Abstract
Menstrual bleeding patterns are considered relevant indicators of reproductive health, though few studies have evaluated patterns among regularly menstruating premenopausal women. The authors evaluated self-reported bleeding patterns, incidence of spotting, and associations with reproductive hormones among 201 women in the BioCycle Study (2005–2007) with 2 consecutive cycles. Bleeding patterns were assessed by using daily questionnaires and pictograms. Marginal structural models were used to evaluate associations between endogenous hormone concentrations and subsequent total reported blood loss and bleeding length by weighted linear mixed-effects models and weighted parametric survival analysis models. Women bled for a median of 5 days (standard deviation: 1.5) during menstruation, with heavier bleeding during the first 3 days. Only 4.8% of women experienced midcycle bleeding. Increased levels of follicle-stimulating hormone (β = 0.20, 95% confidence interval: 0.13, 0.27) and progesterone (β = 0.06, 95% confidence interval: 0.03, 0.09) throughout the cycle were associated with heavier menstrual bleeding, and higher follicle-stimulating hormone levels were associated with longer menses. Bleeding duration and volume were reduced after anovulatory compared with ovulatory cycles (geometric mean blood loss: 29.6 vs. 47.2 mL; P = 0.07). Study findings suggest that detailed characterizations of bleeding patterns may provide more insight than previously thought as noninvasive markers for endocrine status in a given cycle.
Keywords: anovulation, bleeding patterns, menstrual blood loss, metrorrhagia, reproductive hormones
Menstrual bleeding patterns are considered relevant indicators of reproductive health, and changes in bleeding patterns may impact the quality of life for pre- and perimenopausal women (1). Menstruation is often irregular among women of early and late reproductive ages, but its variability among women of mid-reproductive age remains unclear (2, 3). Further, irregular bleeding patterns and midcycle bleeding may be indicative of endocrine dysfunction and uterine abnormalities, and such patterns have been associated with infertility, breast and ovarian cancers, type 2 diabetes, and cardiovascular disease (4–14). Studies of menstrual bleeding have typically been done among women taking hormonal birth control, suffering from bleeding disorders, trying to conceive, or who are perimenopausal (10, 15–21).
Variations in sex hormone levels are hypothesized to be associated with bleeding patterns, as hormones throughout the menstrual cycle influence the proliferation and shedding of the endometrial lining of the uterus (3). During the follicular phase, endometrial cells proliferate under the influence of estrogen, but following ovulation, progesterone secretion stimulates additional morphologic changes in the endometrium. After an ovulatory cycle, menstruation is most often a result of progesterone withdrawal, which induces a series of events involving vasoconstriction, cytokine changes in the endometrium, and programmed cell death (3, 22, 23). Despite these known biologic relations, few studies to our knowledge have evaluated the association between hormonal changes and bleeding patterns among healthy young women who report regular menstrual cycles and are not trying to become pregnant.
Our goal was to analyze patterns of menstrual bleeding, incidence of spotting, and associations with sex hormones among healthy premenopausal women with self-reported regular menstrual cycles. Evaluation of bleeding patterns is important for our understanding of expected bleeding patterns for identification of potentially abnormal cycles or underlying endocrine dysfunction.
MATERIALS AND METHODS
Study design
The BioCycle Study was a prospective cohort of 259 regularly menstruating, healthy female volunteers, recruited from the western New York region. A variety of recruitment methods were used including the following: advertising in clinical practices from the region and the University at Buffalo student health service, placing paid advertisements in local newspapers and other print media, using articles about the study written by the University at Buffalo news bureau, doing radio and television interviews about the study, sending notices about the study to various list serves from the region, and posting flyers at the University and throughout western New York State (mainly in Erie and Niagara counties). Additional information regarding the study population, recruitment, materials, and methods has been described previously in detail (24). In summary, healthy women aged 18–44 years had to be regularly menstruating (self-reported cycle length between 21 and 35 days for each menstrual cycle in the past 6 months) and not currently using hormonal contraception (and for the 3 months prior to study entry) to participate. Exclusion criteria included pregnancy or breastfeeding in the past 6 months; diagnosis of certain chronic conditions, including history of menstrual and ovulation disorders and uterine abnormalities, such as uterine fibroids; and a self-reported body mass index (weight (kg)/height (m)2) of <18 or >35 at screening. Of the 449 women screened, 318 met the eligibility criteria, and 276 were enrolled (9 women completed 1 cycle as part of a pilot study, 250 completed 2 cycles, and 17 withdrew). The University at Buffalo Health Sciences Institutional Review Committee approved the study and served as the internal review board designated by the National Institutes of Health for this study under a reliance agreement. All participants provided written informed consent.
