Abstract
Shigella flexneri is a Gram-negative bacterium causing the diarrhoeal disease shigellosis in humans. The virulence genes required for invasion are clustered on a large 220 kb plasmid encoding type three secretion system (TTSS) apparatus and virulence factors such as adhesions and invasion plasmid antigens (Ipa). The bacterium is transmitted by contaminated food, water, or from person to person. Acanthamoebae are free-living amoebae (FLA) which are found in diverse environments and isolated from various water sources. Different bacteria interact differently with FLA since Francisella tularensis, Vibrio cholerae, Shigella sonnei, and S. dysenteriae are able to grow inside A. castellanii. In contrast, Pseudomonas aeruginosa induces both necrosis and apoptosis to kill A. castellanii. The aim of this study is to examine the role of invasion plasmid of S. flexneri on the interaction with A. castellanii at two different temperatures. A. castellanii in the absence or presence of wild type, IpaB mutant, or plasmid-cured strain S. flexneri was cultured at 30°C and 37°C and the interaction was analysed by viable count of both bacteria and amoebae, electron microscopy, flow cytometry, and statistical analysis. The outcome of the interaction was depended on the temperature since the growth of A. castellanii was inhibited at 30°C, and A. castellanii was killed by invasion plasmid mediated necrosis at 37°C.
1. Introduction
Shigella flexneri is a Gram-negative bacterium and a human intestinal pathogen, causing the diarrhoeal disease bacillary dysentery (shigellosis), which is a worldwide health problem [1–4]. Shigellosis is characterized by invasion of, massive inflammation in, and destruction of the colonic mucosa. The genes required for invasion are clustered on a large 220 kb invasion plasmid that encodes the type three secretion system (TTSS) apparatus and the effectors' proteins, which implicate in the mechanism of characteristic cellular invasion of Shigella virulence such as adhesions and invasion plasmid antigens (Ipa) [5–7].
It is well known that S. flexneri utilises TTSS to invade the epithelial layer of the colonic mucosa [7–9]. Infection of the macrophage and epithelial cells has been shown to be the responsible for TTSS effector Ipa proteins [10–12]. The bacteria escape from the phagocytic vacuoles shortly after entry into infected cells, but the outcome of the invasion process depends on the targeted cell [13]. In macrophages, IpaB activates series of reactions, which triggers the apoptosis of the macrophages. Unlike the situation in macrophages, the invasion of epithelial cells does not lead to apoptosis. Infected epithelial cells remain viable for many hours after infection [13].
Shigella is transmitted by contaminated food, water, or from person to person. Therefore, it is most common in areas with improper sanitation and personal hygiene and lack of a safe supply for drinking water [3, 14].
Acanthamoebae are ubiquitous free-living amoebae that are distributed worldwide, living in diverse environments where different bacteria can be found, and Acanthamoeba species are isolated from various water sources [15]. Different bacteria interact differently with Acanthamoebae. Researchers have shown that many bacteria such as Francisella tularensis, Vibrio cholerae, Shigella sonnei, and S. dysenteriae [16–18] are able to grow inside A. castellanii. In contrast, Pseudomonas aeruginosa was found to kill A. castellanii since TTSS effectors proteins ExoS, ExoT, ExoU, and ExoY induced both necrotic and apoptotic killing [19].
It is known that S. flexneri infect macrophage and cause apoptosis by the effect of IpaB protein encoded by the invasion plasmid. The aim of the present study is to examine the role of invasion plasmid and IpaB protein of S. flexneri during the interaction with A. castellanii at two different temperatures.
2. Material and Methods
2.1. Bacterial Strains and Growth Conditions
S. flexneri serogroup 5a strains, M90T and BS176, are wild type and its virulence plasmid-cured mutant strain [9]. S. flexneri SF620 strain is an avirulent derivative of M90T that contains a nonpolar deletion of the ipaB gene [20]. Sansonetti et al., 1986, verified the presence and absence of the plasmid since the wild type infected >95% of HeLa cells and the plasmid cured strain failed to infect the Hela cells [9]. Moreover, Menard et al., 1993, found that the wild type M90T expressed IpaB protein compared to the nonpolar IpaB deletion mutant SF620 that failed to express the IpaB protein [20]. The strains were grown aerobically in Luria-Bertani medium at 37°C to an absorbance of 0.4 to 0.6 at 600 nm.
2.2. Amoeba Strain and Growth Conditions
Acanthamoeba castellanii (ATCC 30234) was obtained from the American Type Culture Collection, Manassas, VA, USA. A. castellanii was grown stationary at 30°C to a final concentration of 2 × 106 cells/mL in ATCC medium no. 712 (ATCC).
2.3. Cocultivation Assay Conditions
The cocultivation assay was based on a previous method [17]. Briefly, cocultivation of each Shigella strain and A. castellanii was incubated in NUNC tissue culture flasks (75 cm2) purchased from VWR International (Stockholm, Sweden) filled with 50 mL ATCC medium 712 containing a concentration of 2 × 105 cells/mL of A. castellanii and 2 × 106 cells/mL of each Shigella strain and incubated at 30°C and 37°C until the end of experiments.
2.4. Microscopy Analysis
Culture samples were withdrawn for analysis at different time intervals. The numbers of live S. flexneri were determined by viable counts. The numbers of live A. castellanii cells were determined by using Erythrosine B stain (ATCC), which stained dead amoebae only. Briefly, 100 μL of Erythrosine B solution were added to a 100 μL cell suspension of A. castellanii in the absence or presence of S. flexneri. The unstained (live) cells of A. castellanii were counted in a Bürker chamber (Merck Eurolab, Stockholm, Sweden) under a light microscope (Carl Zeiss, Stockholm, Sweden) within 10 min after erythrosine staining.
