Summary
Onyx is increasingly used in endovascular therapy of intracranial arteriovenous malformations (AVMs). However, the embolic effect and post-embolization management are still under discussion. We report our experience in the treatment of supratentorial brain arteriovenous malformations (SBAVMs) with Onyx and discuss post-embolic management.
From June 2006 to July 2008, 20 patients with SBAVM were embolized with Onyx. There were 14 men and six women ranging from 14 to 64 years of age (mean 38.3 years). Initial symptoms included spontaneous hemorrhage (n=12), headaches (n=4), seizure (n=3) and incidentally disclosed after head trauma (n=1). After the endovascular procedure, all had subsequent treatment (follow-up angiogram, stereotactic radiosurgery or microsurgery) according to the obliteration degree.
At angiography, seven patients (35%, 7/20) were completely obliterated (over 95% closure) after embolization while one suffered a small subarachnoid hemorrhage without permanent clinical sequelae. Four patients (20%, 4/20) were subtotally obliterated (over 80% closure), one patient who suffered severe cerebral edema after embolization underwent decompressive craniectomy, two patients had additional radiosurgery and one patient had follow-up angiogram. Nine patients (45%, 9/20) were partially obliterated (20-80% closure), five patients had additional surgery, two patients had additional radiosurgery and two patients had follow-up angiogram (one patient had intraventricular hemorrhage three months after embolization). Of all 20 AVMs, an average of 2.2 ml Onyx was used per patient and average volume reduction was 80% (range, 30%-99%).
Onyx is suitable for embolization of SBAVMs because of its diffuse controllable properties. We suggest clinical follow-up after complete obliteration, additional radiosurgery or angiographic follow-up after subtotal obliteration and additional surgery after partially obliteration. More cases with long-term follow-up are needed to evaluate the long-term prognosis of our postembolization management.
Key words: Onyx, brain arteriovenous malformations, embolization
Introduction
Onyx (ev3, Irvine, CA,USA) is a new liquid polymerizing embolic material, which theoretically permits slower filling, better penetration and obliteration of the nidus, providing a solid nidus cast due to its longer polymerization time and lack of adherence1. It is a biocompatible polymer with a certain mixture percentage of ethylene-vinyl alcohol (EVOH) and dimethylsulfoxide (DMSO). Tantalum powder is added to the mixture for radiopacity, which seems advantageous over n-BCA for embolization of brain arteriovenous malformation (AVM)2. Onyx-18 (with lower viscosity) containing 6% EVOH and 94% DMSO is available for endovascular treatment of AVM3.
In multimodal approaches to AVM, embolization is generally the first step of treatment and several post-embolic treatments may result in permanent cure4. From June 2006 to July 2008, 20 patients were deemed suitable for embolization with Onyx-18 and subsequent treatment with surgery, radiosurgery and follow-up. The purpose of this study was to report our experience in using Onyx and discuss the post-embolic treatment of supratentorial brain arteriovenous malformations (SBAVMs), surgery, radiosurgery and follow-up. Individualized consecutive neurosurgical treatment not only relies on the patient's general situation patient, but is also closely related to the location, size and angiomorphology of the AVM5, which directly affect the percentage effectiveness of the endovascular embolization.
Patients and Methods
Patients and Initial Symptoms
From June 2006 to July 2008, 20 patients underwent embolization procedures using Onyx-18. There were 14 men and six women ranging from 14 to 64 years of age (mean 38.3 years). Initial symptoms included spontaneous hemorrhage in 12 patients, headaches in four patients, seizures in three and a chance finding after head trauma in one patient.
Classification and Location of the SBAVMs
All 20 AVMs were confirmed by experienced neuroradiologists and neurosurgeons with MR imaging (MRI) and angiogram. The AVMs were classified according to the Spetzler-Martin classification, nine were classified as grades I-II, eight as grade III, and three as grade IV. Three patients with small AVMs (diameter <3 cm) achieved complete obliteration; 13 patients with medium-sized AVMs (diameter from 3 to 6 cm) achieved angiographic size reduction to make following surgery or radiosurgery feasible, while four patients with large AVMs (diameter >6 cm) were impossible to cure by simple embolization treatment and required further treatment (surgery, radiosurgery or follow-up angiogram (regular follow-up angiogram every six months after embolization)). Of the 20 patients with SBAVMs, two were located in the frontal lobe, three in the temporal lobe, two in the parietal lobe, seven in the occipital lobe, one in the fronto-temporal lobe, one in the fronto-parietal lobe, one in the temporo-parietal lobe, one in the temporo-occipital lobe, one in the parieto-occipital lobe and one in the callosum. The classification and location of the SBAVMs are listed in Table 1. In addition, six of the AVMs were associated with flow-related aneurysms (four distal and two proximal).
