Abstract
Previous structural neuroimaging studies of bipolar disorder have reported conflicting findings in limbic structures. Medication heterogeneity of patient samples may have contributed to these in-consistencies. Using structural magnetic resonance imaging we assessed whether lithium treatment was associated with differences in amygdala and hippocampal volumes in a sample of bipolar adults. A total of 49 magnetic resonance imaging scans were collected from patients who were currently treated with or without lithium. Amygdala and hippocampal volumes were analyzed using tensor-based morphometry. Statistical between-group comparisons of deformation maps showed that patients treated with lithium exhibited significantly increased volumes of the amygdala and hippocampus compared with patients who were not taking lithium. Our findings may help to explain previous inconsistencies in the bipolar literature.
Keywords: amygdala, bipolar disorder, emotion, hippocampus, lithium, magnetic resonance imaging, mood disorder
Introduction
The amygdala and hippocampus are key components of emotional regulatory networks in the brain. Previous structural neuroimaging studies have reported volumetric increases [1–3], decreases [4,5] or no difference [6] in the amygdala in bipolar patients relative to healthy participants, with similar inconsistencies reported for the hippocampus [1–5,7,8].
One possible cause for discrepancies in findings is the effects of psychotropic medications on grey matter. Lithium, a commonly prescribed mood stabilizer used in the treatment of bipolar disorder, has been associated with increases in cortical grey matter in both cross-sectional [9] and longitudinal [10] neuroimaging studies. Thus, use of this medication may account for increased volume of the amygdala and hippocampus observed in some adult bipolar studies.
To examine this, we employed tensor-based morphometry (TBM) in conjunction with magnetic resonance imaging (MRI) to assess whether amygdala and hippocampus volumes were increased in lithium-treated bipolar patients.
Methods
The study protocol was approved by the Institutional Review Board at UCLA and the VA Greater Los Angeles Healthcare System. Each participant gave written informed consent. Participants with bipolar I disorder were recruited through the UCLA Mood Disorders Clinic, the bipolar Disorders Clinic of the Veterans Affairs Greater Los Angeles Health Care System, and inpatient units of both hospitals. Participants enrolled in other research projects of the UCLA Mood Disorders Research Program were also invited to participate. Patients were evaluated using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders-IV to confirm an accurate diagnosis. Illness duration and medication information were obtained by patient self-report and from medical records when available. Exclusion criteria for all participants included other Axis I comorbidities, left handedness, hypertension, neurologic illness, metal implants and a history of skull fracture or head trauma with loss of consciousness for more than 5 min. Participants were designated as lithium-treated if the participant was treated with lithium at the time of scanning, and lithium-free if the participant was not taking lithium at the time of scanning.
A total of 49 scans were obtained (37 lithium-free, 12 lithium-treated). Lithium-free participants (37.5±10.7 years; 57% women) had been ill for 17.8±12.8 years, had a prior history of 5.2±4.7 manic episodes and 7.6±12.5 depressive episodes. Forty-six percent of these participants were euthymic, 32% were depressed and 22% were manic at the time of scanning and were taking one or more of the following medications: antipsychotics (n=4), selective serotonin reuptake inhibitors (n=12), benzodiazepines (n=4), anticonvulsants (n=25). Lithium-treated participants (42.0±9.1 years; 8% women) were ill for 15.0±11.9 years, and had a prior history of 8.3±8.8 manic episodes and 6.3±4.5 depressive episodes. Fifty-eight percent of these participants were euthymic, 17% were depressed, and 25% were manic at the time of scanning and were taking one or more of the following medications: benzodiazepines (n=1), anticonvulsants (n=4). The two groups did not differ significantly in terms of age (P=0.19), illness duration (P=0.51), prior number of manias (P=0.18) and prior number of depressions (P=0.77).
Structural neuroimaging was performed on a 3-T MRI scanner (General Electric, Waukesha, Wisconsin, USA). A 3-plane gradient echo scan was acquired for alignment and localization, followed by a shimming procedure to improve magnetic field homogeneity. A T-1 weighted three-dimensional volume scan (SPGR, T1 500/TE 3.7/FOV 20/1 NEX) was obtained for each participant that spanned the entire brain, with a slice thickness of 1.2 mm. Precautions were taken to minimize participant motion during scanning.