To study morphology of A. castellanii cell in absence and presence of S. flexneri by electron microscope (EM), positive controls for apoptosis and necrosis as well as negative control in addition samples from amoebae cocultivated with S. flexneri strains were prepared. Cells of A. castellanii at 106 cells/mL in ATCC medium no. 712 were incubated with 50 mM hydrogen peroxide (Merck) for 48 h at 37°C to induce necrosis and with 50 μg/mL Actinomycin D (Sigma, Stockholm, Sweden) for 4 h at 37°C to induce apoptosis as positive controls. Cells of A. castellanii were incubated under the same conditions as negative control. The controls were centrifuged for 10 min at 300 ×g in Labofuge GL centrifuge (VWR International). After centrifugation, the pellets were washed in PBS.
For EM analysis, 5 mL of A. castellanii in absence or presence of S. flexneri cultivated at 30°C and 37°C after 1 day cultivation were centrifuged for 10 min at 300 ×g in Labofuge GL centrifuge (VWR International). After centrifugation, the pellets were washed in PBS. The pellets of the controls and A. castellanii in absence or presence of S. flexneri were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer pH 7.3, with 0.1 sucrose and 3 mM CaCl2 for 30 min at room temperature. Samples were washed in sodium cacodylate buffer and postfixed in 2% osmium tetroxide in the same buffer for 1 h. The samples were centrifuged, dehydrated, and embedded in Epoxy resin, LX-112. The embedded samples were cut into ultrathin sections, put on grids, and stained with uranyl acetate and lead citrate. Sections were examined with a transmission electron microscope. A hundred cells was counted of each alone and cocultivated A. castellanii with wild type or plasmid-cured S. flexneri strain. The percentage of apoptotic, necrotic, and normal amoeba cells was determined by dividing number of each apoptotic, necrotic, and normal amoeba cell separately by the total number of all amoeba cells multiplied by 100.
2.5. Flow Cytometry
Fluorescence activated cell sorting (FACS) analysis of Propidium iodide (PI) staining was used for quantification of necrosis. Briefly, amoebae in the absence and presence of S. flexneri were cultivated for 24 h, samples of the amoebae washed twice in PBS and 100 μL with 5 × 105 amoebae/mL of each sample were incubated in dark for 10 min at room temperature with 10 μg/mL PI reagent (Roch diagnosis GmbH, Mannheim Germany). Finally 500 μL PBS was added to each sample and the samples were analyzed using a FACSCalibur flow cytometer (BD Biosciences, NJ, USA). It was clear from earlier trails that background from PI staining was not elevated in diluted samples or in washed samples. The dilution method was recommended by the manufacture and used to perform FACS analysis in this study.
2.6. Statistical Analysis
The Student t-test and χ 2 test were used to test for statistically significant differences in growth of A. castellanii in the absence or presence of S. flexneri strains.
3. Results
3.1. Growth and Survival of Wild Type S. flexneri in the Presence or Absence of A. castellanii at 30°C
To examine growth of S. flexneri in the presence or absence of A. castellanii at 30°C, viable counts of alone and cocultivated S. flexneri with A. castellanii were performed. It was found that both alone and cocultivated bacteria increased 100 folds on day 1, under the log-growth phase. The stationery growth phase continued from day 2 to day 9 and then the decline phase finished on day 15 for alone-cultivated S. flexneri and on day 18 for cocultivated bacteria. However, A. castellanii enhanced survival of cocultivated bacteria, which survived longer since the alone and cocultivated S. flexneri become nondetectable by viable count assay on days 15 and 18, respectively (Figure 1).
Figure 1.
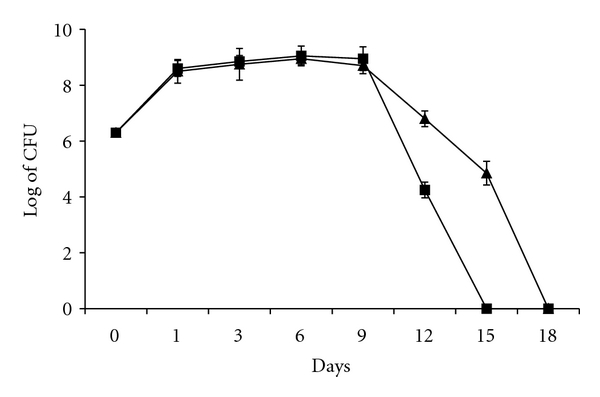
Viable counts of S. flexneri at 30°C. Alone cultivated bacteria (■) and co-cultivated with A. castellanii (▲). The points are mean ± SD of three samples.
3.2. Growth of A. castellanii in the Presence or Absence of S. flexneri at 30°C
Number of viable A. castellanii in absence of S. flexneri increased from 2 × 105 cell/mL on day 0 to 2 × 106 cell/mL from day 6 to day 10 (Figure 2).
Figure 2.