Table 1.
Summary of patients and AVM characteristics.
| AVM | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient No. |
Age, yr/sex |
AVM location |
Primary Symptom |
Spetzler -Martin classification |
Maximum Diameter (mm) |
Feeding artery (No.) |
Draining vein (No.) |
Onyx Solution (ml) |
Angiographic AVM reduction (%) |
| 1 | 32/M | Occipital | Epilepsy | II | 30 MS | 1 | 2 | 1.8 | 99 CO |
| 2 | 48/M | Fronto-temporal | ICH | IV | 50 MS | 6 | 3 | 2.1 | 60 PO |
| 3 | 64/M | Temporo-occipital | ICH | III | 60 LS | 2 | 2 | 1.0 | 35 PO |
| 4 | 45/M | Callosum | Headache | II | 45 MS | 4 | 2 | 1.4 | 45 PO |
| 5 | 34/F | Temporal | Epilepsy | I | 20 SS | 1 | 1 | 0.75 | 98 CO |
| 6 | 20/F | Occipital | ICH | II | 20 SS | 1 | 1 | 0.8 | 99 CO |
| 7 | 43/M | Temporal | ICH | II | 40 MS | 1 | 2 | 2.6 | 99 CO |
| 8 | 29/M | Occipital | Headache | II | 40 MS | 1 | 1 | 2.6 | 90 SO |
| 9 | 38/F | Occipital | Headache | II | 40 MS | 1 | 1 | 1.8 | 78 PO |
| 10 | 49/M | Occipital | ICH | IV | 80 LS | 4 | 4 | 8.1 | 80 PO |
| 11 | 14/M | Frontal | ICH | II | 30 MS | 1 | 1 | 1.5 | 75 PO |
| 12 | 34/M | Frontal | ICH | IV | 60 LS | 4 | 2 | 1.0 | 30 PO |
| 13 | 49/M | Parietal | ICH | III | 30 MS | 3 | 1 | 1.8 | 90 SO |
| 14 | 38/M | Fronto-parietal | ICH | III | 30 MS | 1 | 2 | 1.5 | 98 CO |
| 15 | 37/F | Parietal | ICH | III | 35 MS | 1 | 1 | 3.0 | 99 CO |
| 16 | 46/F | Temporal | Epilepsy | III | 40 MS | 2 | 1 | 1.8 | 75 PO |
| 17 | 37/M | Parieto-occipital | None | III | 60 LS | 1 | 2 | 4.0 | 72 PO |
| 18 | 47/F | Occipital | ICH | I | 20 SS | 1 | 1 | 0.5 | 98 CO |
| 19 | 44/M | Temporo-parietal | ICH | III | 35 MS | 3 | 1 | 2.5 | 90 SO |
| 20 | 18/M | Occipital | Headache | III | 45 MS | 2 | 2 | 3.2 | 85 SO |
|
AVM, arteriovenous malformation; ICH, intracranial hemorrhage; LS, large-size; MS, medium-size; SS, small-size; CO, complete obliteration; SO, subtotal obliteration; PO, partial obliteration. | |||||||||
Embolization Procedure
All embolization procedures were performed under general anesthesia, with infusion of continued calcium antagonist and blood pressure monitoring. Seldinger technique was adopted and a 6F sheath was inserted into the right femoral artery, a 6F guiding catheter was placed in the internal carotid artery with the tip to the lower level of the epistropheus. After the tip of a Marathon microcatheter (ev3, Irvine, CA, USA) was placed in an intra- or perinidal position by blood flow or microwire guidance, superselective angiogram was performed to choose work perspective (to display the tip mark clearly, analyzing nidus structure and assessing reflux situation) and determine the embolization program.