Participants were followed for up to 2 menstrual cycles with blood samples collected for hormonal assessment during the following phases: second day of menstruation; mid- and late-follicular phases; luteinizing hormone (LH)/follicle-stimulating hormone (FSH) surge and predicted ovulation; and early, mid-, and late-luteal phases (approximately corresponding to days 2, 7, 12, 13, 14, 18, 22, and 27 of a 28-day cycle). Fertility monitors (Clearblue Easy Fertility Monitor; Inverness Medical, Waltham, Massachusetts) were used to determine the timing of specimen collection. These monitors measured estrone-3-glucuronide and LH in urine, starting on calendar day 6 after menses and continuing for 10–20 days, depending on whether the woman reached peak levels on the monitor. Monitor indications of low, high, and peak fertility were used to time midcycle visits, with other visits scheduled according to an algorithm that took each woman’s reported cycle length into consideration. Women were highly compliant to the study protocol, with 94% of women completing at least 7 clinic visits per cycle.
Blood flow assessment
BioCycle Study participants maintained a daily diary in which they marked whether they experienced any menstrual bleeding (yes/no). Menses length was defined as a bleeding episode that included at least 2 days of bleeding in a 3-day interval preceded by at least 2 bleed-free days, based on the World Health Organization’s definition, modified by Harlow et al. (25). Although the World Health Organization defines spotting as vaginal bleeding that does not require sanitary protection, we specified spotting based on isolated bleeding days from the daily diary, for which sanitary protection may have been used (26). Only 2% of diary days were missing information on the presence of any menstrual bleeding.
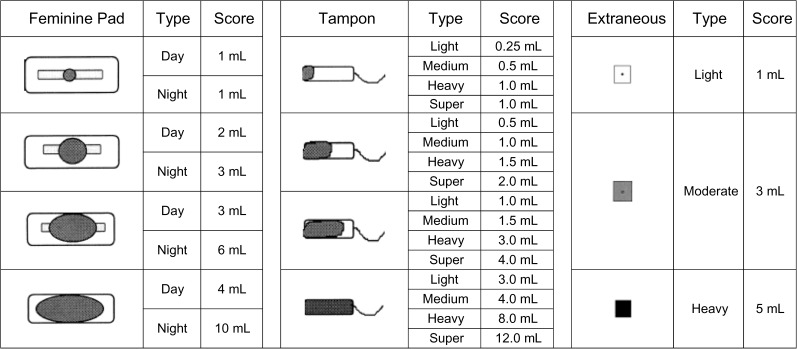
If participants reported any menstrual bleeding on a given day, they were instructed to complete a detailed follow-up menstrual flow questionnaire consisting of the pictograms shown in Figure 1 adapted from Wyatt et al. (27) (without the scales). For each bleeding day, women reported the quantity, size, and observed amount of blood loss for each feminine product used (sanitary napkins or tampons), as well as any extraneous blood loss not captured by sanitary protection. The amount of menstrual blood flow (total blood loss in mL) on each day of bleeding was estimated by using the scales shown in Figure 1, by summing the scores of each sanitary napkin or tampon used on each day. About 15% of reported bleeding days did not have a recorded menses volume in the daily diaries and were marked as missing. If a woman was missing information about either the size or number of sanitary napkins or tampons used for any reported bleeding days, then her total menstrual blood flow was not estimated and was set to missing. These pictograms represent a valid marker for estimating the amount of menstrual blood flow and are highly correlated with blood loss estimates obtained by using the alkaline hematin method (27).
Figure 1.
Assessment of menstrual blood loss by pictograms with blood loss equivalents. Adapted from Wyatt KM, Dimmock PW, Walker TJ, et al. Determination of total menstrual blood loss. Fertil Steril. 2001;76(1):125–131 (27).
