Summary
Polymethylmethacrylate, as a widely used material for vertebroplasty, has several drawbacks such as heat development and high allergenic potential. In order to avoid these drawbacks ceramic cement materials have been developed.
The purpose of this study was to evaluate a new biointegrative material for vertebroplasty in osteoporotic vertebral fractures regarding pain relief, safety aspects and technical feasibility.
The injectable bone substitute Cerament™ SpineSupport has been developed for vertebroplasty of osteoporotic vertebral fractures. The aim of the product is to provide mechanical stability by cured calcium sulfate dehydrate during a period of several weeks and to act as an osteoconductive support by hydroxyl apatite particles.
Inclusion criteria were a stable single vertebral fracture at levels Th5 to L5, verified by CT and MRI, and not older than four weeks, in osteoporotic patients aged 60 years or older. Bipedicular vertebroplasty technique was used. Follow up included CT directly after treatment and after two month and pain assessment (VAS) pre and post procedure after two weeks and one month.
Seven patients (age range 62-96 years, mean 73.9, five women, two men) were treated at levels T 8 (n=1), T12 (n=4) and L1 (n=2). The average injected volume was 1.9 ml (range 0.2-4 ml). No material or procedure-related complications were observed. An average height loss of the treated vertebral bodies of 3.6 mm (range 1.5-5.4) was seen two months after treatment as compared to pre-treatment CT. Pain assessment by VAS resulted in an improvement from mean 69 prior treatment to 37 the day post treatment, 42 after two weeks and 30 after one month.
Initial results indicate that Cerament™ Spine Support is safe and effective in the treatment of acute osteoporotic vertebral body fractures. Further studies with long-term follow-up are needed to confirm these results and to prove the concept of osteoconduction with hydroxyl apatite particles.
Key words: vertebroplasty, biointegrative cement
Introduction
Vertebroplasty is a widely use method for pain treatment and vertebral augmentation in osteoporotic vertebral fractures. The method was introduced by Galibert and Deramont initially for the treatment of painful vertebral hemangiomas and metastases 1. Later the indication was expanded to painful osteoporotic vertebral fractures, which today is by far the most frequent indication for vertebroplasty2,3.
Since the introduction of vertebroplasty polymethylmethaacrylate (PMMA) based cement materials have been used almost exclusively4. There are a number of PMMA cements available adapted to the specific needs in vertebroplasty such as fluoroscopic visibility, injectability and working time. In spite of some differences in mixture composition, the basic properties of PMMA cement materials are rather similar. The use of PMMA is well documented and several studies have shown its effectiveness in vertebral augmentation and pain relief5,6.
However there are a number of drawbacks which query PMMA as an ideal cement material for vertebroplasty for osteoporotic vertebral fractures. PMMA has a high allergenic potential and is locally toxic before hardening. The heat development during hardening may damage nerve structures in case of leakage4,7. Both the toxicity and the heat development are potentially harmful for the local bone viability which might further degrade the bone in the osteoporotic vertebras.
In order to avoid these problems related to PMMA several cement materials based on ceramics have been developed. Beside their good biocompatibility these materials are also potentially osteoconductive 4.
The aim of this prospective patient study was to evaluate a new biointegrative material for vertebroplasty in osteoporotic vertebral fractures regarding pain relief, safety aspects and technical feasibility.
Method and Materials
The investigational bone substitute Cerament™ SpineSupport (BoneSupport AB, Lund, Sweden) based on calcium sulfate hemihydrate, hydroxyl apatite and Iohexol has been developed for percutaneous treatment of osteoporotic vertebral fractures. The basic concept of this cement material is to provide instant mechanical stability by the formation of cured calcium sulfate dehydrate during a period of several weeks and to act as an osteoconductive support for ingrowths of new bone by the hydroxyl apatite particles.
Cerament™ SpineSupport consists of a powder phase comprising calcium sulfate hemihydrate (60 weight %) and hydroxyapatite (40 weight %) and a liquid phase consisting of Iohexol 180 mg Iodine/ml. The powder phase of the cement is provided in a prefilled mixing device and the mixing procedure is started by adding the Iohexol. The injectable paste can thereafter be loaded into 1 m syringes for injection into the vertebral body.
The mixing time is approximately 1 min, the working time up to 10 min and the setting time is approximately 30 min. In contrast to PMMA none of the components is highly allergenic and no significant heat development occurs during the hardening. The compression strength of Cerament™ SpineSupport is 25 MPa in vitro as compared to 50-100 MPa for typical PMMA materials 8.