The microcatheter was flushed with 0.26 ml DMSO. Then Onyx-18 (ev3, Irvine, CA, USA) was slowly injected (at a rate of 0.1~0.15 ml/min adjustable according to the diffusing situation). The injection procedure was interrupted while Onyx entering the main drainage vein or reflux, and another injection was taken after 0.5~2 min to make Onyx further diffused until enough diffusion was achieved (plug and push technology) 6.
A suitable reflux of Onyx (1.0~1.5 cm) at the tip of microcatheter was permitted to form completely block and change the intranidal pressure gradient, then complete or subtotal embolization could be achieved by uninterrupted diffusion of Onyx. The embolization procedure was stopped and the catheter was withdrawn when injection finished or reflux exceeded about 1.5~2.0 cm of the catheter tip. The microcatheter was removed slowly, straightening it and then increasing the tension on the tip until the microcatheter was released from the cast. Another embolization procedure was done as mentioned above if there was another feeding artery.
The distal (intranidal) flow-related aneurysm was dealt with while embolizing the nidus with Onyx at the same time, whereas the proximal flow-related aneurysm was dealt with by microcoils before embolizing the nidus with Onyx.
Additional Therapy after Embolization
After embolization, calcium antagonist infusion was continued to prevent vasospasm and blood pressure was maintained under 120/80 mmHg or 2/3 of preoperative level for three days. Subtotal obliterated patients received additional radiosurgery or follow-up angiogram (regular follow-up angiogram every six months after the embolization) and partially obliterated patients received additional surgery, additional radiosurgery or follow-up angiogram (regular follow-up angiogram every six months after the embolization) according to the situation. At the time when this article was written, the angiographic follow-up of the subtotal and partial obliterated patients was still ongoing, and the clinical follow-up of all patients done by telephone ranged from three months to two years (mean: one year).
Results
Among all 20 AVMs, the average volume of Onyx injected was 2.2 ml per patient (range 0.5~8.1 ml) without any adhesion events, and average volume reduction was 80% (range, 30%-99%). Seven (35%, 7/20) were angiographic completely obliterated (over 95-% closure) after embolization (Figure 1) while one patient had a small subarachnoid hemorrhage without permanent clinical sequelae. There was no angiographic recurrence in five patients who had finished the first follow-up angiogram (six months), the other two have not yet been performed. Four (20%, 4/20) were subtotally obliterated (over 80-% closure)7, one patient who had severe cerebral edema and cerebral hernia after embolization, and was further intracranially infected after decompressive craniectomy, was lost to clinical follow-up. Two patients had additional radiosurgery, the residual nidus disappeared in the angiogram at the end of their hospital stay. One patient had follow-up angiogram (regular follow-up angiogram every six months after the embolization) and the residual nidus had completely disappeared at angiography after six months. Nine lesions (45%, 9/20) were partially obliterated (20-80% closure), five patients had additional surgery (Figure 2) (completely removed in the angiogram at the end of their hospital stay). Two patients had additional radiosurgery: one residual nidus disappeared in the angiogram at the end of hospital stay; the other has not yet been performed. Two patients had follow-up angiogram (regular follow-up angiogram every six months after embolization): one suffered sudden coma caused by intraventricular hemorrhage three months later and recovered after external ventricular drainage, angiogram confirmed the formation of a pseudoaneurysm in the residual nidus; the other has not yet been performed. The 20 patients and AVM characteristics are listed in Table 1. The obliteration degree was classified as different size of AVM and post-embolization treatment are listed in Table 2. The relations between feeding artery and obliteration degree are listed in Table 3. The relations between AVM location and obliteration degree are listed in Table 4.
Table 2.
Degree of Obliteration according to size of AVM and postembolization treatment.
| Size Obliteration Degree |
<3 cm | 3-6 cm | ≥6 cm | Post-Embolization Management (follow-up/radiosurgery/surgery) |
|---|---|---|---|---|
| Complete | 3 | 4 | 0 | 7 / 0 / 0 |
| Subtotal | 0 | 4 | 0 | 1 / 2 / 0 |
| Partial | 0 | 5 | 4 | 2 / 2 / 5 |
| Complete Obliteration Rate |
100% | 30.8% | 0% | |
Table 3.
