Abstract
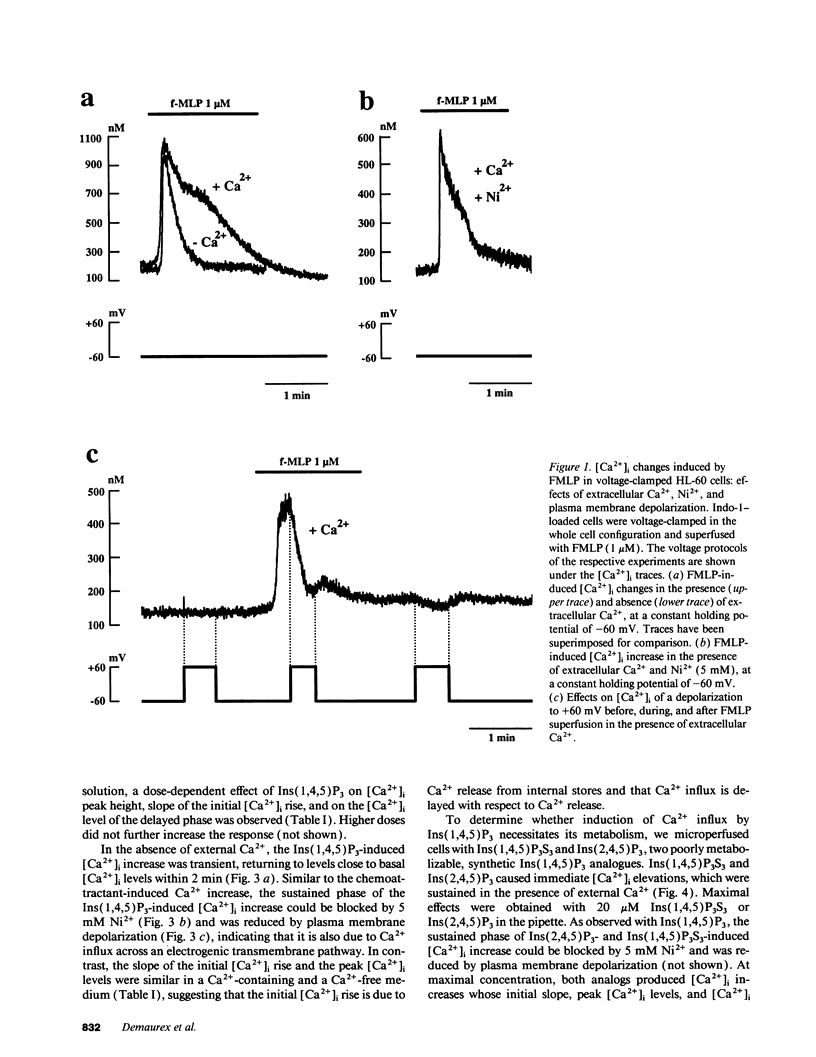
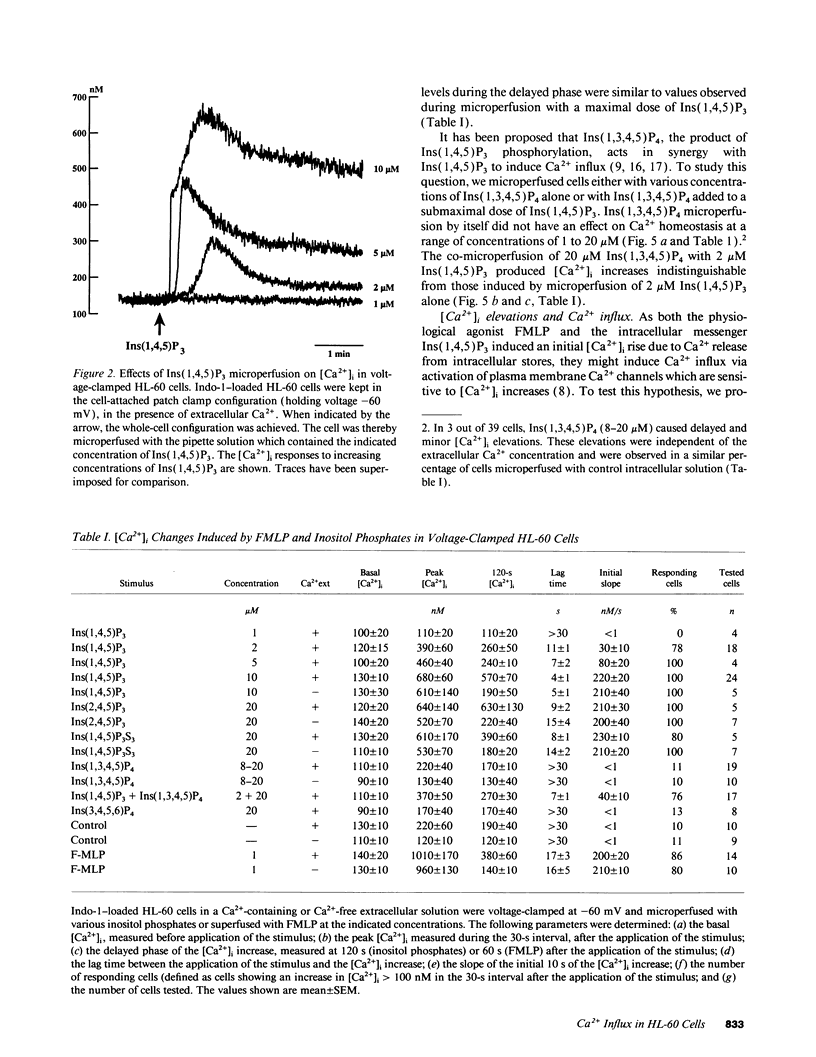
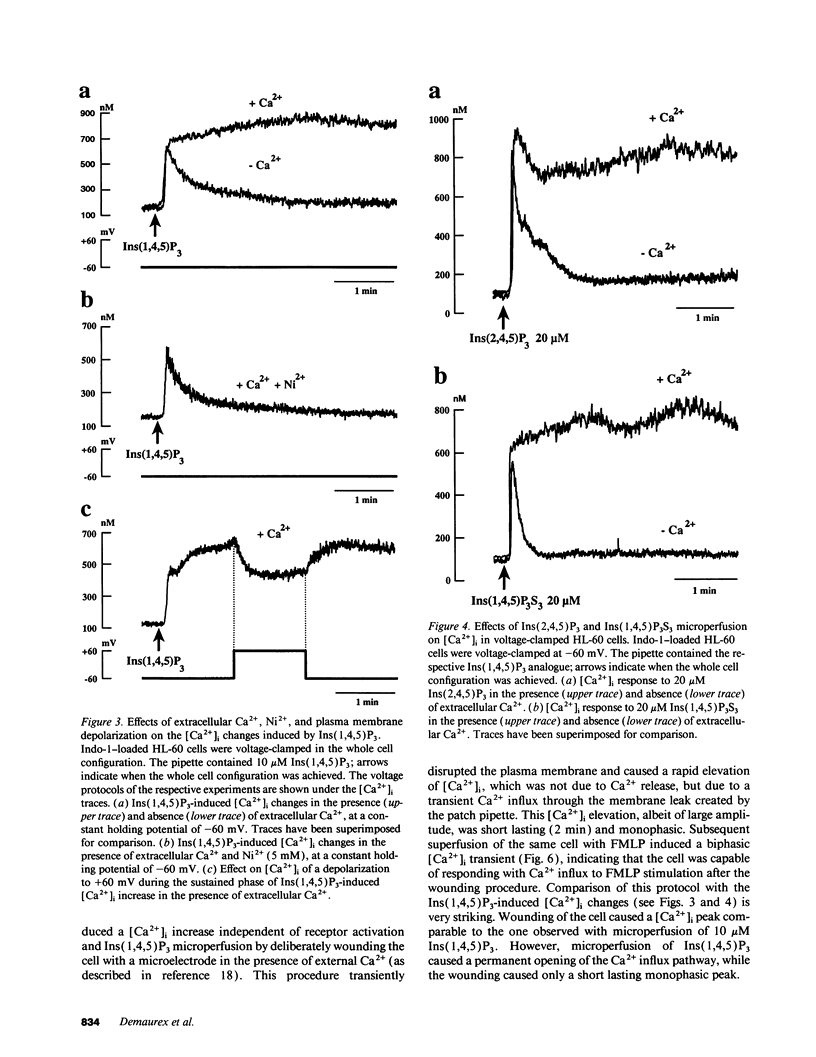
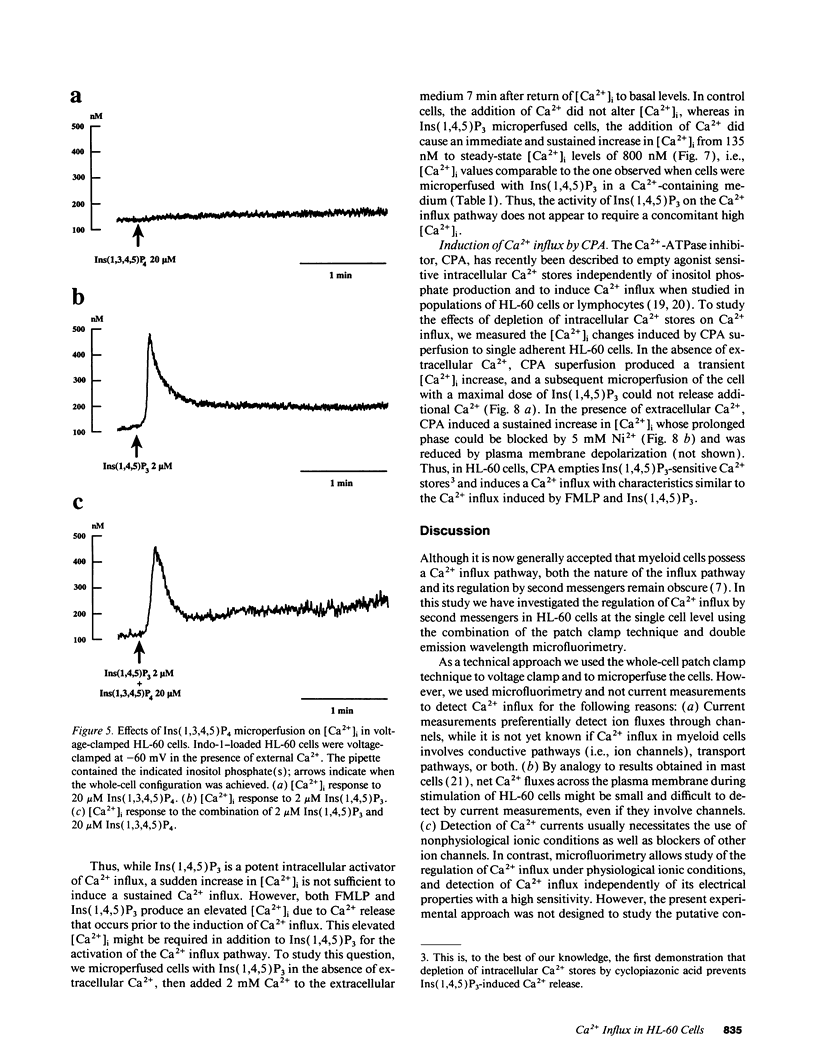
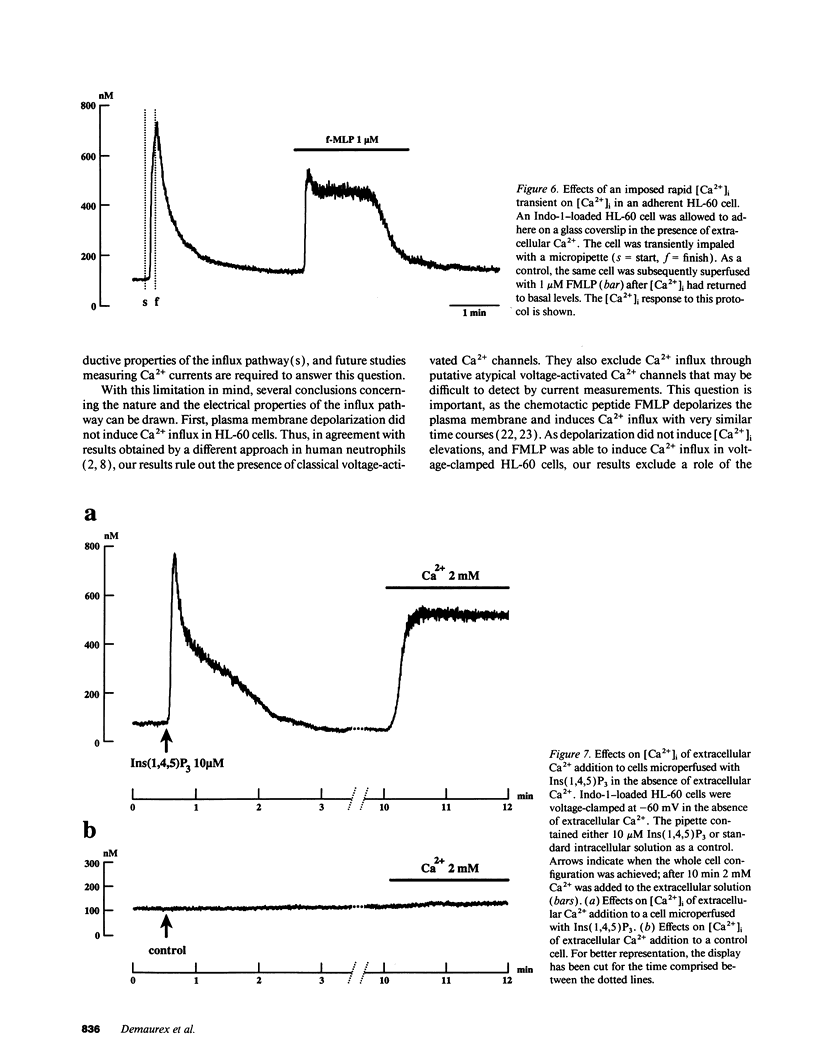
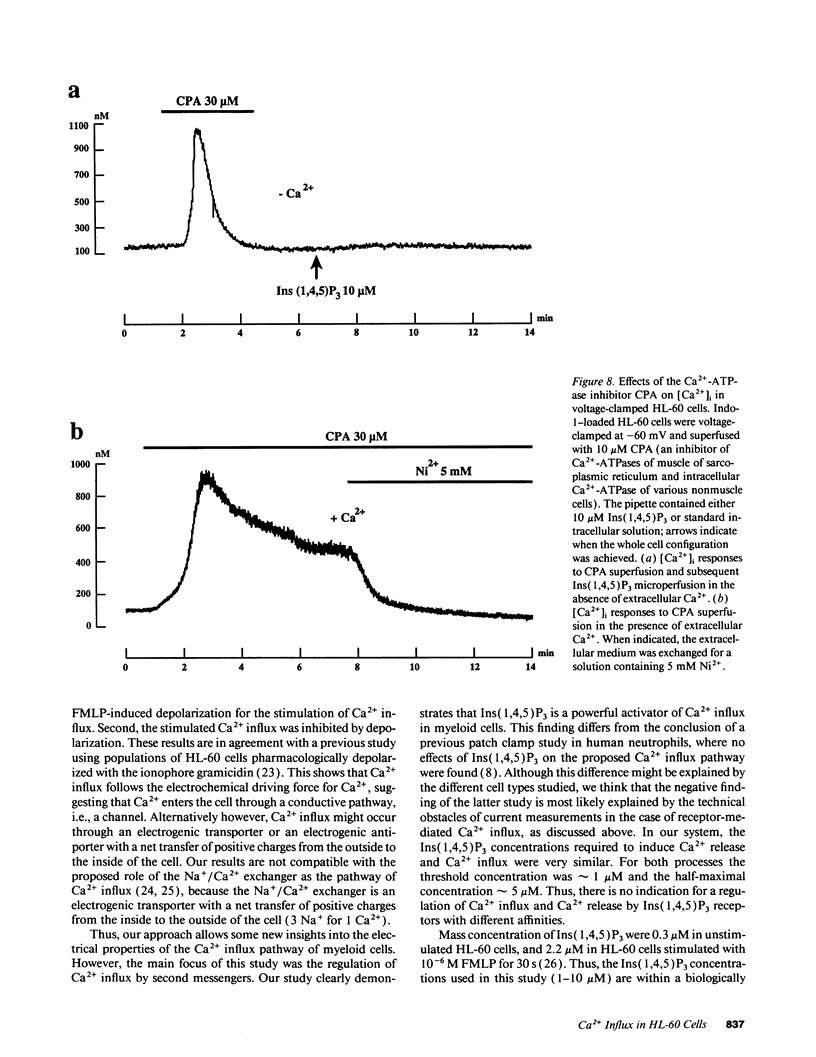
To study the mediation of Ca2+ influx by second messengers in myeloid cells, we have combined the whole-cell patch clamp technique with microfluorimetric measurements of [Ca2+]i. Me2SO-differentiated HL-60 cells were loaded with the fluorescent Ca2+ indicator Indo-1, allowed to adhere to glass slides, and patch-clamped. Receptor agonists and Ca(2+)-ATPase inhibitors were applied by superfusion and inositol phosphates by microperfusion through the patch pipette. In voltage-clamped cells, [Ca2+]i elevations with a sustained phase could be induced by (a) the chemoattractant receptor agonist FMLP, (b) the Ca(2+)-releasing second messenger myo-inositol(1,4,5)trisphosphate [Ins(1,4,5)P3], as well as its nonmetabolizable analogues, and (c) the Ca(2+)-ATPase inhibitor cyclopiazonic acid, which depletes intracellular Ca2+ stores. In