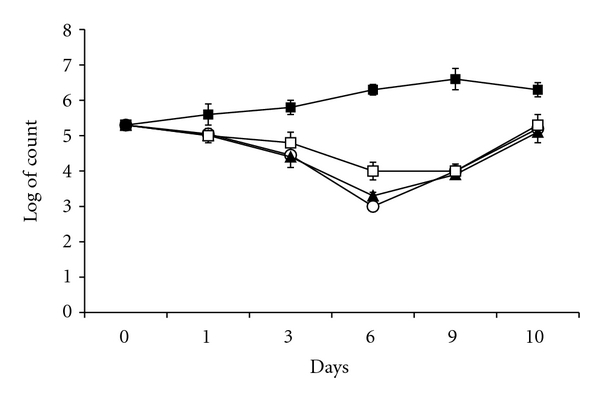
Viable counts of amoeba cells at 30°C. Alone cultivated amoebae (■), co-cultivated with wild type S. flexneri (○), with IpaB mutant (▲), and with plasmid cured (□) strains. The points are mean ± SD of three samples.
Growths of cocultivated A. castellanii (Figure 2) were inhibited under log- and stationery growth phases of the bacteria as shown in Figure 1. The number of viable A. castellanii cocultivated with wild type, IpaB mutant, or plasmid cured strain S. flexneri was 2.0 × 105 cell/mL on day 0 and decreased to 1.0 × 104 cell/mL on day 9 (Figure 2) under log- and stationery growth phases of the bacteria. Interestingly, the number of viable cocultivated amoebae reached their initial numbers (2.0 × 105 cell/mL) when the bacterial decline growth phase started on day 10.
3.3. Growth and Survival of A. castellanii in the Presence or Absence of S. flexneri at 37°C
A. castellanii in the presence of wild type, IpaB mutant, and plasmid-cured S. flexneri strains at 37°C did not grow but survived 0.5, 2.0, and 6.0 days, respectively, compared to A. castellanii in absence of the wild type S. flexneri, which grew from 2 × 105 cell/mL on day 0 to 4 × 105 cell/mL on day 3 and survived at this rate until end of the experiment on day 7.
However, to estimate percentage of survival time of A. castellanii cocultivated with each S. flexneri strain, alone A. castellanii survived 7 days, which considered 100%. The days that cocultivated amoeba survived with each S. flexneri strain are divided by 7 to determine percentage of the survival. Survival times % of A. castellanii cocultivated with wild type S. flexneri, with IpaB mutant, and with plasmid-cured strains were 7%, 28.5%, and 85%, respectively (Figure 3).
Figure 3.
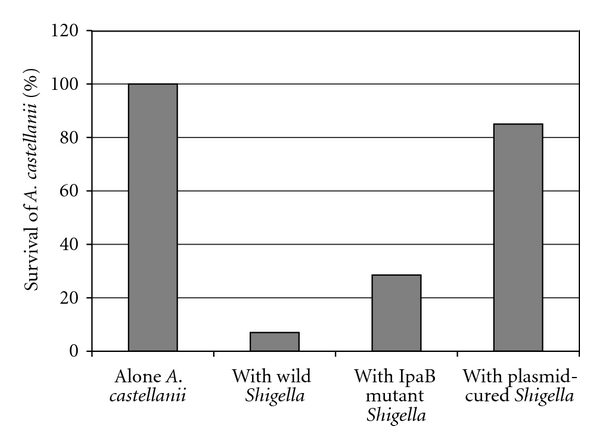
Survival of alone and cocultured A. castellanii. Data indicating mean survival time from three independent experiments expressed in percentage.
The statistical analysis by χ 2 test showed that survival time of alone-cultivated A. castellanii compared to the survival of cocultivated with wild type S. flexneri was very highly significant (P < 0.0000001), but survival of alone-cultivated A. castellanii compared to that of cocultivated with plasmid-cured S. flexneri was not statistically significant (P = 0.88). However, the survival time of alone-cultivated A. castellanii compared to the survival of cocultivated with IpaB mutant S. flexneri was highly significant (P < 0.00001).
On the other hand, survival of A. castellanii cocultivated with wild type S. flexneri compared to that of A. castellanii cocultivated with plasmid-cured S. flexneri was highly significant (P < 0.0001).
3.4. Visualisation of Bacterial Effect on Amoebae at 30°C
To study effect of S. flexneri on A. castellanii, electron microscopy was used to analyse samples of alone-cultivated A. castellanii as well as cocultivated with wild type or with plasmid cured S. flexneri strains.
Electron microscopy estimates percentage of individual apoptotic, necrotic as well as normal cells depending on morphological alteration of nucleus and plasma membrane. Chromatin condensation and reduced size of the nucleus are typical features of apoptosis compared to disruption of plasma membrane and ghost shape of the cell, which are typical features of necrosis.
The analysis at 30°C found that alone-cultivated A. castellanii showed 4 ± 0.5% apoptosis, 2 ± 0% necrosis, and 94 ± 1.4% normal cells. Compared to alone-cultivated A. castellanii (Figure 4(a)), amoebae cocultivated with wild type S. flexneri showed 86 ± 3% necrosis (Figure 4(b)), 6 ± 1% apoptosis, and 8 ± 1 normal cells. Amoebae cocultivated with plasmid-cured S. flexneri showed 26 ± 2.8% necrosis, 72 ± 0 normal cells, which were cysts (Figure 4(c)), and 2 ± 1% apoptosis (Figure 4(d)).
Figure 4.