Relationship between feeding artery and obliteration degree.
| Number Obliteration Degree |
1 | 2 | 3 | 4 | 6 |
|---|---|---|---|---|---|
| Complete | 7 | 0 | 0 | 0 | 0 |
| Subtotal | 1 | 1 | 2 | 0 | 0 |
| Partial | 3 | 2 | 0 | 3 | 1 |
| Complete Obliteration Rate |
63.6% | 0% | 0% | 0% | 0% |
Table 4.
Relationship between AVM location and obliteration degree.
| Location Obliteration Degree |
Deep in brain hemisphere |
Supratentorial cortex |
|---|---|---|
| Complete | 0 | 7 |
| Subtotal | 0 | 4 |
| Partial | 2 | 7 |
| Complete Obliteration Rate |
0% | 38.9% |
Figure 1.
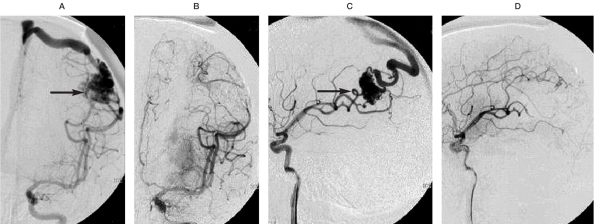
A 37-year-old woman suffered coma and right limb weakness after cerebral hemorrhage, the nidus located in left parietal area (arrow), about 3.5 cm in size. A,B) Before and after embolization with Onyx, left middle cerebral artery (MCA), anteroposterior projection. C,D) Before and after embolization with Onyx, left MCA, lateral projection. Injection through 2 branches of left MCA supplying the nidus, using Onyx about 3.0 ml in total, the nidus was completely obliterated.
Figure 2.
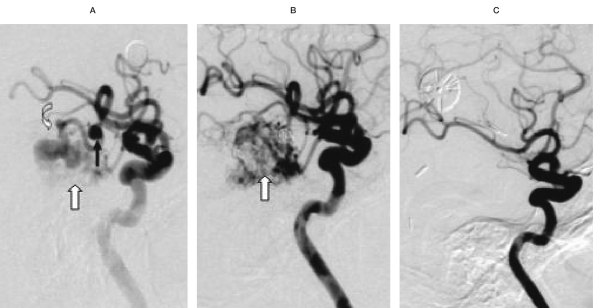
A 64-year-old man suffered sudden coma and weakness, the nidus located in right temporal-occipital area, about 6 cm in size. A) Before embolization with Onyx, right middle cerebral artery (MCA), lateral projection; indicated aneurysm (arrow), fistula (curved arrow) and nidus (white arrow) respectively. B) After embolization with Onyx, right MCA, lateral projection;indicated complete embolized aneurysm and fistula, flow reduced nidus (white arrow). C) Surgical excision after embolization, the nidus was completely disappeared, right MCA, anteroposterior and lateral projection. After embolization of aneurysm by GDC, injection through right MCA supplying the nidus, using Onyx about 1.0 ml in total, the nidus was partially obliterated and was completely removed after embolization.
Discussion
The micro-morphology of Onyx is that of soft sponge-like particles after precipitation, non-adhesive and better controllable, which seems advantageous over n-BCA and makes slow injection possible. So Onyx is better for AVM embolization 8. Ding et Al9 reported their retrospective analysis of the AVM obliteration rate between Onyx and n-BCA based on the following features: size of nidus, type and number of feeding arteries. They found that both embolic agents are suitable for small AVMs. On the other hand, Onyx is superior to n-BCA for medium AVMs which located in supratentorial cortex and with several supplying feeders.
As a new, non-adhesive liquid embolic agent, Onyx is available in mainly two different viscosities, Onyx-18 and Onyx-34 (18 and 34 are units of centipoise), Panagiotopoulos et Al1 mentioned that Onyx-18 was used in AVMs with a small feeding vessel (only slightly above normal vessel diameter), while Onyx-34 was selected for occlusion of a very high-flow fistulous nidus. In our opinion, Onyx-18 is also feasible for slow-flow fistulous nidus (Figure 2), and for a high-flow fistulous nidus or very large fistulous vessels, micro-coils could help to slow down the flow speed and make Onyx-18 embolization possible. So we have achieved ideal effects in intravascular embolization with Onyx-18 based on this principle.