Automated extraction of brain tissue was performed using the Brainsuite software package [11], following which manual corrections were made by an image analyst blind to participant characteristics. All scans were then corrected for intensity inhomogeneity [12] and spatially smoothed using a 1.5-mm full-width at half-maximum Gaussian kernel. Each brain was then linearly transformed to the International Consortium for Brain Mapping-27 brain template using a 9-parameter transformation matrix to maximize the mutual information between the participant’s image and the standard template.
TBM was used to measure differences in amygdalar and hippocampal volumes between groups. This technique derives regional brain volumes from the amount of warping needed to match each patient’s subcortical structures to that of a common template [13]. This template was the average of 27 T1-weighted MRI acquisitions from a single healthy participant, aligned within the stereotaxic space of the International Consortium for Brain Mapping [14]. Rather than using a multiparticipant average as a template in this deformation, we preferred registration to a single participant’s image given the improved contrast and better spatial resolution. Template optimization for TBM, however, is the subject of further on-going study [15]. For each individual, this warping was encoded in a deformation map with expansion or contraction encoded at every voxel (sometimes called the ‘Jacobian determinant’). Values less than 1 indicated volume decreases in the patient relative to the template, whereas values greater than 1 indicated volume increases. Overall scaling of these deformation maps was removed and retained for use as a covariate in subsequent statistical analyses to control for total brain volume.
Using the deformation maps, group differences in the volume of the amygdala and hippocampus were obtained by first averaging across point values contained within the amygdala and hippocampus, manually defined on the template brain by a trained neuroanatomist. Regional averages were then entered into a second-level statistical analysis in SAS (PROC mixed SAS, Version 8.1, SAS Institute Inc., Cary, North Carolina, USA) to predict volume difference as a function of lithium status, controlling for age, sex, mood state and total brain scale. As nine patients were scanned in two different mood states, within-subject variability was accounted for using a mixed-effects model, with subject modeled as a random effect, nested within treatment group. Treatment was modeled as a between-subjects factor.
Spearman rank correlation was used to examine the effects of specific clinical variables on the subcortical volumes across patients within lithium-treated and lithium-free bipolar subgroups.
Results
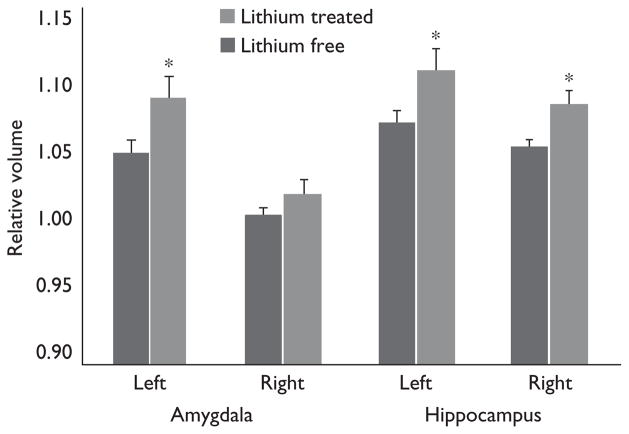
Global brain scaling factors were not significantly different between groups (P=0.23). Lithium-treated patients, however, showed significantly greater total amygdala (P=0.0258) and hippocampal volumes (P<0.0084) compared with lithium-free patients (Table 1) [16]. Both left (P=0.0356) and right (P=0.0053) hippocampus were found to be significantly larger in lithium-treated patients, whereas only the left amygdala was significantly larger in lithium-treated versus lithium-free patients (P=0.0353; Fig. 1). These findings remained significant even after covarying for age, sex, total brain scaling and mood state.
Table 1.
