Abstract
Animals will acquire an operant task using sensory stimuli as a primary reinforcer. Many operant tasks use sensory stimuli as cues that are paired with other primary reinforcers. Recent studies have called attention to this potential confound, but there has not been a parametric assessment of the effect of stimulus variability on operant responding. We found that stimulus variability increased the amount of operant responding exhibited by mice, a phenomenon observed under both fixed- and progressive-ratio schedules.
Keywords: Operant sensation seeking, sensory stimuli, operant learning, novelty seeking
Introduction
Sensory stimuli are self-administered by numerous species, including rats, mice, and non-human primates [1, 4, 5, 7, 11, 21]. These non-drug reinforcers are unique in that they are not driven by vegetative states (such as hunger, thirst, or reproductive drive) [4], and that they are modulated by some of the same processes as psychostimulants [12, 14]. While this is an intriguing phenomenon, the fact that some stimuli are reinforcing is a potential confound when using sensory stimuli as “neutral” cues to pair with primary reinforcers in studies of operant learning or drug self-administration. Sensory stimuli have been demonstrated to enhance the self-administration of nicotine [6, 9, 19] and increase the locomotor response of psychostimulants [16, 18]. Recent reports have called attention to these synergistic effects as potentially confounding drug self-administration studies [8, 22]. The goal of the current study was to examine the effect of stimulus variability on the ability of auditory and visual stimuli to support self-administration.
Materials and Methods
Subjects
Male C57BL/6J mice (Jackson, Bar Harbor, ME; 4 weeks of age at time of experiment) were housed 4 per cage in a temperature and humidity controlled environment (lights on 1500-0300 h) within AAALAC approved Vanderbilt University Animal Care Facilities with food and water available ad libitum throughout the experiment. Mice were handled for at least three days prior to the beginning of experiments. All procedures were approved by the Vanderbilt University Animal Care and Use Committee.
Apparatus and Behavioral Procedures
Operant training chambers were as described [10, 13, 17], with levers mounted 2.2 cm above the grid floor and cue lamps (yellow LEDs) mounted 2 cm above them. At the beginning of each session, both levers were extended, and the house light and exhaust fan were turned on. Each mouse was assigned either the left or right lever as the active lever, and the side of the active lever was counterbalanced between mice within each group. Completion of the required ratio on the active lever resulted in a presentation of one type of sensory stimulus (see Table 1 for stimulus types), while presses on the inactive lever were counted but had no programmed consequence. Compound visual/auditory stimuli were as described [12, 14, 15], where our previous studies used frequency and duration parameters that were classified as group four in the present study. The stimulus types used in the present study ranged from no stimuli (type 1) to highly variable stimuli (type 5), which consisted of flashing cue lights of random frequency and an auditory stimulus provided by activation of an infusion pump located within the cubicle (no infusion was made). To maximize stimulus variability, duration of the compound stimuli was also of a value randomly chosen from a list.
Table 1.
Properties of each stimulus type. There is no programmed consequence of lever pressing with stimulus type 1. Auditory stimulus remained constant for the duration of the stimulus presentation. Frequency of light flashing was randomly chosen from four or six different values in stimulus types 4 or 5, respectively. Duration of compound stimuli was also randomly chosen from 4 or 6 different values in stimulus types 4 or 5, respectively.
| Stimulus Type | Audio Stimuli | Visual Stimuli | Frequencies (Hz) | Durations (sec) | N |
|---|---|---|---|---|---|
| 1 | No | None | None | None | 8 |
| 2 | No | 1 second on | None | None | 6 |
| 3 | Yes | Flashing | 1.25 | 2 | 9 |
| 4 | Yes | Flashing | 0.625, 1.25, 2.5, or 5 | 2, 4, 6, or 8 | 8 |
| 5 | Yes | Flashing | 0.313, 0.625, 1.25, 2.5, 5, 10 | 1, 2, 4, 6, 8 or 12 | 9 |
Data Analysis
Data were analyzed by one-way ANOVA using SigmaPlot 11 (repeated measures when appropriate). Post hoc analyses were performed using the Holms-Sidak test. In the case of unequal variances, the Kruskal-Wallis ANOVA followed by Dunn’s post hoc tests was employed. No performance criteria were imposed; therefore all mice were included in analysis.
