Abstract
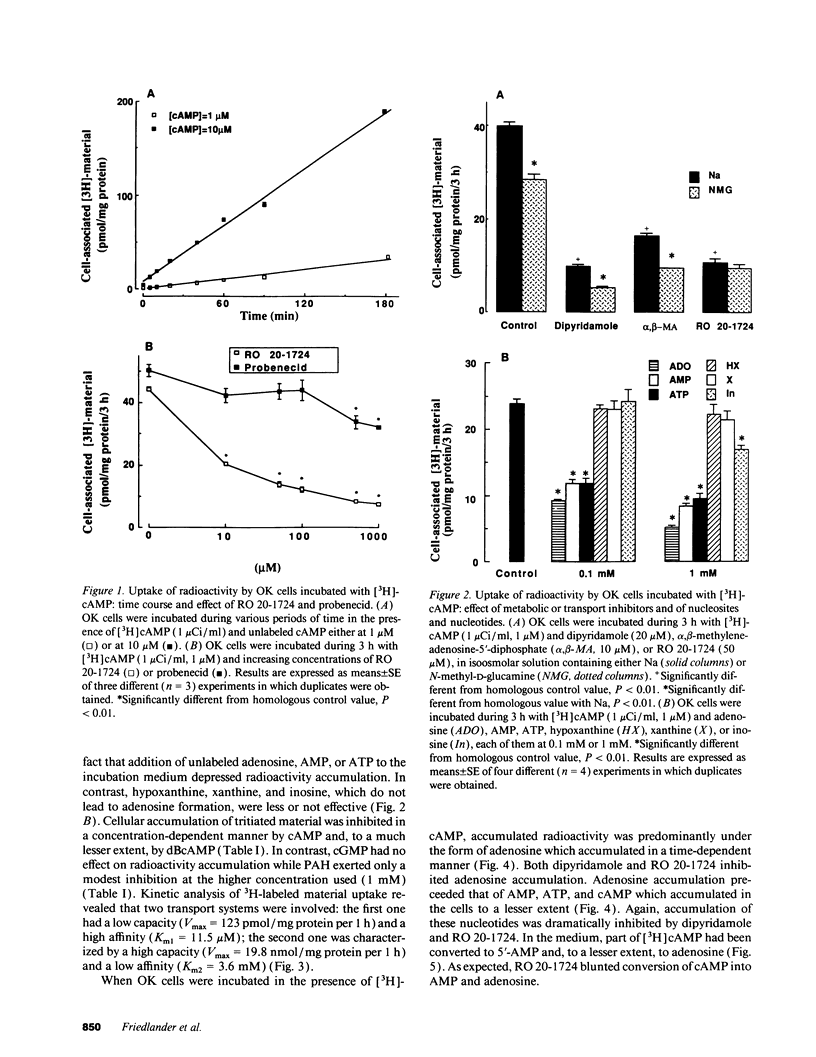
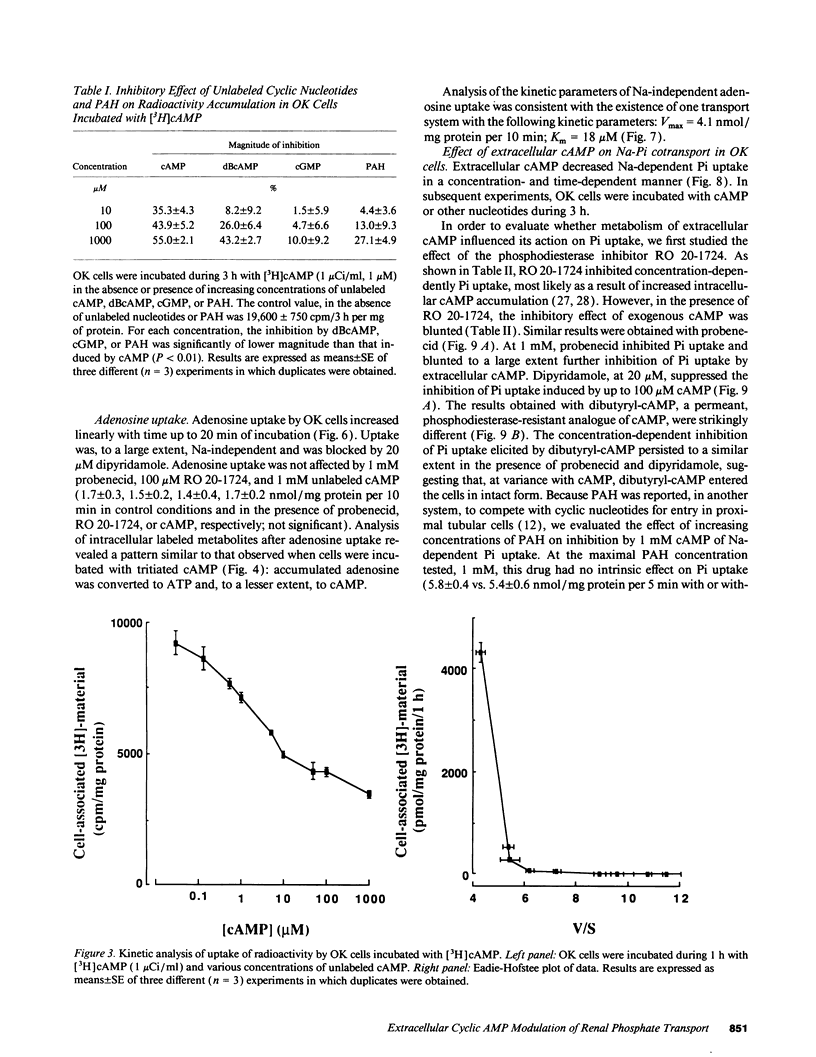
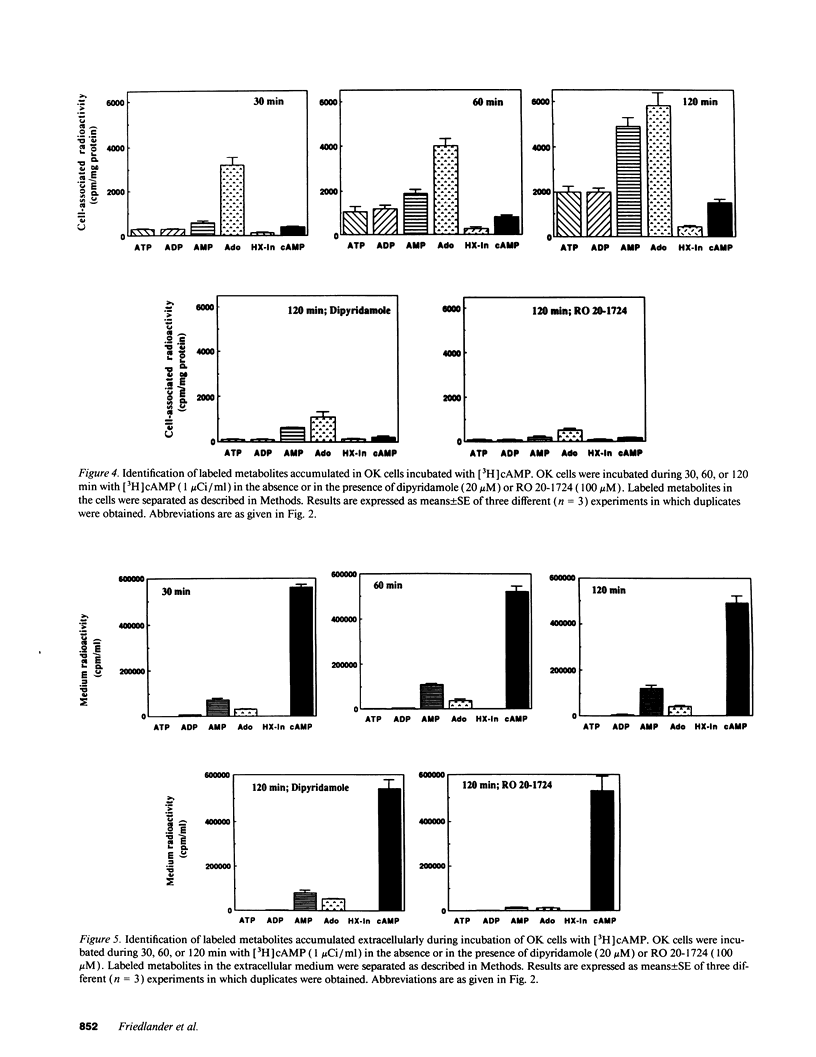
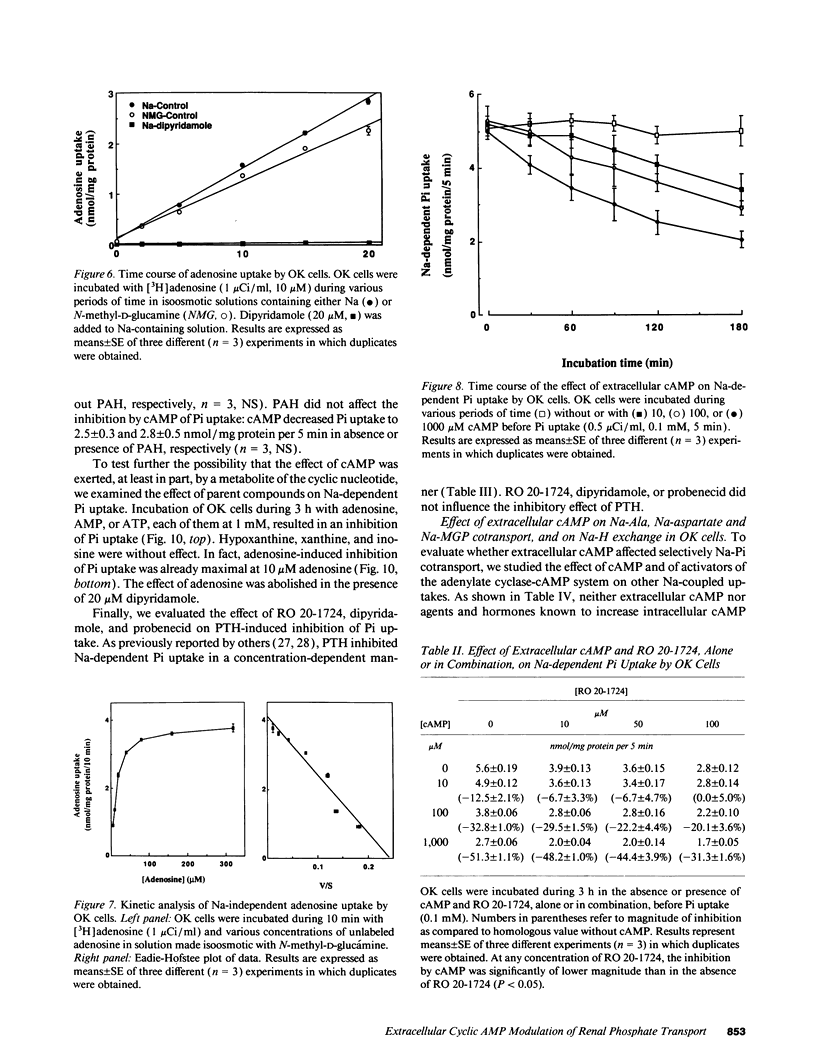
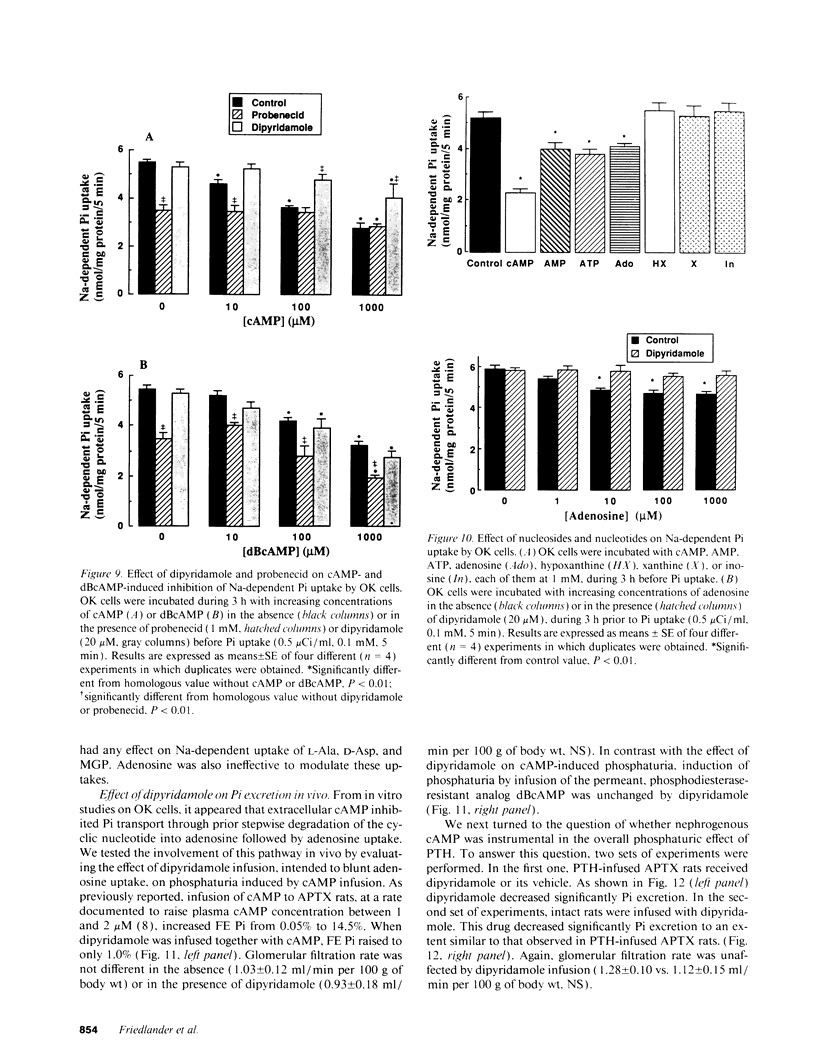
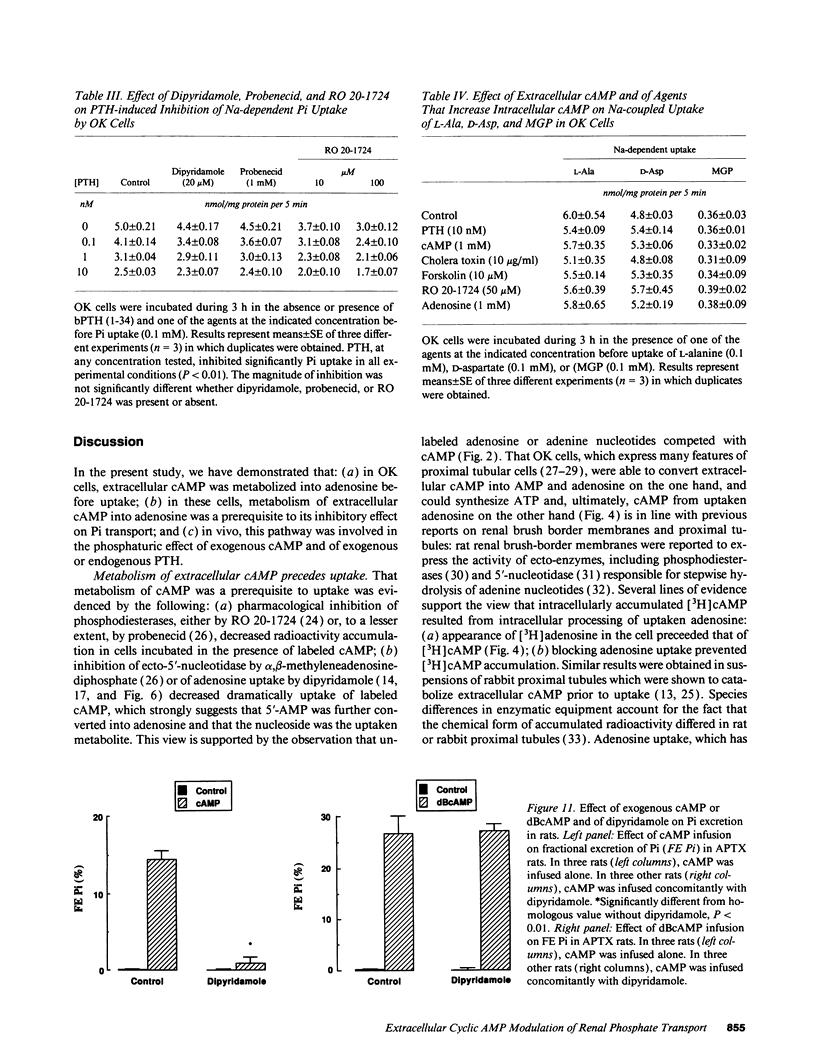
The mechanism of phosphaturia induced by cAMP infusion and the physiological role of extracellular cAMP in modulation of renal phosphate transport were examined. In cultured opossum kidney cells, extracellular cAMP (10-1,000 microM) inhibited Na-dependent phosphate uptake in a time- and concentration-dependent manner. The effect of cAMP was reproduced by ATP, AMP, and adenosine, and was blunted by phosphodiesterase inhibitors or by dipyridamole which inhibits adenosine uptake. [3H]cAMP was degraded extracellularly into AMP and adenosine, and radioactivity accumulated in the cells as labeled adenosine and, subsequently, as adenine nucleotides including cAMP. Radioactivity accumulation was decreased by dipyridamole and by inhibitors of phosphodiesterases and ecto-5'-nucleotidase, assessing the existence of stepwise hydrolysis of extracellular cAMP and intracellular processing of taken up adenosine. In vivo, dipyridamole abolished the phosphaturia induced by exogenous cAMP infusion in acutely parathyroidectomized (APTX) rats, decreased phosphate excretion in intact rats, and blunted phosphaturia induced by PTH infusion in APTX rats. These results indicate that luminal degradation of cAMP into adenosine, followed by