The study was approved by the local Ethics Committee and the Swedish Medical Products Agency. Informed consent was obtained from all patients. Inclusion criteria were:
1. Stable, painful vertebral fracture at a single level from T 5 to L 5, verified by computed tomography (CT) and magnetic resonance imaging (MR).
2. Age of the fracture not older than four weeks confirmed by patient history.
3. Signs of osteoporosis verified by radiography, CT or by medical history (e.g. bone density measurements).
4. Patients aged 60 years or older. Seven patients (mean age 73.9 years, range 62-96, five women, two men) were included and treated at levels T 8 (n=1),T 12 (4) and L 1 (2) (Table 1).
Table 1.
Patient age, treated level, injected cement volume and anterior vertebral height loss two months after treatment compared to preprocedural imaging.
| Patient No. |
Age | Level | Injected volume |
Height loss, mm |
Height loss, % |
|---|---|---|---|---|---|
| 1 | 78 | T 12 | 0.2 | 2.6 | 17 |
| 2 | 96 | T 8 | 1.5 | ||
| 3 | 65 | T 12 | 0.6 | 2.9 | 16 |
| 4 | 62 | L1 | 0.8 | 1.5 | 8 |
| 5 | 73 | L1 | 2.2 | 5.0 | 29 |
| 6 | 68 | T 12 | 3.9 | 5.4 | 25 |
| 7 | 75 | T 12 | 4.0 | 4.3 | 32 |
| mean | 73.9 | 1.9 | 3.6 | 21 | |
Bipedicular vertebroplasty technique was used with 13G or 11G needle size and Cerament™ SpineSupport was used exclusively in all patients. The procedure was performed under conscious sedation. Periprocedural arterial blood gas sampling was performed to monitor pulmonary embolisation.
Preprocedural and postprocedural imaging included x-ray one month after treatment, CT the day before treatment, within one day after treatment and after two months. MRI within two weeks before treatment.
Postprocedural compression of the treated vertebral body was assessed by measuring the anterior vertebral height on mid sagittal reconstructions on the CT examinations before treatment and after eight weeks.
Pain was assessed on a 100 graded (0 = no pain, 100 = worst imaginable pain) visual analogue scale (VAS) on the day before treatment, within one day after treatment, after two weeks and one month. The patients were also asked to assess their general health status using a visual analogue global self-rated health assessment (EQ5Dvas, 0 = worst imaginable health status, 100 = best imaginable health status) on the day before treatment, within one day after treatment, after two weeks and one month9.
Results
Procedural behavior of the material
Due to the prefilled mixing device the mixing procedure was convenient. In contrast to PMMA no measures to prevent monomere fume exposure were needed. The injection was performed with 1 mm syringes.
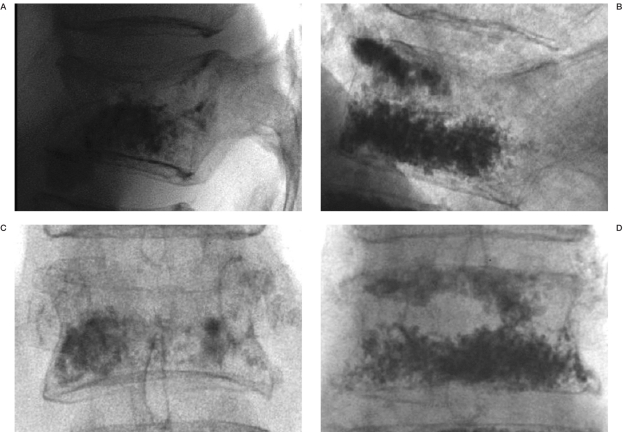
During the treatment of the first cases plugging of the injection needles occurred, which ruled out further injection of the cement. Because of this, the injected cement volumes were unintentionally low in the first four cases (Table 1). Probably a separation of the powder and liquid phase caused by filter pressing created a particle jam inside the injection needle. These initial problems were partially solved by a minor modification of the cement material decreasing its susceptibility to pressure and by saline flushing of the vertebral body through the vertebroplasty needles prior to the cement injection. These measures markedly reduced the occurrence of needle plugging. The average injected volume was 1.9 ml (range 0.2-4.0) (Table 1). The fluoroscopic visibility of the cement was inferior to typical PMMA cements for vertebroplasty, which made fluoroscopic monitoring of the injection difficult especially in obese patients (Figure 1).
Figure 1.
Radiographic visibility of 4.0 ml Cerament™ SpineSupport (A,C) as compared to 4.2 ml PMMA (Osteofirm™; Cook Europe, Bjaeverskov, Denmark) (B,D) directly postprocedural in two different cases.