Results
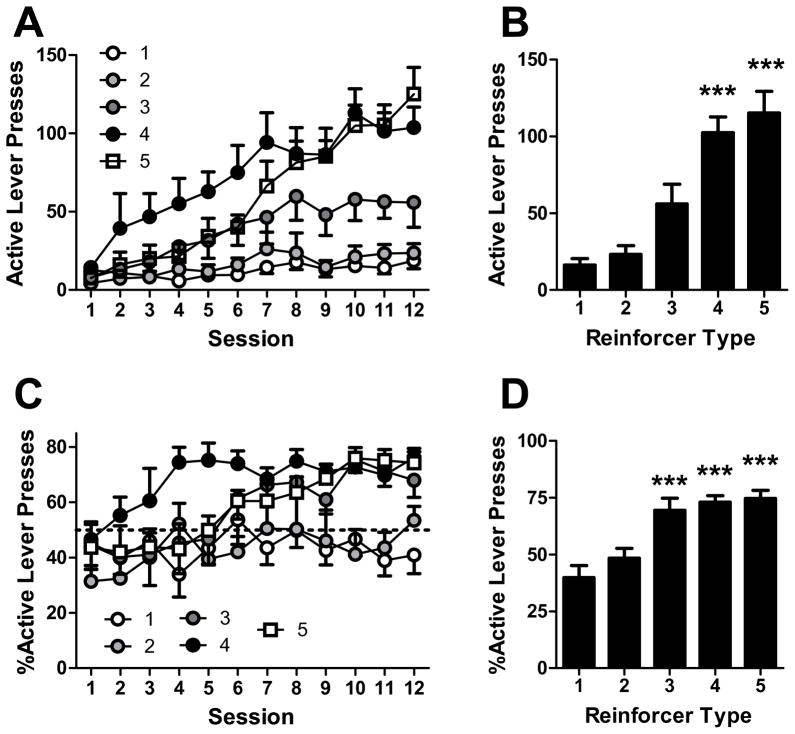
To test the effect of stimulus variability on the ability of sensory stimuli to support operant conditioning, 40 mice were assigned to one of five groups, corresponding to the stimulus type used in conditioning (Table 1). Mice were tested in 1-hour operant fixed ratio-1 (FR-1) sessions with no maximum number of reinforcers 5–6 days per week for 12 sessions (Figure 1). To assess levels of operant responding following acquisition, group means of sessions 11–12 were compared (Figure 1B). There was a significant effect of stimulus type on active lever responding (H = 21.2, p<0.001). Compared to type 1 (non-reinforced) mice, active lever pressing was significantly greater in animals responding for type 4 (Q=3.3, p<0.001) and type 5 (Q=3.7, p<0.001) stimuli (Figure 1B). Active lever preference (percent of lever presses on the active lever) following acquisition was also compared for sessions 11–12 (Figure 1D). Stimulus type had a significant effect on lever accuracy (F(4,34)=13.2, p<0.001), where accuracy was greater in stimulus types 3–5 (t=4.9–5.9, p<0.001, Figure 1D) compared to type 1. Thus, under an FR-1 schedule of reinforcement, operant responding increased with increasing stimulus variability. Further, mice had a significant preference for the active lever when visual stimuli were flashing and the auditory stimulus was present, regardless of stimulus variability. This preference was not observed when the visual stimulus was not flashing (type 2).
Figure 1.
Fixed ratio-1 responding for sensory stimuli. A. Active lever pressing during operant sessions for stimuli with different levels of variability. B. Mean of sessions 10–12 for each group in A. C. Lever accuracy for operant sessions shown in A. D. Mean lever accuracy of sessions 10–12 for each group in A. Points and bars represent mean ± SEM. N = 6–9 per group.
***p<0.001.
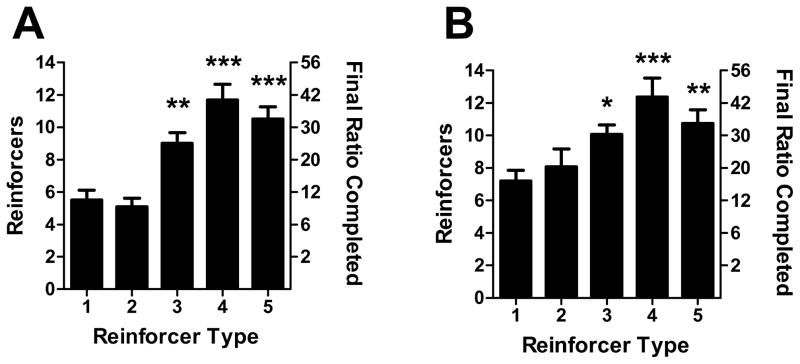
Following the 12 FR-1 sessions, mice were tested on a progressive ratio (PR) schedule of reinforcement where the ratio was increased in the following pattern: 1, 2, 4, 6, 9, 12, 16, 20, 25, 30, and so on [13, 14] (Figure 2A). Mice remained in the same stimulus group as in the FR-1 sessions and were tested in 5 daily 2-hour PR sessions. As observed with the FR-1 reinforcement schedule, analysis of the mean number of reinforcers earned for sessions 4–5 revealed a significant effect of stimulus type (F(4,34)=14.6, p<0.001). Unlike the results of FR-1, however, PR responding for type 3 stimuli was greater than for type 1 (t=3.3, p<0.01). Responding for type 4 and 5 stimuli was also significantly greater than for type 1 (t=4.9–5.9, p<0.001). Thus, under fixed and progressive ratio schedules of reinforcement, visual and auditory stimuli with greater variability elicit greater rates of operant responding.
