Abstract
CD70 (CD27L) has been shown to be preferentially expressed on Th1, but not Th2, CD4+ lymphocytes in murine contact sensitivity. The CD70-CD27 co-stimulatory pathway as well as the Th17 subset of lymphocytes have also been identified in human contact sensitivity reactions. The authors have previously reported increased expression of CD70 and the Th17-specific transcription factor retinoid orphan receptor gamma T in the elicitation phase of allergic contact dermatitis by reverse transcriptase-polymerase chain reaction. The manipulation of these pathways has potential for ameliorating autoimmune and inflammatory disorders such as allergic contact dermatitis, psoriasis, and rheumatoid arthritis. Also, upregulation of the CD70-CD27 and Th17 pathways has been associated with the remarkable ability of topical sensitizers to treat warts and skin cancers including melanoma. As natural killer and natural killer T cells are also involved in contact sensitivity, future studies investigating the function of these cells are necessary to elucidate the transition between innate and acquired immune responses in the context of the Th1/Th2/Th17 and regulatory T cell paradigm.
CD70 (CD27L) has recently been shown to be preferentially expressed on Th1, but not Th2, CD4+ lymphocytes in murine contact sensitivity.1 The CD70-CD27 co-stimulatory pathway as well as the Th17 subset of lymphocytes have also been identified in human contact sensitivity reactions.2, 3 The authors have previously reported increased expression of CD70 and the Th17-specific transcription factor retinoid orphan receptor gamma T in the elicitation phase of allergic contact dermatitis (Figure 1, Figure 2).
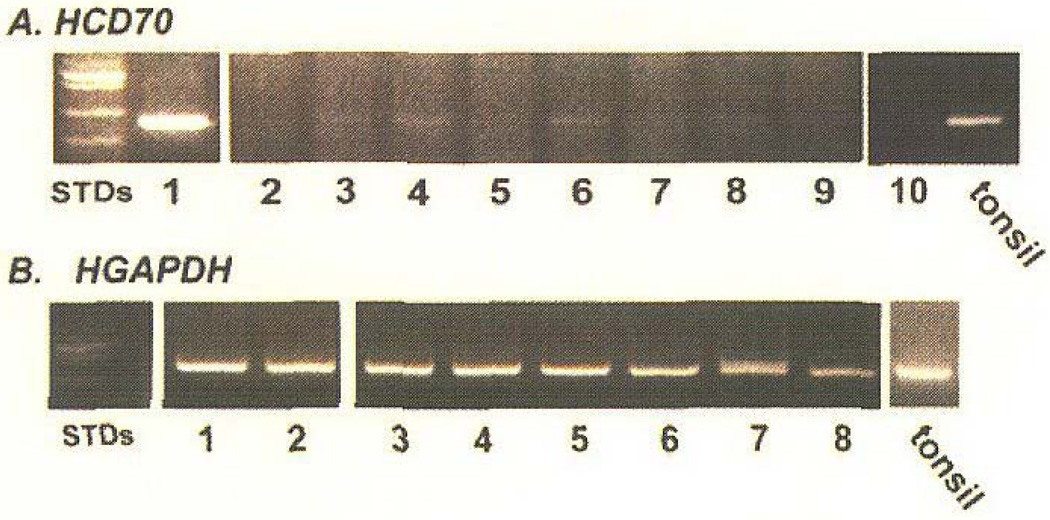
Figure 1.
Agarose gel electrophoresis of HCD70 and HGAPDH expression in contact dermatitis specimens. RNA was extracted from contact dermatitis and clinically normal skin biopsy specimens and was reverse transcribed to cDNA. Polymerase chain reaction amplicons were electrophoresed on agarose gel: a) Polymerase chain reaction amplification for HCD70 seen positive in lanes 1, 3, 4, 6, and 8 and negative in lane 9 (normal); lanes 1–9 are respectively: CD1, CD2, CD3, CD4, CD5, CD6 (24 hours), CD6 (48 hours), CD7, and normal. b) Polymerase chain reaction for HGAPDH band in lanes 1–9 of the agarose gel. Lanes 1–8, respectively: CD1, CD2, CD4, CD5, CD6 (24 hours), CD6 (48 hours), CD7, and normal. CD3 is not shown. Lane 10 is a negative control (no template DNA) and a positive control of tonsil is indicated. Reproduced, with permission, from Martiniuk et al, 2008.