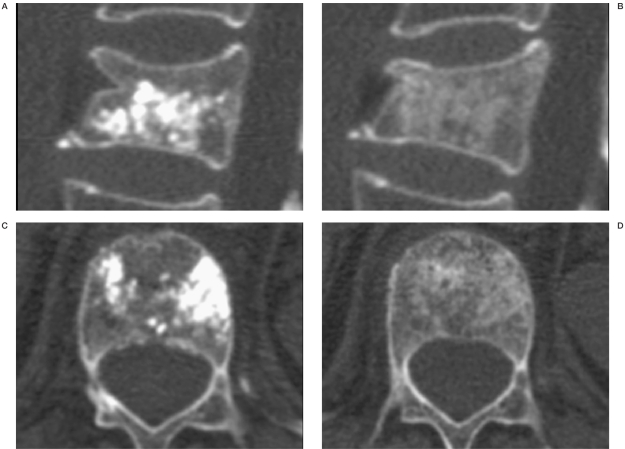
The visibility on CT directly after the procedure was good without any difficulties ruling out material leakage. However on CT two months after treatment almost nothing of the material was seen, probably due to resorption and elimination of the Iohexol, indicating that the remaining components barely contribute to the radioopacity of the material (Figure 2).
Figure 2.
Visibility on CT of Cerament™ SpineSupport directly after treatment (A,C) and after 2 months (B,D).The markedly decreased visibility after 2 months is probably due to the resorption of the Iohexol. As in this case, in all measured cases an additional anterior compression was seen after 2 months (B).
Pain relief, quality of life
The VAS score was reduced for all patients by at least 50% except for two patients. In patient 1, treated at level T 12, a marked increase in pain was seen at follow-up at two week caused by a new fracture at level L 2. Patient 6 had no pain reduction but an increase in the VAS score was seen both the day after treatment and at two and four weeks follow-up. CT at eight weeks revealed the development of an osteonecrosis in the treated level. The mean VAS score decreased from 69 preoperatively to 30 after four weeks. However this improvement was not statistically significant (p > 0.05; Wilcoxon signed rank test) (Table 1).
Regarding the self-assessed general health status (EQ5Dvas) the mean status improved from preprocedural 45 (range 20-60) to 54.7 (range 20-81) at two weeks with a further improvement to 71.1 (range 51-95) at four weeks. The improvement from preprocedural to four weeks was statistically significant (p <0.05; Wilcoxon signed rank test).
Height stabilization
Measurements of the anterior height of the treated vertebral bodies on CT before and eight weeks after treatment showed an increased anterior compression in all cases. The mean height loss was 3.6 mm (range 1.5-5.5) or 21% (range 8-32). Due to medical complications follow-up CT after eight weeks was not performed in patient 2, so the height loss could not assessed in this case (Table 1, Figure 2).
Table 2.
VAS values the day before treatment, within one day after treatment, after two weeks and one month.
| Patient No. | Preop | Postop | 2 weeks | 1 month |
|---|---|---|---|---|
| 1 | 65 | 5 | 90 | 49 |
| 2 | 30 | 15 | 0 | 1 |
| 3 | 80 | 18 | 10 | 0 |
| 4 | 70 | 45 | 30 | 37 |
| 5 | 89 | 54 | 65 | 42 |
| 6 | 70 | 80 | 100 | 77 |
| 7 | 80 | 40 | 0 | 6 |
| mean | 69 | 37 | 42 | 30 |
Safety
Measurement of periprocedural arterial blood gases showed no signs of pulmonary embolism. Patient 2 was admitted 11 days after treatment because of sudden onset of paraparesis. Subsequent MRI showed edema in the thoracic medulla and further investigation revealed a thoracic aortic aneurysm. The condition was interpreted as medullary ischemia due to the thoracic aneurysm and not related to the vertebroplasty. Due to the complicated course no follow-up CT after eight weeks was performed in this patient. In patient 1, treated at level T 12, a new vertebral fracture occurred at level L 2. Patient 6 showed the typical radiological features of osteonecrosis on follow-up CT eight weeks after treatment. The patient did not show any pain relief following the treatment.
Discussion
The presented study shows that vertebroplasty in osteoporotic vertebral fractures with the ceramic bone substitute Cerament™ Spine Support is feasible with promising results.
The use of ceramic cements like Cerament™ SpineSupport in vertebroplasty in osteoporotic vertebral fractures avoids some of the drawbacks related to PMMA, namely the toxic and thermal effect during the procedure and the allergenic effect especially for personnel. However further improvements of the material are needed to reach injectability and fluoroscopic visibility comparable with PMMA for vertebroplasty.