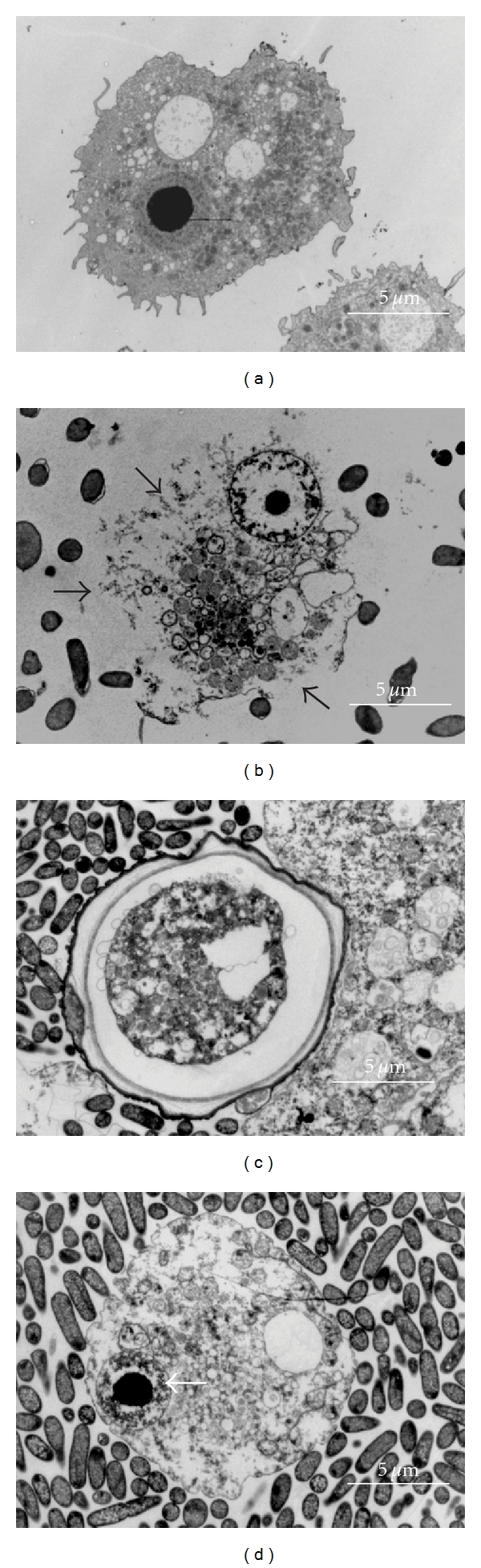
Electron microscopy at 30°C, black arrows point out necrosis and white apoptosis. A. castellanii trophozoite cultivated alone (a), A. castellanii trophozoite cocultivated with wild type S. flexneri showing necrosis (b), A. castellanii cyst cocultivated with plasmid-cured S. flexneri (c), and A. castellanii trophozoite cocultivated with plasmid-cured S. flexneri showing apoptosis (d).
3.5. Visualisation of Bacterial Effect on Amoebae at 37°C
Alone-cultivated A. castellanii showed 3 ± 1% apoptosis, 5 ± 1% necrosis, and 92 ± 1% normal cells. Amoebae cocultivated with wild type S. flexneri showed 93 ± 1% necrosis (Figure 5(a)), 4 ± 1% apoptosis, and 3 ± 1% normal cells. All the normal cells were cysts (Figure 5(b)). Amoebae cocultivated with plasmid-cured S. flexneri showed 90 ± 5% necrosis, 6 ± 1% apoptosis (Figure 5(c)), and 4 ± 1% normal cells. Again, all the normal cells were cysts (Figure 5(d)).
Figure 5.
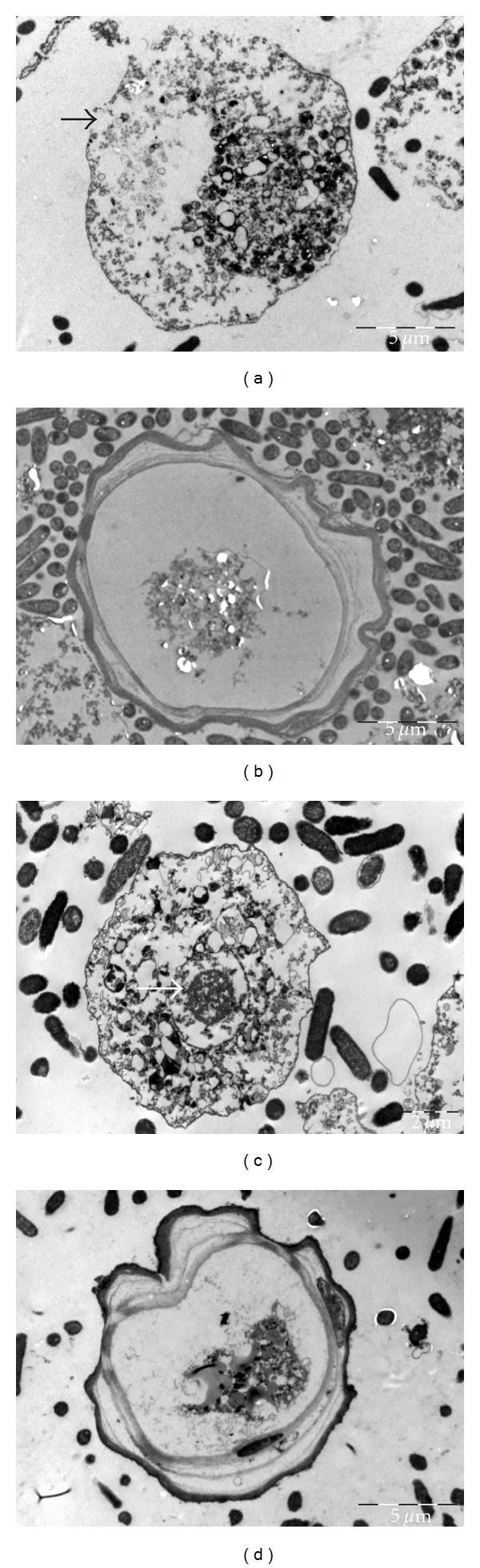
Electron microscopy at 37°C, black arrows point out necrosis and white apoptosis. A. castellanii trophozoite cocultivated with wild type S. flexneri showing necrosis (a), A. castellanii cyst cocultivated with wild type S. flexneri (b), A. castellanii trophozoite cocultivated with plasmid-cured S. flexneri showing apoptosis (c), and A. castellanii cyst cocultivated with plasmid-cured S. flexneri (d).
