwick J., Li S. W., Camp R. D. Novel neutrophil-stimulating peptides. Immunol Today. 1989 May;10(5):146–147. doi: 10.1016/0167-5699(89)90164-3. [DOI] [PubMed] [Google Scholar]