cellular uptake of the nucleoside by tubular cells, is a key event which accounts for the phosphaturic effect of exogenous cAMP and for the part of the phosphaturic effect of PTH which is mediated by cAMP added to the tubular lumen under the influence of the hormone.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud H. E., Dousa T. P. Action of adenosine on cyclic 3',5'-nucleotides in glomeruli. Am J Physiol. 1983 Jun;244(6):F633–F638. doi: 10.1152/ajprenal.1983.244.6.F633. [DOI] [PubMed] [Google Scholar]
- Amiel C., Kuntziger H., Couette S., Coureau C., Bergounioux N. Evidence for a parathyroid hormone-independent calcium modulation of phosphate transport along the nephron. J Clin Invest. 1976 Feb;57(2):256–263. doi: 10.1172/JCI108276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Bonnafous J. C., Dornand J., Mani J. C. Adenosine-induced cyclic AMP increase in pig lymphocytes is not related to adenylate cyclase stimulation. Biochim Biophys Acta. 1979 Oct 4;587(2):180–191. doi: 10.1016/0304-4165(79)90352-0. [DOI] [PubMed] [Google Scholar]
- Boumendil-Podevin E. F., Podevin R. A. Transport and metabolism of adenosine 3':5'-monophosphate and N6, O2'-dibutyryl adenosine 3':5'-monophosphate by isolated renal tubules. J Biol Chem. 1977 Oct 10;252(19):6675–6681. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broadus A. E., Mahaffey J. E., Bartter F. C., Neer R. M. Nephrogenous cyclic adenosine monophosphate as a parathyroid function test. J Clin Invest. 