3.6. S. flexneri Induces Rapid Membrane Damage to A. castellanii
Flow cytometry measures fluorescence intensity of a cell population in this case stained with PI which is a DNA-binding dye not able to penetrate the cell membrane and thus only stains cells with disrupted membrane integrity.
Analysis at 30°C showed that the percentages of normal and necrotic A. castellanii in absence of S. flexneri stains were 88.2% and 11.7%, respectively. The percentages of necrotic amoeba cells cocultivated with S. flexneri wild type, IpaB mutant and plasmid-cured strains were 99.8%, 99.7%, and 83.3%, respectively. Moreover, percentage of normal amoeba cells cocultivated with plasmid-cured strain was 15.6% (Data not shown).
Analysis at 37°C showed that the percentages of normal and necrotic A. castellanii in absence of S. flexneri stains were 71.3% and 28.7%, respectively. The percentages of necrosis in amoeba cells cocultivated with S. flexneri wild type, IpaB mutant and plasmid-cured strains were 94.85%, 93.70%, and 94.82%, respectively (Figure 6).
Figure 6.
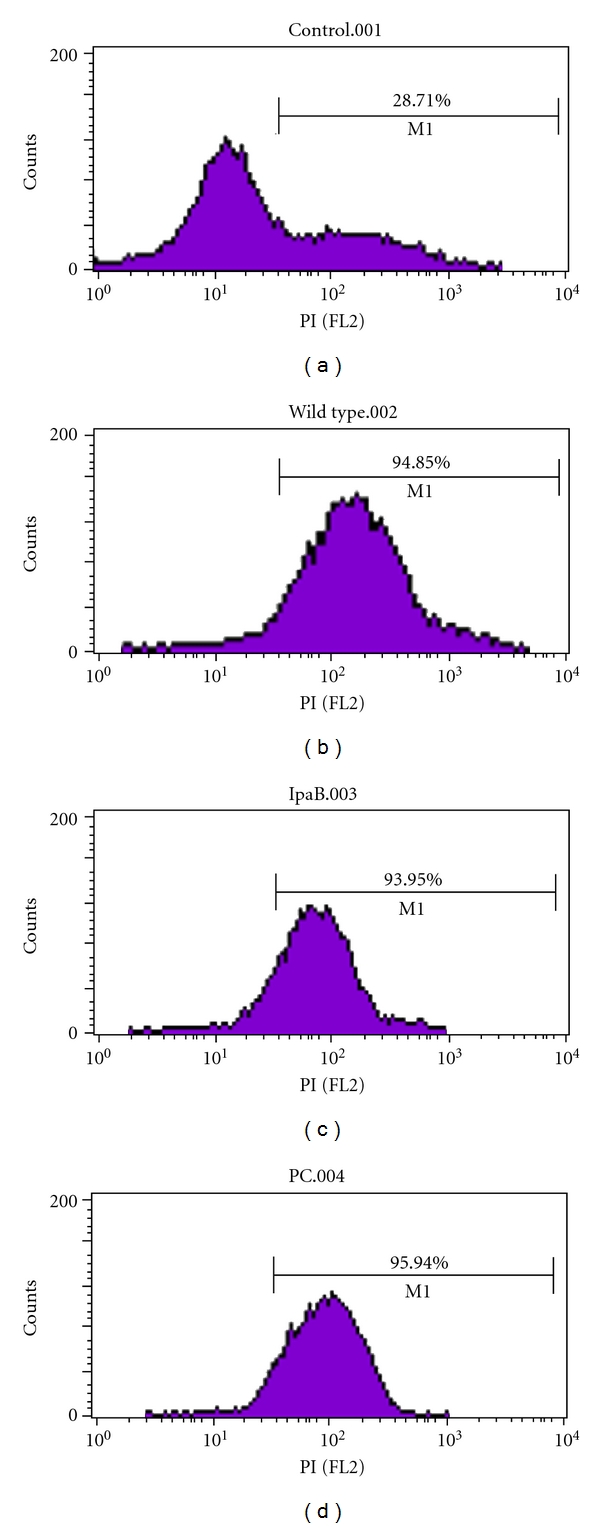
Necrosis analysis by flow cytometry at 37°C. A. castellanii alone (a), A. castellanii co-cultivated with wild type S. flexneri (b), A. castellanii co-cultivated with IpaB mutant S. flexneri (c) and A. castellanii co-cultivated with plasmid-cured S. flexneri (d).