Regional brain volumes in patients with bipolar disorder treated with and without lithium medication
| Brain region | Lithium-free bipolar patients
|
Lithium-treated bipolar patients
|
% difference | P | ||
|---|---|---|---|---|---|---|
| cm3 (SE) | Mean deformation | cm3 (SE) | Mean deformation | |||
| Bilateral amygdala | 4.04 (0.02) | 1.0279 (0.005600) | 4.16 (0.04) | 1.0570 (0.010310) | 2.75 | 0.023 |
| Left amygdala | 2.09 (0.02) | 1.0496 (0.008310) | 2.17 (0.03) | 1.0902 (0.015250) | 3.72 | 0.035 |
| Right amygdala | 1.95 (0.01) | 1.0049 (0.005551) | 1.98 (0.02) | 1.0216 (0.010230) | 1.63 | 0.163 |
| Bilateral hippocampus | 6.29 (0.03) | 1.0614 (0.005516) | 6.49 (0.06) | 1.0961 (0.010350) | 3.17 | 0.008 |
| Left hippocampus | 3.04 (0.02) | 1.0697 (0.007836) | 3.15 (0.04) | 1.1079 (0.014430) | 3.45 | 0.036 |
| Right hippocampus | 3.24 (0.01) | 1.0534 (0.004752) | 3.33 (0.03) | 1.0830 (0.008926) | 2.73 | 0.005 |
Mean deformation, average across point values contained within the amygdala and hippocampus manually defined on the template brain by a trained neuroanatomist; cm3, regional estimated group mean volume obtained by multiplying the mean deformation by regional brain volume of the template (as in Ref. [16]); SE, standard error; P, significance calculated using a linear mixed model, with age, sex and brain scale as controlling variables.
Fig. 1.
Amygdala and hippocampus volumes in bipolar patients treated with and without lithium. Volumes are reported in terms of deformation values, which indicate amount of contraction or expansion that is required to match each patient’s subcortical structure to a common template. Significant group differences in mean regional deformations reveal group differences in hippocampal and amygdala volume after covarying for age, sex, mood state and total brain scaling. *P<0.05.
No significant correlations were found between amygdala and hippocampus volumes and illness duration, prior number of manias or prior number of depressions (P>0.2).
Discussion
We found significant increases in the volume of the amygdala and hippocampus among bipolar patients treated with lithium compared with bipolar patients who were lithium-free. Current lithium medication was associated with volume increases of both the left and right hippocampus, whereas volume increases of the amygdala were constrained to the left side only.
Our finding of lithium-associated increases in left amygdala volume is consistent with a recent study of bipolar youths that assessed the effects of this medication [17]. To our knowledge, this study is the first to reveal significant lithium effects on amygdala volume in an adult sample. Our finding of bilateral increases in hippocampal volume is consistent with results from a recent study examining lithium effects in an adult bipolar sample [7].
The current study’s findings may help to explain some discrepancies in the structural neuroimaging literature of bipolar disorder, which has reported increases [1–3] and decreases [4,5] in amygdala volumes of patients compared with healthy participants. Although some of these reports did not include medication information for their bipolar samples [2], those that did yielded findings consistent with the volumetric differences observed here. For example, Altshuler et al. [1] and Brambilla et al. [3], who found increases in amygdala volume in patients compared with healthy participants included mostly lithium-treated subjects in their patient samples, whereas Blumberg et al. [4,5] who found volumetric decreases in this structure among patients relative to healthy participants included mostly subjects who were lithium-free. Moreover, studies of lithium-naive pediatric [17] and first-episode bipolar patients [18] have more consistently shown amygdala volume to be reduced in patients compared with healthy age-matched controls. Thus, our findings, taken in context with previous studies suggest current lithium exposure may contribute to increased amygdala volumes in studies of bipolar adults.
Similar associations can also be seen between medication characteristics and hippocampal findings. Specifically, groups that reported increases [8] or no difference [1,3,7] in hippocampal volumes among patients versus healthy participants have sampled from higher proportions of lithium-treated patients whereas groups reporting volume decreases included mostly patients who were lithium-free [4,5].