the absence of extracellular Ca2+, responses to all stimuli were short-lasting, monophasic transients; however, subsequent addition of Ca2+ to the extracellular medium led to an immediate [Ca2+]i increase. In all cases, the sustained phase of the [Ca2+]i elevations could be inhibited by millimolar concentrations of extracellular Ni2+, and its amplitude could be decreased by depolarization of the plasma membrane. Thus, the sustained phase of the Ca2+ elevations was due to Ca2+ influx through a pathway sensitive to the electrical driving force and to Ni2+. No Ca2+ influx could be observed after (a) plasma membrane depolarization in resting cells, (b) an imposed [Ca2+]i transient independent of receptor activation, or (c) microperfusion of myo-inositol(1,3,4,5)tetrahisphosphate (Ins(1,3,4,5)P4). Also, Ins(1,3,4,5)P4 did not have additive effects when co-perfused with a submaximal concentration of Ins(1,4,5)P3. Our results suggest that, in myeloid cells, activation of chemoattractant receptors induces an electrogenic, Ni(2+)-sensitive Ca2+ influx via generation of Ins(1,4,5)P3. Ins(1,4,5)P3 might activate Ca2+ influx directly, or by depletion of intracellular Ca2+ stores, but not via [Ca2+]i increase or Ins(1,3,4,5)P4 generation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Alvarez J., Montero M., García-Sancho J. Cytochrome P-450 may link intracellular Ca2+ stores with plasma membrane Ca2+ influx. Biochem J. 1991 Feb 15;274(Pt 1):193–197. doi: 10.1042/bj2740193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson T., Dahlgren C., Pozzan T., Stendahl O., Lew P. D. Characterization of fMet-Leu-Phe receptor-mediated Ca2+ influx across the plasma membrane of human neutrophils. Mol Pharmacol. 1986 Nov;30(5):437–443. [PubMed] [Google Scholar]
- Bird G. S., Rossier M. F., Hughes A. R., Shears S. B., Armstrong D. L., Putney J. W., Jr Activation of Ca2+ entry into acinar cells by a non-phosphorylatable inositol trisphosphate. Nature. 1991 Jul 11;352(6331):162–165. doi: 10.1038/352162a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Berridge M. J., Hoyle P. C., Putney J. W., Jr Inositol 1,4,5-trisphosphate may be a signal for