The technology and experience in Onyx embolization for AVM is still very limited. When Onyx-18 enters blood vessels and contacts aqueous solutions, the diffusion and absorption of DMSO promotes copolymer precipitation and forms a soft, non-absorbable mass, resulting in permanent vascular occlusion. This procedure begins on the surface while the core remains liquid, so Onyx has a lava-like flow pattern within blood vessels without any fragmentation during injection10. The discovery and application of plug and push technology6 is exactly based on this property. Also, variation of intranidal pressure gradient during Onyx embolization procedure brings a better diffusion effect. So before application of Onyx to embolize brain AVMs, we should be familiar with the nidus structure, choose the largest feeder permitting the longest reflux and determine the beginning of draining vein. Sufficient patience is necessary during injection and we should pause for 0.5~2 minutes rather than extubate hastily when any reflux of Onyx into the feeding pedicle or venous migration is noted, and then injection is continued. Continued injection is suggested to facilitate embolic agent to fill different portions of the nidus and achieve satisfied nidus occlusion when resistance and reflux are not obvious 3. The waiting time is directly proportional to the length of reflux. Through several manipulation of inject, reflux, pause and re-inject, the diffuse effect and obliteration rate obviously improved. Song et Al 11 described 70 AVM embolization procedures with Onyx: 13 (18.6%) were angiographic completely occluded, 38 (54.3%) were subtotally obliterated, 17/70 (24.3%) suffered perioperative complications. Vasilios et al12 reported 101 AVM embolizations with Onyx: 28 (27.7%) were angiographically completely occluded, 18 (17.8%) were subtotally obliterated, 32/101 (31.7%) suffered perioperative complications. Panagiotopoulos et Al1 described 82 AVM embolizations with Onyx: 20/82 (24.4%) were achieved complete obliteration, with an average of 75% (range, 30%-100%) volume reduction. Our report of 20 AVM embolizations with Onyx is that seven (35%) were angiographically completely occluded, four (20%) were subtotally obliterated, with an average of 80% (range, 30%-99%) volume reduction, and three (15%) suffered perioperative complications. The obliteration rate is markedly improved in our report compared with previous literature and it based on application of the above-mentioned technology and embolization experience with Onyx.
When reflux of Onyx is more than 1.0~1.5 cm, the sticking of the catheter increases. Injection must be stopped with immediate extubation. The reflux length is not the only indication to extubate, more crucial is vascular diameter and tortuosity, which decide extubation time. There was no complication of indwelling catheter or catheter perforation in our report, which is attributed to control reflux strictly in relatively small tortuous feeders and good control of extubation time. On the other hand, we should stop embolization to prevent rupture of the microcatheter or vessels and sudden reflux when the injection resistance increases significantly, indicating complete or subtotal occlusion of the nidus compartment supplied by the embolic feeder. In our report, we continued injecting Onyx with high injection resistance in one AVM that had unexpectedly large reflux, fortunately without any serious consequence after post-operative therapy. It deserves reference and we learnt from it.
The most feared complication during embolization or afterwards is hemorrhage, ranging from 5.9% to 6.8% in some reports12,13. Besides the possible reasons such as normal perfusion pressure breakthrough and rupture of the vessels caused by extubation, we consider inappropriate migration of the glue into the draining veins and thrombosis of the draining veins followed by slow blood flow after subtotal nidus occlusion are more common, causing drainage obstruction of the residual nidus and immediate or delayed hemorrhage. So Van et Al 13 considered embolization principle is that during embolization with Onyx, it seems sensible either to leave a substantial part of the nidus open to assure sufficient flow in the draining veins, or to proceed to complete ocelusion. A good working perspective is needed to distinguish the beginning of draining veins and avoid too much migration of the glue into the main and large draining veins. There was no perioperative hemorrhage except one mild subarachnoid hemorrhage in our report. We think this is related to appropriate working perspective, especially repeated comparison between suitable angiogram images and corresponding embolization images to confirm nidus structure and location of draining veins. Some draining veins of the brain AVMs drain both nidus and normal blood flow, so except for cerebral hemorrhage, postoperative brain edema is also correlated with obstruction of draining veins. In one of the cases subtotally obliterated in our group, the intact draining veins were not found in the postoperative angiogram. The obstruction of normal blood flow was caused by thrombosis of the draining vein and then resulted in severe brain edema. Thrombosis of draining vein is an important factor towards AVM embolization complications and no effective way has been found except controlling the obliteration rate of the nidus, which deserves attention and further discussion.