1977 Oct;60(4):771–783. doi: 10.1172/JCI108831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caverzasio J., Rizzoli R., Bonjour J. P. Sodium-dependent phosphate transport inhibited by parathyroid hormone and cyclic AMP stimulation in an opossum kidney cell line. J Biol Chem. 1986 Mar 5;261(7):3233–3237. [PubMed] [Google Scholar]
- Chase L. R., Aurbach G. D. Parathyroid function and the renal excretion of 3'5'-adenylic acid. Proc Natl Acad Sci U S A. 1967 Aug;58(2):518–525. doi: 10.1073/pnas.58.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson R. Metabolism and excretion of exogenous adenosine 3':5'-monophosphate and guanosine 3':5'-monophosphate. Studies in the isolated perfused rat kidney and in the intact rat. J Biol Chem. 1976 Aug 25;251(16):4958–4967. [PubMed] [Google Scholar]
- Culić O., Sabolić I., Zanić-Grubisić T. The stepwise hydrolysis of adenine nucleotides by ectoenzymes of rat renal brush-border membranes. Biochim Biophys Acta. 1990 Nov 30;1030(1):143–151. doi: 10.1016/0005-2736(90)90249-n. [DOI] [PubMed] [Google Scholar]
- Dunlay R., Hruska K. PTH receptor coupling to phospholipase C is an alternate pathway of signal transduction in bone and kidney. Am J Physiol. 1990 Feb;258(2 Pt 2):F223–F231. doi: 10.1152/ajprenal.1990.258.2.F223. [DOI] [PubMed] [Google Scholar]
- Filburn C. R., Sacktor B. Cyclic nucleotide phosphodiesterases of rabbit renal cortex. Characterization of brush border membrane activities. Arch Biochem Biophys. 1976 May;174(1):249–261. doi: 10.1016/0003-9861(76)90344-1. [DOI] [PubMed] [Google Scholar]