Figure 2.
Progressive ratio responding for sensory stimuli. A. Between-groups comparison: mice were tested for progressive ratio responding for the same stimulus type as for FR-1. B. Within-groups comparison: mice that responded for type 5 stimuli in FR-1 sessions were subsequently tested for PR responding for all 5 stimulus types. Bars represent mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.
To test whether mice trained on one stimulus type remained sensitive to changes in stimulus type, mice that underwent FR-1 and PR sessions with type 5 stimuli were subsequently tested with all other stimulus types in a Latin square design. Mice were tested in PR operant sessions using each stimulus type for 4 days, and the mean number of reinforcers earned during the final 2 sessions for each stimulus type was analyzed (Figure 2B). There was a significant effect of stimulus type on operant responding within the same animals (F(4,32)=8.5, p<0.001), indicating that mice remained sensitive to the type of stimulus following extensive training on type 5 stimuli. Comparison of type 1 to all other types revealed that, like the between-groups comparison, stimulus types 3–5 all significantly increased PR responding above type 1 (t=2.9–5.1, p<0.05-0.001; Figure 2B). Therefore, after FR and PR experience with one stimulus type, mice remained sensitive to alterations in the stimuli; there was not a significant perseverance of operant responding following initial experience with type 5 stimuli.
Discussion
In conclusion, we found that stimulus dynamics contribute to the effectiveness of visual and auditory stimuli to serve as primary reinforcers in an operant learning task. Despite this finding, we did not observe a significant increase in responding for type 5 stimuli compared with the type 4 stimuli that we have previously characterized [12, 14, 15]. This may be due to a ceiling effect of sensory stimuli that can be delivered by the current apparatus (i.e. two LEDs and a single static audio source). Future studies will employ more dynamic methods of sensory stimulation to study this phenomenon. Nonetheless, the current findings are consistent with an earlier study in primates which demonstrated increased preference for dynamic movie clips over still pictures [4]. These data also reiterate the importance of evaluating sensory stimuli that are presumed neutral and paired with other primary reinforcers in operant self-administration procedures [6, 8, 19, 22]. While a simple static visual cue (type 2) did not influence lever pressing in any of our measures, a static flashing visual stimulus (type 3) significantly elevated active lever preference under an FR-1 schedule of reinforcement and significantly increased lever pressing under a PR schedule. Finally, with the emergence of in vivo optogenetic methods, the possibility of unshielded high-intensity light sources stimulating locomotor activity and/or reward-related processing must be considered when designing experiments using these methods [20].
Although sensory self-administration may be a form of novelty seeking, there is little data on how the variability (and presumably the novelty) of sensory stimuli contributes to the degree of reinforced behavior they elicit. In mice, operant responding was elevated for higher complexity line drawings [1]. In primates, the variability and complexity of visual stimuli (i.e. film clips vs. static images) were positively correlated with the degree of self-administration [4]. These data are consistent with the present findings and collectively warrant further investigations into the association between sensory self-administration and other measures of novelty seeking, such as novelty-induced place conditioning [2, 3].
Highlights.