Bleeding amount was measured per day and per cycle for each woman in the study. Cycle blood flow was classified in tertiles as light (≤36.5 mL), medium (>36.5 and ≤72.5 mL), or heavy (>72.5 mL). Individual bleeding days were classified in tertiles as light (≤4 mL), medium (>4 and ≤14 mL), or heavy (>14 mL) blood flow. We compared the number of light, medium, heavy, and total bleeding days per cycle, as well as the amounts of bleeding on each calendar day of the cycle.
Hormone and ovulation assessment
Reproductive hormones were measured in fasting serum blood samples collected at each cycle visit (8 visits per cycle for 2 cycles) at the Kaleida Health Center for Laboratory Medicine (Buffalo, New York). Estradiol concentrations were measured by radioimmunoassay. FSH, LH, and progesterone were measured using solid-phase competitive chemiluminescent enzymatic immunoassays by Specialty Laboratories, Inc. (Valencia, California) on the DPC Immulite 2000 analyzer (Siemens Medical Solutions Diagnostics, Deerfield, Illinois). Across the study period, the coefficients of variation for these tests were <10% for estradiol, <5% for LH and FSH, and <14% for progesterone. The limits of detection (LODs) for estrogen and progesterone were 20 pg/dL (2% < LOD) and 0.20 ng/dL (4% < LOD), respectively, and no values were below the LODs for FSH and LH. Cycles were defined as anovulatory if peak progesterone concentrations were ≤5 ng/mL on any given day during the cycle and no serum LH peak was observed during the mid- or late-luteal phase (42 of 509 cycles (8.3%)) (28).
Covariate assessment
At study enrollment, body mass index was obtained by using standardized protocols by trained study staff, while age, race, smoking, and reproductive history were obtained by using validated questionnaires (24). Participants completed questionnaires regarding lifestyle and health history, as well as physical activity using the International Physical Activity Questionnaire (IPAQ) (29), with high, moderate, and low physical activity categories formed on the basis of standard Questionnaire cutoffs. Cycle length was defined as the number of days from the first day of bleeding (menstruating by 4 PM) until the day before the next onset of bleeding. All of the covariates assessed had at least a 95% response rate.
Statistical analysis
Of the 259 women in the BioCycle Study, 250 women recorded complete information on duration and amount of bleeding for 470 cycles (220 women for 2 cycles, 30 women for 1 cycle). Analysis of demographics and bleeding patterns was based on data from all 470 cycles with bleeding information. For analysis of the associations between hormone levels and bleeding patterns in the subsequent cycle, we further restricted our sample to women with 2 consecutive cycles (n = 201 women).
Descriptive statistics were calculated for demographic characteristics. Exact chi-square tests and analysis of variance were used to test for associations between demographic variables and tertiles of total menstrual blood loss per cycle. Alternative cutoffs were explored to assess sensitivity of results to this categorization. Geometric mean blood loss was calculated overall and for cycles classified as light, medium, and heavy bleeding. The duration and amount of blood flow were compared following ovulatory and anovulatory cycles. We calculated the percentage of women who experienced a midcycle bleeding episode (here defined as “spotters”). The median number of bleed days, cycle length, and ovulatory status were compared between spotting and nonspotting cycles. P values are based on geometric mean values and take repeated measures into account.
As hormone levels are hypothesized to influence bleeding patterns, we evaluated the association between hormone levels and menstrual blood loss as shown in Web Figure 1, posted on the Journal’s website (http://aje.oxfordjournals.org/).
Marginal structural models with inverse-probability-of-exposure weights were applied to estimate the effects of hormones on menstrual bleeding amount and length (30, 31). Marginal structural models were used to appropriately account for the time-varying confounding factors affected by prior exposure. Specifically, because estrogen levels change over the cycle in response to complex feedback mechanisms with other hormones, traditional adjustment for LH, FSH, and progesterone is inadequate.
Linear mixed models with inverse-probability-of-exposure weights were used to estimate the parameters of the final marginal structural model to evaluate the associations between log hormone concentrations throughout the cycle and total blood loss (on the log scale). Random-intercept models were used to account for the variation in baseline hormone concentrations between women and the correlation between visits of the same woman. As menses length can be considered as time-to-event data (time to end of bleeding), parametric survival models with inverse-probability-of-exposure weights were applied to model the associations between log hormone concentrations and time to end of menstrual bleeding. The parametric distribution of the random disturbance was modeled as a Weibull distribution, and no censoring was observed in the data.