- Franco R., Centelles J. J., Kinne R. K. Further characterization of adenosine transport in renal brush-border membranes. Biochim Biophys Acta. 1990 May 24;1024(2):241–248. doi: 10.1016/0005-2736(90)90350-w. [DOI] [PubMed] [Google Scholar]
- Friedlander G., Amiel C. Protein kinase C activation has dissimilar effects on sodium-coupled uptakes in renal proximal tubular cells in primary culture. J Biol Chem. 1989 Mar 5;264(7):3935–3941. [PubMed] [Google Scholar]
- Friedlander G., Le Grimellec C., Sraer J., Amiel C. 12-HETE modulates Na-coupled uptakes in proximal tubular cells: role of diacylglycerol kinase inhibition. Am J Physiol. 1990 Nov;259(5 Pt 2):F816–F822. doi: 10.1152/ajprenal.1990.259.5.F816. [DOI] [PubMed] [Google Scholar]
- Gmaj P., Murer H. Cellular mechanisms of inorganic phosphate transport in kidney. Physiol Rev. 1986 Jan;66(1):36–70. doi: 10.1152/physrev.1986.66.1.36. [DOI] [PubMed] [Google Scholar]
- Insel P., Balakir R., Sacktor B. The binding of cyclic AMP to renal brush border membranes. J Cyclic Nucleotide Res. 1975;1(2):107–122. [PubMed] [Google Scholar]
- Kather H. Beta-adrenergic stimulation of adenine nucleotide catabolism and purine release in human adipocytes. J Clin Invest. 1990 Jan;85(1):106–114. doi: 10.1172/JCI114399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kather H. Pathways of purine metabolism in human adipocytes. Further evidence against a role of adenosine as an endogenous regulator of human fat cell function. J Biol Chem. 1990 Jan 5;265(1):96–102. [PubMed] [Google Scholar]