f-Met-Leu-Phe-induced intracellular Ca mobilisation in human leucocytes (HL-60 cells). FEBS Lett. 1984 Oct 15;176(1):193–196. doi: 10.1016/0014-5793(84)80939-4. [DOI] [PubMed] [Google Scholar]
- Changya L., Gallacher D. V., Irvine R. F., Potter B. V., Petersen O. H. Inositol 1,3,4,5-tetrakisphosphate is essential for sustained activation of the Ca2+-dependent K+ current in single internally perfused mouse lacrimal acinar cells. J Membr Biol. 1989 Jul;109(1):85–93. doi: 10.1007/BF01870793. [DOI] [PubMed] [Google Scholar]
- Demaurex N., Lew D. P., Krause K. H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J Biol Chem. 1992 Feb 5;267(4):2318–2324. [PubMed] [Google Scholar]
- Di Virgilio F., Lew P. D., Andersson T., Pozzan T. Plasma membrane potential modulates chemotactic peptide-stimulated cytosolic free Ca2+ changes in human neutrophils. J Biol Chem. 1987 Apr 5;262(10):4574–4579. [PubMed] [Google Scholar]
- Gallin E. K. Ion channels in leukocytes. Physiol Rev. 1991 Jul;71(3):775–811. doi: 10.1152/physrev.1991.71.3.775. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Paranjape S., Tsien R. Y. Generation of calcium oscillations in fibroblasts by positive feedback between calcium and IP3. Science. 1991 Jan 4;251(4989):75–78. doi: 10.1126/science.1986413. [DOI] [PubMed] [Google Scholar]
- Henne V., Mayr G. W., Grabowski B., Koppitz B., Söling H. D. Semisynthetic derivatives of inositol 1,4,5-trisphosphate substituted at the 1-phosphate group. Effects on calcium release from permeabilized guinea-pig parotid acinar cells and comparison with binding to aldolase A. Eur J Biochem. 1988 May 16;174(1):95–101. doi: 10.1111/j.1432-1033.1988.tb14067.x. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K. H., Schlegel W., Wollheim C. B., Andersson T., Waldvogel F. A., Lew P. D. Chemotactic peptide activation of human neutrophils and HL-60 cells. Pertussis toxin reveals correlation between inositol trisphosphate generation, calcium ion transients, and cellular activation. J Clin Invest. 1985 Oct;76(4):1348–1354. doi: 10.1172/JCI112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K. H., Welsh M. J. Voltage-dependent and Ca2(+)-activated ion channels in human neutrophils. J Clin Invest. 1990 Feb;85(2):491–498. doi: 10.1172/JCI114464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Nunn D. L., Taylor C. W. Liver inositol, 1,4,5-trisphosphate-binding sites are the Ca2(+)-mobilizing receptors. Biochem J. 1990 Aug 15;270(1):227–232. doi: 10.1042/bj2700227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Pittet D., Lew D. P., Mayr G. W., Monod A., Schlegel W. Chemoattractant receptor promotion of Ca2+ influx across the plasma membrane of HL-60 cells. A role for cytosolic free calcium elevations and inositol 1,3,4,5-tetrakisphosphate production. J Biol Chem. 1989 May 5;264(13):7251–7261. [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Cragoe E. J., Jr Na+-Ca2+ exchange in human neutrophils. Am J Physiol. 1988 Jan;254(1 Pt 1):C150–C164. doi: 10.1152/ajpcell.1988.254.1.C150. [DOI] [PubMed] [Google Scholar]
- von Tscharner V., Prod'hom B., Baggiolini M., Reuter H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. 1986 Nov 27-Dec 3Nature. 324(6095):369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]