In this study, no significant correlations were seen between subcortical volumes and illness duration, prior number of manias or prior number of depressions. It remains possible that the association between prior manias and subcortical volumes found by some studies [19] was driven in part by the effects of lithium. This medication remains a popular first line of treatment for acute mania [20]. Thus, a higher number of manias may correlate with increased volume of the amygdala owing to repeated exposure to this medication. Future studies however, involving first episode and multi-episode bipolar individuals treated with and without lithium are needed to help clarify any differential effects of these factors on subcortical brain volumes.
Although the exact mechanisms of lithium medication remain unknown, several human and animal studies suggest that lithium works at the molecular level to boost various factors, such as bcl-2 [21] and brain-derived neurotrophic factor [22] which help to protect against stress-induced reductions in neuronal integrity [23]. How these changes may work to impact brain morphology and stabilize mood is not yet known.
Results of this study should be interpreted in light of several methodological limitations. First, because this study was not designed to examine the effects of lithium on brain structure, the number of patients in the lithium-treated sample was small. Second, increased volume of the amygdala and hippocampus among lithium-treated patients could be the result of having a higher proportion of male patients in the lithium-treated sample. We however consider this possibility unlikely because both sex and total brain scale were included as covariates in our statistical analysis. Third, patients in the lithium-treated group were older than patients in the lithium-free group. Such a difference in age is unlikely to have contributed to our findings of increased subcortical volumes in lithium-treated patients, because volumes of medial-temporal brain regions have been found to decline with age [24]. Age however was included as a covariate in our statistical model to control for any such effects. Fourth, many of our patients were taking other medications, of which the long and short-term effects on brain structure are not known. In particular, much of our lithium-free sample was taking valproic acid at the time of scanning. Thus, a larger volume of the amygdala and hippocampus in lithium-treated patients could potentially be driven by a valproic-acid-induced shrinkage of these structures in the lithium-free sample. We however consider this possibility unlikely in light of research showing valproic acid to be neuroprotective [25]. Fifth, it is unknown whether patients in our lithium-free sample had taken this medication in the past, although if they had, that may have worked against detecting the differences reported here. Finally, because this study did not include a healthy comparison group, it cannot be determined here whether lithium-treated patients had a larger than normal amygdala and hippocampus (i.e. compared with healthy control participants), or whether the volume of these structures in lithium-free patients were smaller than normal. Future studies that incorporate a healthy comparison group, as well as a group of medication-naive bipolar patients would be able to assess the direction of volumetric change better.
Conclusion
This study is the first to our knowledge to evaluate the effects of lithium use on both amygdala and hippocampal volumes in an adult bipolar sample. The limitations imposed by the small sample size are clear and as such, the current findings should be considered preliminary. Nevertheless, our findings emphasize the need for future studies of bipolar to control for this medication and suggest that further research is needed to tease apart the effects of bipolar illness from the effects medication on brain structure.
Acknowledgments
The authors thank Allen Lu and Susan Bookheimer, for methodological consultations and technical expertise. This study was supported by research grants from the Stanley Medical Research Institute (L.L.A.), the National Alliance for Research on Schizophrenia and Depression (L.L.A.) and the National Institutes of Mental Health (MH01848, L.L.A.; MH078556, L.C.F.). Additional support was provided by the Brain Mapping Medical Research Organization, the Brain Mapping Support Foundation, Pierson-Lovelace Foundation, Ahmanson Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, Northstar Fund, the National Center for Research Resources (RR019771, P.M.T.), the National Institute for Biomedical Imaging and Bioengineering (EB01651, P.M.T.) and the National Institute for Child Health and Development (HD050735, P.M.T.).
Dr Altshuler reports having received research funding and honoraria from Abbott, honoraria from Bristol-Myers Squibb, Forest laboratories and GlaxoSmithKline, is an advisory board member for Abbott and Forest Laboratories and is on the speaker’s bureau for Forest Laboratories and GlaxoSmithKline.
Footnotes
All other authors reported no biomedical financial interests or conflicts of interest.