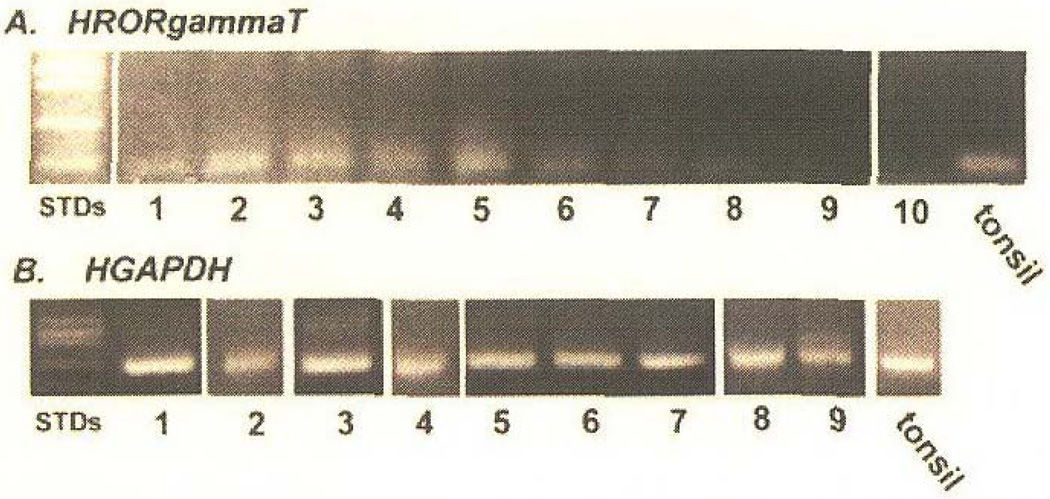
Figure 2.
Human retinoid orphan receptor gamma T (HRORgammaT) expression in contact dermatitis specimens. RNA was extracted from contact dermatitis and clinically normal skin biopsy specimens and was reverse transcribed to cDNA. Polymerase chain reaction amplicons were electrophoresed on an agarose gel: a) Polymerase chain reaction for HRORgammaT is shown positive in lanes 1–8 and negative in lane 9. Lanes 1–9, respectively: CD8, CD9, CD10, CD11, CD12, CD13, CD14, CD15, CD16, and normal. b) Polymerase chain reaction for HGAPDH in lanes 1–9 for: CD8, CD9, CD10, CD11, CD12, CD13, CD14, CD15, CD16, and normal. Lane 10 is a negative control (no template DNA) and a positive control of tonsil is indicated. Reproduced, with permission, from Martiniuk et al, 2008.
Manipulation of these pathways has potential for ameliorating autoimmune and inflammatory disorders such as allergic contact dermatitis, psoriasis, psoriatic arthritis, and rheumatoid arthritis.4 Experimental treatment of murine collagen-induced arthritis and colitis with anti-CD70 antibody therapy has proven promising.5, 6 Several biotechnology companies, including Seattle Genetics and Bristol-Myers Squibb, have already developed humanized anti-CD70 monoclonal antibodies and antibody-drug conjugates, which are currently being studied in the treatment of renal cell carcinoma and non-Hodgkin lymphoma in early-phase clinical trials. These agents also have the potential to treat autoimmune disorders.
Upregulation of the CD70-CD27 and Th17 pathways has been associated with the remarkable ability of topical sensitizers to treat warts and skin cancers including melanoma.7 CD70 and major histocompatibility complex class II localize in the late endosome and are jointly delivered to the immunological synapse at the cell surface, which is important for antigen-specific T cell activation.8 Activation of the CD70-CD27 co-stimulatory pathway has been implicated in adoptive transfer of interleukin 2-treated CD8+ T lymphocytes responsible for melanoma tumor regression.9 Byrne et al. recently reported durable CD8+ T lymphocyte immunity to melanoma in mice with vitiligo, strengthening the hypothesis that autoimmunity enhances antitumor immunity.10 Th17 cells, which have been shown to mediate destruction of advanced B16 melanoma in a transgenic mouse model,11 have been identified in the active margins of vitiligo lesions in humans.12 Given our current understanding of Th17 effector function, it is likely that this pathway enhances both neutrophil recruitment and CD8+ T cell killing activity in topical immunotherapy with contact sensitizers.
Natural killer T (NKT) cells are CD1d-restricted T cells that, in activated form, infiltrate the skin at elicitation sites of allergic contact dermatitis.13 In a murine model, activated NKT cells have been shown to be capable of inducing the expression of CD70 on dendritic cells.14 The presence or absence of CD27 differentiates two functionally distinct NK cell subsets. NK cells positive for this receptor have lower cytolytic potential than those that are negative.15 Different NKT cell subsets also have been demonstrated to have important functional distinctions, especially with regards to tumor immunosurveillance. Classical type I or invariant NKT cells protect against tumor cell growth while type II NKT cells suppress immunosurveillance.16 The role of NK and NKT cell subsets on the CD70-CD27 pathway warrants further investigation as these cells are involved in contact sensitivity.