- Kuntziger H., Amiel C., Roinel N., Morel F. Effects of parathyroidectomy and cyclic AMP on renal transport of phosphate, calcium, and magnesium. Am J Physiol. 1974 Oct;227(4):905–911. doi: 10.1152/ajplegacy.1974.227.4.905. [DOI] [PubMed] [Google Scholar]
- Kübler D., Pyerin W., Bill O., Hotz A., Sonka J., Kinzel V. Evidence for ecto-protein kinase activity that phosphorylates Kemptide in a cyclic AMP-dependent mode. J Biol Chem. 1989 Aug 25;264(24):14549–14555. [PubMed] [Google Scholar]
- Küntziger H., Cailla H., Amiel C., Delaage M. Influence of parathyroid status of rats on renal tubular handling of adenosine 3'5'-monophosphate: a micropuncture study. J Cyclic Nucleotide Res. 1981;7(5):313–319. [PubMed] [Google Scholar]
- Le Hir M., Angielski S., Dubach U. C. Properties of an ecto-5'-nucleotidase of the renal brush border. Ren Physiol. 1985;8(6):321–327. doi: 10.1159/000173064. [DOI] [PubMed] [Google Scholar]
- Le Hir M., Dubach U. C. Concentrative transport of purine nucleosides in brush border vesicles of the rat kidney. Eur J Clin Invest. 1985 Jun;15(3):121–127. doi: 10.1111/j.1365-2362.1985.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Malmström K., Murer H. Parathyroid hormone inhibits phosphate transport in OK cells but not in LLC-PK1 and JTC-12.P3 cells. Am J Physiol. 1986 Jul;251(1 Pt 1):C23–C31. doi: 10.1152/ajpcell.1986.251.1.C23. [DOI] [PubMed] [Google Scholar]