As a consequence of this study the liquid phase in the final CE marked product Cerament™ SpineSupport has been changed to Iohexol 300 mg Iodine/ml.
Regarding the therapeutic effect, the results indicate that the efficiency in pain relief within the first month after treatment is comparable to results reported with PMMA6. A slight to moderate additional anterior compression of the treated vertebras was seen in all measured cases indicating that the stabilizing effect probably is less than that of PMMA10. On the other hand, the physiological compression strength of Cerament™ SpineSupport might help to prevent new fractures at adjacent levels, which has be discussed as a consequence of vertebroplasty with PMMA11,12. These aspects are still speculative and have to be evaluated with larger studies with long-term follow-up.
No complications likely related to the procedure or the material were seen. The new vertebral fracture in patient 1 occurred at a non adjacent level and was most probably related to the osteoporosis. The osteonecrosis at the treated level in patient 6 was primarily judged as spontaneous. Osteonecrosis is a known complication of vertebral fractures. However a contribution of the cement material to the development of the osteonecrosis cannot be ruled out and the lack of pain relief in this patient indicates that Cerament™ SpineSupport probably is not suitable for this fracture type.
Regarding the basic concept of Cerament™ SpineSupport, with a short-term stabilizing effect with pain relief provided by cured calcium sulfate dehydrate and a long-term osteoconductive effect by the hydroxyl apatite particles, only the pain relief could be demonstrated in our study with short-term follow-up. Whether this bone substitute is able to induce a local increase in bone mass in an osteoporotic patient has to be shown with long-term follow-up studies including longitudinal measurements of the local bone mass.
Conclusions
Initial results indicate that Cerament™ SpineSupport is safe and effective in the treatment of acute osteoporotic vertebral body fractures. Further studies with long-term follow-up are needed to confirm these results and to prove the concept of osteoconduction with hydroxyl apatite particles.
References
- 1.Galibert P, Deramond H, et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33(2):166–168. [PubMed] [Google Scholar]
- 2.Jensen ME, McGraw, et al. Position statement on percutaneous vertebral augmentation: a consensus statement developed by the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, American Association of Neurological Surgeons/Congress of Neurological Surgeons, and American Society of Spine Radiology. J Vasc Interv Radiol. 2007;18(3):325–330. doi: 10.1016/j.jvir.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Jensen ME, Evans AJ, et al. Percutaneous polymethyl-methacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. Am J Neuroradiol. 1997;18(10):1897–1904. [PMC free article] [PubMed] [Google Scholar]
- 4.Lieberman IH, Togawa D, et al. Vertebroplasty and kyphoplasty: filler materials. Spine J. 2005;5(6 Sup):305S–316S. doi: 10.1016/j.spinee.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Guglielmi G, Andreula C, et al. Percutaneous vertebroplasty: indications, contraindications, technique, and complications. Acta Radiol. 2005;46(3):256–268. doi: 10.1080/02841850510021049. [DOI] [PubMed] [Google Scholar]
- 6.Gill JB, Kuper M, et al. Comparing pain reduction following kyphoplasty and vertebroplasty for osteoporotic vertebral compression fractures. Pain Physician. 2007;10(4):583–590. [PubMed] [Google Scholar]
- 7.Nussbaum DA, Gailloud P, et al. A review of complications associated with vertebroplasty and kyphoplasty as reported to the Food and Drug Administration medical device related web site. J Vasc Interv Radiol. 2004;15(11):1185–1192. doi: 10.1097/01.RVI.0000144757.14780.E0. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson M, Wang JS, et al. Biodegradation and biocompatability of a calcium sulphate-hydroxyapatite bone substitute. J Bone Joint Surg Br. 2004;86(1):120–125. [PubMed] [Google Scholar]
- 9.EuroQol-a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 10.Lin WC, Lee YC, et al. Refractures in cemented vertebrae after percutaneous vertebroplasty: a retrospective analysis. Eur Spine J. 2008;17(4):592–599. doi: 10.1007/s00586-007-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voormolen MH, Lohle PN, et al. The risk of new osteoporotic vertebral compression fractures in the year after percutaneous vertebroplasty. J Vasc Interv Radiol. 2006;17(1):71–76. doi: 10.1097/01.RVI.0000190910.43602.3C. [DOI] [PubMed] [Google Scholar]
- 12.Uppin AA, Hirsch JA, et al. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226(1):119–124. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]