4. Discussion
Shigellosis is a global health problem mostly affecting young children [3]. S. flexneri is a waterborne pathogen, it may interact with A. castellanii present in water and this may prime the S. flexneri for infection of the host cell. In this study, role of the IpaB protein and the invasion plasmid on the interaction of S. flexneri with A. castellanii was examined. It is thought that any findings may uncover a better understanding of the pathogenesis of S. flexneri and contribute to improved treatment of the infection.
The results showed that wild type S. flexneri at 30°C inhibited growth of A. castellanii to a rate that is lower than alone-grown amoebae (P < 0.001), but the effect did not result in killing of the amoebae. However, at 37°C it was found that the wild type, IpaB mutant, and plasmid cured strains of S. flexneri killed A. castellanii. In this context, it is well known that the invasive property of S. flexneri depended on the 220-kb plasmid, which is strongly temperature regulated. Maurelli et al. found that virulent strains of S. flexneri, S. sonnei, and S. dysenteriae at 37°C were invasive, and usually greater than 90% of Henle 407 human intestinal epithelial cells could be seen to have bacteria in the cytoplasm. However, these virulent Shigella strains at 30°C failed to invade the Henle cells, which were also free of adherent bacteria. Interestingly, it was found that growth of the virulent strains of S. flexneri, S. sonnei, and S. dysenteriae at 30°C inhibited expression of invasiveness of these strains for Henle cells [21]. This finding may explain why growth of the cocultivated amoebae with S. flexneri is inhibited at 30°C and the amoebae are killed at 37°C.
Our result showed that wild type, IpaB mutant and plasmid-cured strains of S. flexneri killed A. castellanii in 0.5, 2.0, and 6.0 days, respectively. Interestingly, the statistical analysis found that survival of alone-cultivated amoebae compared to that of cocultivated with wild type S. flexneri was very highly significant (P value of χ 2 test was <0.0000001), but survival of alone-cultivated amoebae compared to that of cocultivated with plasmid-cured S. flexneri was not statistically significant (P = 0.88). The very highly statistical significant explains the important role of invasion plasmid on amoebae killing and as a potent virulence factor for S. flexneri.
However, the survival time of alone-cultivated A. castellanii compared to the survival of the cocultivated with IpaB mutant S. flexneri was highly significant (P < 0.00001), which suggests clearly presence of other Ipa genes rather than IpaB such as IpaC and IpaD. On the other hand, the significant different survival time of amoebae cocultivated with wild type S. flexneri compared to that of amoebae cocultivated with plasmid-cured S. flexneri (P < 0.0001) suggests strongly presence of gene/genes for other virulence factors not located in the invasion plasmid but in the chromosome. The other plasmid-located TTSS genes (rather than IpaB) and chromosome-located TTSS genes that will be activated at 37°C may affect the outcome of interaction between S. flexneri and FLA.
The aim of this study was to examine the role of invasion plasmid and IpaB protein of S. flexneri during the interaction with A. castellanii at two different temperatures. Anyhow, role of other plasmid-located and chromosome-located TTSS genes in interaction between S. flexneri and FLA will be subjects to next study.
The result has shown that S. flexneri inhibits growth of the amoebae when invasion plasmid was not activated at 30°C (Figure 2). The inhibition occurred while the bacteria were in log- and stationary growth phases (Figure 1). However, the amoebae returned to its initial viable number when the bacteria entered the decline phase (Figures 2 and 1). This may explain that the bacteria produce virulence factors not mediated by the invasion plasmid and these factors are not able to kill the amoebae but can inhibit growth of the amoebae. In this context, it was shown that A. castellanii cocultivated with S. sonnei or with S. dysenteriae was not inhibited but grew at 30°C from 105 cell/mL to 106 cell/mL while these Shigella species killed the amoebae at 37°C [18]. There are differences between Shigella species (S. dysenteriae and S. sonnei) as studied in [18] and S. flexneri studied in the current study. The log- and stationary growth of both alone and cocultivated S. flexneri lasted 9 days. However, the log- and stationary growth phases of both alone-cultivated S. dysenteriae and S. sonnei lasted 6 days compared to that of cocultivated S. dysenteriae and S. sonnei lasted 18 and 15 days, respectively. Moreover, numbers of alone and cocultivated S. flexneri were nondetectable by viable count method at day 15 and day 18, respectively. Numbers of alone-cultivated S. dysenteriae and S. sonnei become nondetectable by viable count method at day 18 and day 15, respectively. Interestingly, viable counts of each S. dysenteriae and S. sonnei cocultivated with amoebae were 108 CFU/mL at day 21. The important difference is that both S. dysenteriae and S. sonnei did not inhibit growth of the amoebae at 30°C compared to S. flexneri that inhibited growth of the amoeba. Surprisingly, S. dysenteriae, S. sonnei, and S. flexneri killed the amoeba population by induction necrosis at 37°C. This finding may indicate that S. sonnei and S. dysenteriae are less virulent against the amoeba at 30°C, which may allow these strains to grow and survive inside A. castellanii [18].