The embolization effects differ according to nidus size, location and structural properties. Weber et Al14 described 94 AVMs embolized with Onyx, the features of all AVMs were evaluated: type of nidus and shunt, draining veins and feeding arteries. They concluded that the injection of Onyx resulted in high occlusion rates (volume reduction >90%) when the AVM was in a supratentorial and cortical location, the nidus was compact and plexiform, and when there was a small number of supplying (direct) feeders and one superficial draining vein. There was a low occlusion rate (<70%) for Onyx in AVMs with multiple compartmental draining veins, multiple supplying arteries (especially leptomenigeal, en-passant, or perforating feeders) and when the nidus was diffuse. In our report, two AVMs located deep in the brain hemisphere were partially obliterated. Among 18 AVMs located in the supratentorial cortex, seven were completely obliterated, four were subtotally obliterated, seven were partially obliterated (four of them were large AVMs with diameters >6 cm with multiple supplying arteries and draining veins). After comparing and analyzing the AVM morphological characteristics among completely embolization, subtotal embolization and partially embolization, we found better a AVM obliteration rate with Onyx when the nidus was compact, located in the supratentorial cortex with fewer supplying feeders and one superficial draining vein. The AVM obliteration rate was worse when the nidus was diffuse, located deep, and compartmental with multiple supplying arteries (especially perforating feeders) and multiple draining veins, consistent with Weber's report14. According to the result, we suggest that radiosurgery is the first choice for deep-located AVMs with diameters <3 cm to avoid new hemodynamic instability of AVMs caused by embolization.
Sabareesh et Al15 suggest that after Onyx embolization, surgery was frequently performed within a few days after the last embolization session because a much greater AVM volume was embolized and there was concern about the prospect of hemorrhage from the AVM while awaiting surgery. So it is reasonable to predict that once AVM could not be embolized completely, formation of a new AVM with new morphologic properties and unstable hemodynamics would occur. The post-embolic treatment for AVMs not completely obliterated is very important.
Duffner et Al 17 found that the nidus embolized with Onyx intraoperatively remains elastic and formable and could be dissected from the surrounding brain tissue clearly by microsurgery. Vasilios et Al12 suggested that patients with near-total occlusion should avoid embolic treatment again for the following reasons: the inability to visualize the new Onyx through the previously injected material cast, the inability to catheterize any more feeding pedicles and the presence of feeders supplying the malformation as well as eloquent brain. So we chose different subsequent treatments for AVMs according to the obliteration degree with Onyx.
Subtotal embolization patients received additional radiosurgery or follow-up, and additional surgery is first considered after partial embolization to avoid bleeding again. In our group, one patient with a partially obliterated AVM had intraventricular hemorrhage three months later and angiogram confirmed the formation of a pseudoaneurysm in a residual nidus. The feasibility of our post-embolic treatment strategy had been demonstrated and more experiences must be accumulated.
Conclusions
Onyx is suitable for the embolization of compact, small and superficial AVMs with fewer, larger, straighter supplying feeders. Better results and fewer complications are available. Embolic treatment using Onyx could be chosen as the primary treatment for various brain AVMs and subsequent treatment should be adopted according to obliteration degree. We suggest clinical follow-up after complete obliteration, additional radiosurgery or angiographic follow-up after subtotal obliteration and additional surgery after partially obliteration. Needless to say, more cases with long-term follow-up are needed to evaluate the long-term prognosis of our post-embolization management.