References
- 1.Altshuler L, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Arch Gen Psychiatry. 1998;55:663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- 2.Strakowski S, DelBello M, Sax K, Zimmerman M, Shear P, Hawkins J, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- 3.Brambilla P, Harenski K, Nicoletti M, Sassi R, Mallinger A, Frank E, et al. MRI investigation of temporal lobe structures in bipolar patients. J Psychiatry Res. 2003;37:287–295. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg H, Kaufman J, Martin A, Whiteman R, Zhang J, Gore J, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg H, Fredericks C, Wang F, Kalmar J, Spencer L, Papademetris X, et al. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord. 2005;7:570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swayze V, Andreasen N, Alliger R, Yuh W, Ehrhardt J. Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 1992;31:221–240. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- 7.Yucel K, Taylor V, McKinnon M, Macdonald K, Alda M, Young L, et al. Bilateral hippocampal volume increase in patients with bipolar disorder and short-term lithium treatment. Neuropsychopharmacology. 2007;195:357–367. doi: 10.1038/sj.npp.1301405. [DOI] [PubMed] [Google Scholar]
- 8.Beyer J, Kuchibhatla M, Payne M, Moo-Young M, Cassidy F, Macfall J, et al. Hippocampal volume measurement in older adults with bipolar disorder. Am J Geriatr Psychiatry. 2005;12:613–620. doi: 10.1176/appi.ajgp.12.6.613. [DOI] [PubMed] [Google Scholar]
- 9.Bearden C, Thompson P, Dalwani M, Hayashi K, Lee A, Nicoletti M, et al. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry. 2007;62:7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore G, Bebchuk J, Wilds I, Chen G, Manji H. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- 11.Shattuck D, Leahy R. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002;6:129–142. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- 12.Sled J, Zijdenbos A, Evans A. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imag. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 13.Leow A, Huang S, Geng A, Becker J, Davis S, Toga A, et al. Inverse consistent mapping in 3D deformable image registration: its construction and statistical properties. Inf Process Med Imaging. 2005;19:493–503. doi: 10.1007/11505730_41. [DOI] [PubMed] [Google Scholar]
- 14.Holmes C, Hoge R, Collins L, Woods R, Toga A, Evans A. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 15.Studholme C, Cardenas V. A template-free approach to volumetric spatial normalization of brain anatomy. Pattern Recogn Lett. 2004;25:1191–1202. [Google Scholar]
- 16.Lee A, Leow A, Lu A, Reiss A, Hall S, Chiang M, et al. 3D pattern of brain abnormalities in Fragile X syndrome visualized using tensor-based morphometry. NeuroImage. 2007;34:924–938. doi: 10.1016/j.neuroimage.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova D, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2003;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 18.Rosso I, Killgore W, Cintron C, Gruber S, Tohen M, Yurgelun-Todd D. Reduced amygdala volumes in first-episode bipolar disorder and correlation with cerebral white matter. Biol Psychiatry. 2007;61:743–749. doi: 10.1016/j.biopsych.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Altshuler L, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, et al. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- 20.Cookson J. Use of antipsychotic drugs and lithium in mania. Br J Psychiatry. 2001;178:S148–S156. [PubMed] [Google Scholar]
- 21.Chen G, Zeng W, Yuan P, Huang L, Jiang Y, Zhao Z, et al. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- 22.Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S. Lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology. 2001;158:100–106. doi: 10.1007/s002130100871. [DOI] [PubMed] [Google Scholar]
- 23.Manji H, Quiroz J, Payne J, Singh J, Lopes B, Viegas J, et al. The underlying neurobiology of bipolar disorder. World Psychiatry. 2003;2:136–146. [PMC free article] [PubMed] [Google Scholar]
- 24.Moorhead T, McKirdy J, Sussmann J, Hall J, Lawrie S, Johnstone E, et al. Progressive gray matter loss in patients with bipolar disorder. Biol Psychiatry. 2007;62:894–900. doi: 10.1016/j.biopsych.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Manji H, Lenox R. Signaling: cellular insights into the pathophysiology of bipolar disorder. Biol Psychiatry. 2000;48:518–530. doi: 10.1016/s0006-3223(00)00929-x. [DOI] [PubMed] [Google Scholar]