Our understanding of contact sensitivity has increased considerably since the discovery of what became referred to as major histocompatibility complex class I-restricted killing and Th1 immunity in the 1970s and 80s.17 More recently characterized effector mechanisms, including those of Th17, NK, and NKT cells, as well as co-stimulatory pathways such as the CD70-CD27 pathway, appear to contribute to an integrated immune response to antigenic challenge that is not yet fully understood. Future studies investigating the function of more primitive cell lineages (i.e., NK and NKT cells) in contact sensitivity are necessary to elucidate the transition between innate and acquired immune responses in the context of the Th1/Th2/Th17 and regulatory T cell paradigm.
Acknowledgments
This work was supported in part by a grant from the GCRC grant-NIH M01RR00096 and by NIH MSTP grant GM07739 (N.G.).
Footnotes
Conflict of Interest: The authors state no conflict of interest.
Contributor Information
David S. Lee, Email: davidsyngyulee@gmail.com.
Nicholas Gulati, Email: nicholasgulati@gmail.com.
Frank Martiniuk, Email: Frank.Martiniuk@nyumc.org.
William R. Levis, Email: william_levis@yahoo.com.
References
- 1.Kawamura T, Ogawa Y, Shimozato O, et al. CD70 is selectively expressed on Th1 but not on Th2 cells and is required for Th1-type immune responses. J Invest Dermatol. 2011;131:1252–1261. doi: 10.1038/jid.2011.36. [DOI] [PubMed] [Google Scholar]
- 2.Martiniuk F, Lee DS, Gaspari A, et al. Expression of CD70 and the TH17 transcription factor RORgammaT in human contact dermatitis. J Drugs Dermatol. 2008;7:956–960. [PubMed] [Google Scholar]
- 3.Zhao Y, Balato A, Fishelevich R, et al. Th17/Tc17 infiltration and associated cytokine gene expression in elicitation phase of allergic contact dermatitis. Br J Dermatol. 2009;161:1301–1306. doi: 10.1111/j.1365-2133.2009.09400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jabbari A, Johnson-Huang LM, Krueger JG. Role of the immune system and immunological circuits in psoriasis. G Ital Dermatol Venereol. 2011;146:17–30. [PubMed] [Google Scholar]
- 5.Manocha M, Rietdijk S, Laouar A, et al. Blocking CD27-CD70 costimulatory pathway suppresses experimental colitis. J Immunol. 2009;183:270–276. doi: 10.4049/jimmunol.0802424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oflazoglu E, Boursalian TE, Zeng W, et al. Blocking of CD27-CD70 pathway by anti-CD70 antibody ameliorates joint disease in murine collagen-induced arthritis. J Immunol. 2009;183:3770–3777. doi: 10.4049/jimmunol.0901637. [DOI] [PubMed] [Google Scholar]
- 7.Martiniuk F, Damian DL, Thompson JF, et al. TH17 is involved in the remarkable regression of metastatic malignant melanoma to topical diphencyprone. J Drugs Dermatol. 2010;9:1368–1372. [PMC free article] [PubMed] [Google Scholar]
- 8.Keller AM, Groothuis TA, Veraar EA, et al. Costimulatory ligand CD70 is delivered to the immunological synapse by shared intracellular trafficking with MHC class II molecules. Proc Natl Acad Sci USA. 2007;104:5989–5994. doi: 10.1073/pnas.0700946104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Kerstann KW, Ahmadzadeh M, et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176:7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrne KT, Côté AL, Zhang P, et al. Autoimmune melanocyte destruction is required for robust CD8+ memory T cell responses to mouse melanoma. J Clin Invest. 2011;121:1797–1809. doi: 10.1172/JCI44849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang CQ, Cruz-Inigo AE, Fuentes-Duculan J, et al. Th17 cells and activated dendritic cells are increased in vitiligo lesions. PLoS One. 2011;6(4):e18907. doi: 10.1371/journal.pone.0018907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gober MD, Fishelevich R, Zhao Y, et al. Human natural killer T cells infiltrate into the skin at elicitation sites of allergic contact dermatitis. J Invest Dermatol. 2008;128:1460–1469. doi: 10.1038/sj.jid.5701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taraban VY, Martin S, Attfield KE, et al. Invariant NKT cells promote CD8+ cytotoxic T cell responses by inducing CD70 expression on dendritic cells. J Immunol. 2008;180:4615–4620. doi: 10.4049/jimmunol.180.7.4615. [DOI] [PubMed] [Google Scholar]
- 15.Vossen MT, Matmati M, Hertoghs KM, et al. CD27 defines phenotypically and functionally different human NK cell subsets. J Immunol. 2008;180:3739–3745. doi: 10.4049/jimmunol.180.6.3739. [DOI] [PubMed] [Google Scholar]
- 16.Ambrosino E, Terabe M, Halder RC, et al. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179:5126–5136. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- 17.Shaw S, Levis WR, Dattner AM, et al. Specificity of human cytotoxic responses to chemically modified autologous cells. Transplant Proc. 1978;10:937–941. [PubMed] [Google Scholar]