- Malström K., Stange G., Murer H. Identification of proximal tubular transport functions in the established kidney cell line, OK. Biochim Biophys Acta. 1987 Aug 20;902(2):269–277. doi: 10.1016/0005-2736(87)90305-1. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Takano T., Soltoff S. P., Murdaugh S. Mechanisms whereby exogenous adenine nucleotides improve rabbit renal proximal function during and after anoxia. J Clin Invest. 1988 Apr;81(4):1255–1264. doi: 10.1172/JCI113443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel F., Doucet A. Hormonal control of kidney functions at the cell level. Physiol Rev. 1986 Apr;66(2):377–468. doi: 10.1152/physrev.1986.66.2.377. [DOI] [PubMed] [Google Scholar]
- Osswald H., Spielman W. S., Knox F. G. Mechanism of adenosine-mediated decreases in glomerular filtration rate in dogs. Circ Res. 1978 Sep;43(3):465–469. doi: 10.1161/01.res.43.3.465. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Aran J. M. Characterization of Na(+)-dependent, active nucleoside transport in rat and mouse peritoneal macrophages, a mouse macrophage cell line and normal rat kidney cells. Biochim Biophys Acta. 1990 Oct 19;1028(3):289–298. doi: 10.1016/0005-2736(90)90178-q. [DOI] [PubMed] [Google Scholar]
- Podevin R. A., Boumendil-Podevin E. F., Bujoli-Roche J., Priol C. Effects of probenecid on transport and metabolism of cyclic AMP by isolated rabbit renal tubules. Biochim Biophys Acta. 1980 Apr 17;629(1):135–142. doi: 10.1016/0304-4165(80)90272-x. [DOI] [PubMed] [Google Scholar]