Animals will acquire an operant task using sensory stimuli as a primary reinforcer
Stimulus variability increases self-administration on fixed and progressive ratio
Mice remain sensitive to changes in stimulus variability after training
Acknowledgments
Financial support was provided by NIH grant DA026994 (CMO). Behavioral work was performed at the Vanderbilt Murine Neurobehavioral Laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barnes GW, Baron A. Stimulus complexity and sensory reinforcement. J Comp Physiol Psychol. 1961;54:466–469. doi: 10.1037/h0046708. [DOI] [PubMed] [Google Scholar]
- 2.Besheer J, Jensen HC, Bevins RA. Dopamine antagonism in a novel-object recognition and a novel-object place conditioning preparation with rats. Behav Brain Res. 1999;103:35–44. doi: 10.1016/s0166-4328(99)00021-2. [DOI] [PubMed] [Google Scholar]
- 3.Bevins RA, Bardo MT. Conditioned increase in place preference by access to novel objects: antagonism by MK-801. Behav Brain Res. 1999;99:53–60. doi: 10.1016/s0166-4328(98)00069-2. [DOI] [PubMed] [Google Scholar]
- 4.Blatter K, Schultz W. Rewarding properties of visual stimuli. Exp Brain Res. 2006;168:541–546. doi: 10.1007/s00221-005-0114-y. [DOI] [PubMed] [Google Scholar]
- 5.Butler RA. The responsiveness of Rhesus monkeys to motion pictures. J Genet Psychol. 1961;98:239–245. doi: 10.1080/00221325.1961.10534374. [DOI] [PubMed] [Google Scholar]
- 6.Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 7.Cain ME, Green TA, Bardo MT. Environmental enrichment decreases responding for visual novelty. Behavioural Processes. 2006;73:360–366. doi: 10.1016/j.beproc.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contet C, Whisler KN, Jarrell H, Kenny PJ, Markou A. Patterns of responding differentiate intravenous nicotine self-administration from responding for a visual stimulus in C57BL/6J mice. Psychopharmacology (Berl) 2010;212:283–299. doi: 10.1007/s00213-010-1950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- 10.Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, Winder DG. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006;26:3210–3219. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marx MH, Henderson RL, Roberts CL. Positive reinforcement of the bar-pressing response by a light stimulus following dark operant pretests with no after effect. J Comp Physiol Psychol. 1955;48:73–76. doi: 10.1037/h0045062. [DOI] [PubMed] [Google Scholar]
- 12.Olsen CM, Childs DS, Stanwood GD, Winder DG. Operant sensation seeking requires metabotropic glutamate receptor 5 (mGluR5) PLoS One. 2010;5:e15085. doi: 10.1371/journal.pone.0015085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen CM, Winder DG. A method for single-session cocaine self-administration in the mouse. Psychopharmacology (Berl) 2006;187:13–21. doi: 10.1007/s00213-006-0388-1. [DOI] [PubMed] [Google Scholar]
- 14.Olsen CM, Winder DG. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology. 2009;34:1685–1694. doi: 10.1038/npp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsen CM, Winder DG. Operant sensation seeking in the mouse. J Vis Exp. 2010 doi: 10.3791/2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pum ME, Rubio AR, Carey RJ, Silva MA, Muller CP. The effects of cocaine on light-induced activity. Brain Res Bull. 2011;84:229–234. doi: 10.1016/j.brainresbull.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Schramm-Sapyta NL, Olsen CM, Winder DG. Cocaine self-administration reduces excitatory responses in the mouse nucleus accumbens shell. Neuropsychopharmacology. 2006;31:1444–1451. doi: 10.1038/sj.npp.1300918. [DOI] [PubMed] [Google Scholar]
- 18.Shin R, Cao J, Webb SM, Ikemoto S. Amphetamine administration into the ventral striatum facilitates behavioral interaction with unconditioned visual signals in rats. PLoS ONE. 2010;5:e8741. doi: 10.1371/journal.pone.0008741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorge RE, Pierre VJ, Clarke PB. Facilitation of intravenous nicotine self-administration in rats by a motivationally neutral sensory stimulus. Psychopharmacology (Berl) 2009;207:191–200. doi: 10.1007/s00213-009-1647-8. [DOI] [PubMed] [Google Scholar]
- 20.Sparta DR, Stamatakis AM, Phillips JL, Hovelso N, van Zessen R, Stuber GD. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat Protoc. 2011;7:12–23. doi: 10.1038/nprot.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart J. Reinforcing effects of light as a function of intensity and reinforcement schedule. Journal of comparative and physiological psychology. 1960;53:187–193. doi: 10.1037/h0047315. [DOI] [PubMed] [Google Scholar]
- 22.Thomsen M, Caine SB. False positive in the intravenous drug self-administration test in C57BL/6J mice. Behav Pharmacol. 2011;22:239–247. doi: 10.1097/FBP.0b013e328345f8f2. [DOI] [PMC free article] [PubMed] [Google Scholar]