Abd et al. reviewed in [19] that cell death is classified by either apoptosis or necrosis. Distinguishing features of apoptosis are chromatin condensation (pyknosis) and DNA fragmentation (karyorrhexis), and features of necrosis are plasma membrane disruption and nuclear disintegration (karyolysis). The apoptotic process in mammalian cells, as well as in unicellular eukaryotes such as Acanthamoeba polyphaga, and Dictyostelium discoideum, depends on activation of caspases and mitochondrial outer membrane permeabilisation. Microbial pathogens improve their ability to persist in infected hosts by competing with host defence systems. Extracellular bacteria, such as Corynebacterium diphtheriae, Pseudomonas aeruginosa, and Bacillus anthracis, are sensitive to phagocytosis; therefore, they may benefit from killing macrophages before they are ingested. Moreover, infection of epithelial cells by P. aeruginosa resulted in a surface upregulation of CD95 and CD95 ligand. The upregulation depends on the function of the TTSS of P. aeruginosa. Binding of CD95 by the CD95 ligand upon upregulation induces the activation of caspases 8 and 3, and the release of mitochondrial cytochrome c, which leads to apoptosis. On the other hand, facultative intracellular bacteria may employ different strategies to prevent cell death during their replication. However, macrophage apoptosis is an end stage for bacterial proliferation [19].
The cell death is triggered by different biochemical pathways and it is involved in the interaction between host cells and bacterial pathogens [22]. Shigella species have been reported to be able to induce apoptosis in macrophages by secreting effector proteins such as IpaB protein into the host cell by TTSS [23–27]. Moreover, Monocyte-derived macrophages infected with S. flexneri undergo a rapid cytolytic event resulted in cell death (necrosis) characterised by rupture of the plasma membrane, cell swelling, disintegration of the cellular ultra structure, and generalized karyolysis [28].
It has been reported that TTSS proteins of the extracellular bacterium P. aeruginosa induced apoptosis to kill macrophages [29] and it induced both apoptosis and necrosis in A. castellanii [19]. While, the facultative intracellular bacterium, L. pneumophila did not induce apoptosis but necrosis in A. polyphaga [30].
Different methods are used in this paper such as viable count assay, electron microscopy, and flow cytometry. These methods estimate absolute number of the individual viable cells, percentage of individual apoptotic, necrotic as well as normal cells, and percentage of fluorescence intensity emitted by the IP stained cell population, respectively. Regarding the methodology, viable count determines number of living amoebae in 1 μL cell suspension compared to total number of cells in pellet of 2-3 mL cell suspension by electron microscopy and to percentage of the fluorescence intensity emitted by cell population by FACS, which examines 60 μL/min.
In addition to the methodological considerations, there are biological considerations regarding growth and encystation of A. castellanii that may play a role in the interaction. A. castellanii trophozoites have a wide growth temperature range of 12°C to 45°C, but the optimal growth temperature is 30°C [31]. Our practical experience showed that A. castellanii grew highest up to 2.0 × 106 cell/mL at 30°C and up to 4.0 × 105 cell/mL at 37°C. That is why the percentage of the normal A. castellanii in absence of S. flexneri was 88.2% at 30°C and decreased to 71.3% at 37°C, compared to the percentage of the necrotic A. castellanii in absence of S. flexneri, which was 11.7% at 30°C and increased to 28.7% at 37°C, respectively.
Whatever, despite these different analysis methods did not give same exact percentages of necrosis, these methods showed that inhibition and killing of A. castellanii was due to the necrosis caused mostly by the invasion plasmid of S. flexneri under different temperatures 30°C and 37°C.
5. Conclusions
Findings in this paper together with findings of other researcher may conclude that Shigella has the ability to control the way it kills the host cells, as it clearly commands mechanisms that lead to either apoptosis or necrosis. Thus, such ability must play a crucial role in the interaction of Shigella with different host cells at different temperatures since invasion plasmid playing very important role.
Acknowledgments
The authors thank Professor Hubert Hilbi, Institute of Microbiology, Swiss Institute of Technology, Zürich, Switzerland, for his generosity in supplying the S. flexneri strains. Karolinska Institute, Stockholm, Sweden, financed this project and they were supported by the Swedish Agency for Research Cooperation with Developing Countries, Project no. SWE 2006-044.
References
- 1.Jin Q, Yuan Z, Xu J, et al. Genome sequence of Shigella flexneri 2a: insights into pathogenicity through comparison with genomes of Escherichia coli K12 and O157. Nucleic Acids Research. 2002;30(20):4432–4441. doi: 10.1093/nar/gkf566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotloff KL, Winickoff JP, Ivanoff B, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bulletin of the World Health Organization. 1999;77(8):651–666. [PMC free article] [PubMed] [Google Scholar]
- 3.Niyogi SK. Shigellosis. Journal of Microbiology. 2005;43(2):133–143. [PubMed] [Google Scholar]
- 4.Wei J, Goldberg MB, Burland V, et al. Complete genome sequence and comparative genomics of Shigella flexneri serotype 2a strain 2457T. Infection and Immunity. 2003;71(5):2775–2786. doi: 10.1128/IAI.71.5.2775-2786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews GP, Hromockyj AE, Coker C, Maurelli AT. Two novel virulence loci, mxiA and mxiB, in Shigella flexneri 2a facilitate excretion of invasion plasmid antigens. Infection and Immunity. 1991;59(6):1997–2005. doi: 10.1128/iai.59.6.1997-2005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sansonetti PJ, Arondel J, Cantey JR, Prevost MC, Huerre M. Infection of rabbit Peyer’s patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infection and Immunity. 1996;64(7):2752–2764. doi: 10.1128/iai.64.7.2752-2764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsot C. Shigella flexneri: genetics of entry and intercellular dissemination in epithelial cells. Current Topics in Microbiology and Immunology. 1994;192:217–241. doi: 10.1007/978-3-642-78624-2_10. [DOI] [PubMed] [Google Scholar]