- Podevin R. A., Boumendil-Podevin E. F. Inhibition by cyclic AMP and dibutyryl cyclic AMP of transport of organic acids in kidney cortex. Biochim Biophys Acta. 1975 Jan 14;375(1):106–114. doi: 10.1016/0005-2736(75)90075-9. [DOI] [PubMed] [Google Scholar]
- Samir Amer M., Kreighbaum W. E. Cyclic nucleotide phosphodiesterases: properties, activators, inhibitors, structure--activity relationships, and possible role in drug development. J Pharm Sci. 1975 Jan;64(1):1–37. doi: 10.1002/jps.2600640106. [DOI] [PubMed] [Google Scholar]
- Strewler G. J. Release of cAMP from a renal epithelial cell line. Am J Physiol. 1984 Mar;246(3 Pt 1):C224–C230. doi: 10.1152/ajpcell.1984.246.3.C224. [DOI] [PubMed] [Google Scholar]
- Trimble M. E., Coulson R. Adenosine transport in perfused rat kidney and renal cortical membrane vesicles. Am J Physiol. 1984 Jun;246(6 Pt 2):F794–F803. doi: 10.1152/ajprenal.1984.246.6.F794. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Papavassiliou F., Klöss S., Fritzsch G. Contraluminal p-aminohippurate transport in the proximal tubule of the rat kidney. VII. Specificity: cyclic nucleotides, eicosanoids. Pflugers Arch. 1991 May;418(4):360–370. doi: 10.1007/BF00550874. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M. The cell biology of ischemic renal injury. Kidney Int. 1991 Mar;39(3):476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]