References
- 1.Panagiotopoulos V, Gizewski E, et al. Embolization of Intracranial Arteriovenous Malformations with Ethylene-Vinyl Alcohol Copolymer (Onyx) Am J Neuroradiol. 2009:1–8. doi: 10.3174/ajnr.A1314. [doi: 10.3174/ajnr. A1314] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierot L, Januel AC, et al. Endovascular treatment of brain arteriovenous malformation using Onyx: preliminary results of a prospective multicenter study. Interventional Neuroradiology. 2005;11:159–64. doi: 10.1177/15910199050110S119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jahan R, Murayama Y, et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery. 2001;48(5):984–995. doi: 10.1097/00006123-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Yu SC, Chan MS, et al. Complete obliteration of intracranial arteriovenous malformation with endovascular cyanoacrylate embolization: initial success and rate of permanent cure. Am J Neuroradiol. 2004;25:1139–1143. [PMC free article] [PubMed] [Google Scholar]
- 5.Weber W, Kis B, et al. Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery. 2007;61(2):244–52. doi: 10.1227/01.NEU.0000255473.60505.84. [DOI] [PubMed] [Google Scholar]
- 6.Cekrige S, Arat A, Soatei L. Long intranidal injections of Onyx in the endovascular treatment of brain AVMs, presented at the 6th congress of world federation of interventional and therapeutic. Neuroradiology (WFITN) 2001:102–103. [Google Scholar]
- 7.Perez-Higueras A, Lopez RR, Tapia DQ. Endovascular treatment of cerebral AVM: Our experience with Onyx. Interventional Neuroradiology. 2005;11:141–157. doi: 10.1177/15910199050110S118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akin ED, Perkins E, Ross IB. Surgical handling characteristics of an ethylene vinyl alcohol copolymer compared with N-butyl cyanoacrylate used for embolization of vessels in an arteriovenous malformation resection model in swine. J Neurosurg. 2003;98(2):366–370. doi: 10.3171/jns.2003.98.2.0366. [DOI] [PubMed] [Google Scholar]
- 9.Ding X, Wang ZG, et al. The study of different type of cerebral arteriovenous malformation embolization with NBCA and ONYX. Chin J Neurosurg. 2006;8:464–466. [Google Scholar]
- 10.Murayama Y, Viñuela F, et al. Nonadhesive liquid embolic agent for cerebral arteriovenous malformations: preliminary histopathological studies in swine rete mirabile. Neurosurgery. 1998;43:1164–75. doi: 10.1097/00006123-199811000-00081. [DOI] [PubMed] [Google Scholar]
- 11.Song DL, Leng B, et al. Clinical experience of 70 cases of cerebral arteriovenous malformations embolization with Onyx, a novel liquid embolic agent. Chin J Surg. 2007;4:223–225. (in Chinese) [PubMed] [Google Scholar]
- 12.Vasilios K, Chrysanthi P, Enrico A. Curative embolization of cerebral arteriovenous malformations(AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50:589–597. doi: 10.1007/s00234-008-0382-x. [DOI] [PubMed] [Google Scholar]
- 13.Van Rooij WJ, Sluzewski M, Beute GN. Brain AVM embolization with Onyx. Am J Neuroradiol. 2007;28:172–177. [PMC free article] [PubMed] [Google Scholar]
- 14.Weber W, Kis B, et al. Endovascular treatment of intracranial arteriovenous malformations with Onyx: Technical aspects. Am J Neuroradiol. 2007;28:371–377. [PMC free article] [PubMed] [Google Scholar]
- 16.Natarajan SK, Ghodke B, et al. Multimodality treatment of brain arteriovenous malformations with microsurgery after embolization with onyx: single-center experience and technical nuances. Neurosurgery. 2008;62(6):1213–1226. doi: 10.1227/01.neu.0000333293.74986.e5. [DOI] [PubMed] [Google Scholar]
- 16.Natarajan SK, Ghodke B, et al. Multimodality treatment of brain arteriovenous malformations with microsurgery after embolization with onyx: single-center experience and technical nuances. Neurosurgery. 2008;62(6):1213–1226. doi: 10.1227/01.neu.0000333293.74986.e5. [DOI] [PubMed] [Google Scholar]
- 17.Duffner F, Ritz R, Bornemann A. Combined therapy of cerebral arteriovenous malformations: histological differences between a non-adhesive liquid embolic agent and n-butyl 2-cyanoacrylate (NBCA) Clin Neuropathol. 2002;21(1):13–17. [PubMed] [Google Scholar]