- 8.Maurelli AT, Baudry B, D’Hauteville H. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri . Infection and Immunity. 1985;49(1):164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sansonetti PJ, Ryter A, Clerc P. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infection and Immunity. 1986;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuwae A, Yoshida S, Tamano K, Mimuro H, Suzuki T, Sasakawa C. Shigella invasion of macrophage requires the insertion of IpaC into the host plasma membrane. Functional analysis of IpaC. Journal of Biological Chemistry. 2001;276(34):32230–32239. doi: 10.1074/jbc.M103831200. [DOI] [PubMed] [Google Scholar]
- 11.Skoudy A, Mounier J, Aruffo A, et al. CD44 binds to the Shigella IpaB protein and participates in bacterial invasion of epithelial cells. Cellular Microbiology. 2000;2(1):19–33. doi: 10.1046/j.1462-5822.2000.00028.x. [DOI] [PubMed] [Google Scholar]
- 12.Watarai M, Funato S, Sasakawa C. Interaction of Ipa proteins of Shigella flexneri with α5β1 integrin promotes entry of the bacteria into mammalian cells. Journal of Experimental Medicine. 1996;183(3):991–999. doi: 10.1084/jem.183.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucchini S, Liu H, Jin Q, Hinton JCD, Yu J. Transcriptional adaptation of Shigella flexneri during infection of macrophages and epithelial cells: insights into the strategies of a cytosolic bacterial pathogen. Infection and Immunity. 2005;73(1):88–102. doi: 10.1128/IAI.73.1.88-102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moulder JW. Comparative biology of intracellular parasitism. Microbiological Reviews. 1985;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker CWB. Acanthamoeba: ecology, pathogenicity and laboratory detection. British Journal of Biomedical Science. 1996;53(2):146–151. [PubMed] [Google Scholar]
- 16.Abd H, Johansson T, Golovliov I, Sandström G, Forsman M. Survival and growth of Francisella tularensis in Acanthamoeba castellanii . Applied and Environmental Microbiology. 2003;69(1):600–606. doi: 10.1128/AEM.69.1.600-606.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abd H, Saeed A, Weintraub A, Nair GB, Sandström G. Vibrio cholerae O1 strains are facultative intracellular bacteria, able to survive and multiply symbiotically inside the aquatic free-living amoeba Acanthamoeba castellanii . FEMS Microbiology Ecology. 2007;60(1):33–39. doi: 10.1111/j.1574-6941.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- 18.Saeed A, Abd H, Edvinsson B, Sandström G. Acanthamoeba castellanii an environmental host for Shigella dysenteriae and Shigella sonnei . Archives of Microbiology. 2009;191(1):83–88. doi: 10.1007/s00203-008-0422-2. [DOI] [PubMed] [Google Scholar]
- 19.Abd H, Wretlind B, Saeed A, Idsund E, Hultenby K, Sandström G. Pseudomonas aeruginosa utilises its type III secretion system to kill the free-living amoeba Acanthamoeba castellanii . Journal of Eukaryotic Microbiology. 2008;55(3):235–243. doi: 10.1111/j.1550-7408.2008.00311.x. [DOI] [PubMed] [Google Scholar]
- 20.Menard R, Sansonetti PJ, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. Journal of Bacteriology. 1993;175(18):5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurelli AT, Blackmon B, Curtis R. Temperature-dependent expression of virulence genes in Shigella species. Infection and Immunity. 1984;43(1):195–201. doi: 10.1128/iai.43.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao LY, Abu Kwaik Y. Hijacking of apoptotic pathways by bacterial pathogens. Microbes and Infection. 2000;2(14):1705–1719. doi: 10.1016/s1286-4579(00)01326-5. [DOI] [PubMed] [Google Scholar]
- 23.Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(5):2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hilbi H, Moss JE, Hersh D, et al. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. Journal of Biological Chemistry. 1998;273(49):32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 25.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Prada CM, Hoover DL, Tall BD, Venkatesan MM. Human monocyte-derived macrophages infected with virulent Shigella flexneri in vitro undergo a rapid cytolytic event similar to oncosis but not apoptosis. Infection and Immunity. 1997;65(4):1486–1496. doi: 10.1128/iai.65.4.1486-1496.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Prada CM, Hoover DL, Tall BD, Hartman AB, Kopelowitz J, Venkatesan MM. Shigella flexneri IpaH7.8 facilitates escape of virulent bacteria from the endocytic vacuoles of mouse and human macrophages. Infection and Immunity. 2000;68(6):3608–3619. doi: 10.1128/iai.68.6.3608-3619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nonaka T, Kuwabara T, Mimuro H, Kuwae A, Imajoh-Ohmi S. Shigella-induced necrosis and apoptosis of U937 cells and J774 macrophages. Microbiology. 2003;149(9):2513–2527. doi: 10.1099/mic.0.26341-0. [DOI] [PubMed] [Google Scholar]
- 29.Hauser AR, Engel JN. Pseudomonas aeruginosa induces type-III-secretion-mediated apoptosis of macrophages and epithelial cells. Infection and Immunity. 1999;67(10):5530–5537. doi: 10.1128/iai.67.10.5530-5537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao LY, Kwaik YA. The mechanism of killing and exiting the protozoan host Acanthamoeba polyphaga by Legionella pneumophila . Environmental Microbiology. 2000;2(1):79–90. doi: 10.1046/j.1462-2920.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- 31.Abd H. Interaction between waterborne pathogenic bacteria and Acanthamoeba castellanii. Stockholm, Sweden: Karolinska Institutet; 2006. Doctoral thesis. [Google Scholar]