Stabilized inverse-probability-of-exposure weights for estradiol were calculated for each individual at each cycle visit (8 total per cycle) by using the conditional density of estradiol obtained by ordinary least-squares regression and estimated by the normal distribution to account for the transient effects of other reproductive hormones at each visit (30, 31). The predicted probability for each woman was included as a weight term in the linear mixed and survival models. All models were adjusted for age, race, and body mass index (32–34), as well as for the time-varying hormone levels FSH, LH, and progesterone and past measurements of estradiol (30, 31). Weights for the other hormones were calculated in a similar manner. We further evaluated whether these effects were modified by age, race, and body mass index. It should be noted that parity and marital status were highly correlated with age and, thus, we adjusted only for age in the models.
In addition, linear mixed models were used to compare hormone concentrations during each cycle phase between spotters and nonspotters. Sensitivity analysis using multiple imputation was used to assess the impact of missing diary information of bleeding patterns on study findings. SAS, version 9.2, statistical software (SAS Institute, Inc., Cary, North Carolina) was used for all statistical analyses.
RESULTS
Demographics
Overall, women with complete bleeding information (n = 470 cycles) were young (mean age: 27.7 years), physically active (moderate to high physical activity: 90.6%), and nonsmokers (95.7%), as well as of healthy weight (mean body mass index: 24.1) (Table 1). Menstrual blood loss varied significantly according to age, marital status, and parity, with older, married, and parous women more likely to report heavier bleeding. In addition, menstrual blood loss varied significantly by age at menarche with light bleeding associated with a later age at menarche. Body mass index, cycle length, and physical activity were not significantly associated with bleeding amount.
Table 1.
Demographic Characteristics of Women Participating in the BioCycle Study (250 Women, 470 Cycles) by Volume of Menstrual Blood Loss per Cycle, Buffalo, New York, 2005–2007a
| Menstrual Blood Loss by Tertile of Menstrual Flow |
P Valueb | ||||||||||||
| Total Cohort |
Light (≤36.5 mL) |
Medium (>36.5–≤72.5 mL) |
Heavy (>72.5 mL) |
||||||||||
| Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | ||
| Age, years | 27.7 (8.3) | 25.6 (7.0) | 28.5 (9.0) | 29.1 (8.4) | 0.0005 | ||||||||
| Age at menarche, years | 12.6 (1.2) | 12.9 (1.1) | 12.5 (1.3) | 12.5 (1.2) | 0.02 | ||||||||
| Body mass indexc | 24.1 (3.9) | 23.8 (4.0) | 23.9 (3.7) | 24.5 (4.0) | 0.2 | ||||||||
| Cycle length, days | 28.8 (4.1) | 29.1 (3.8) | 28.7 (4.0) | 28.6 (4.6) | 0.6 | ||||||||
| Race | |||||||||||||
| White | 285 | 61 | 89 | 57 | 95 | 59 | 101 | 66 | 0.3 | ||||
| Black | 87 | 19 | 25 | 16 | 33 | 21 | 29 | 19 | |||||
| Other | 98 | 21 | 42 | 27 | 32 | 20 | 24 | 16 | |||||
| High school education or less | 60 | 13 | 17 | 11 | 22 | 14 | 21 | 14 | 0.7 | ||||
| Married | 126 | 27 | 29 | 19 | 39 | 24 | 58 | 38 | 0.009 | ||||
| Nulliparous | 331 | 72 | 119 | 80 | 112 | 71 | 100 | 66 | 0.09 | ||||
| Current smoker | 20 | 4 | 6 | 4 | 8 | 5 | 6 | 4 | 0.9 | ||||
| Physical activity | |||||||||||||
| Low | 44 | 9 | 19 | 12 | 13 | 8 | 12 | 8 | 0.9 | ||||
| Moderate | 166 | 35 | 48 | 31 | 61 | 38 | 57 | 37 | |||||
| High | 260 | 55 | 89 | 57 | 86 | 54 | 85 | 55 | |||||
| Ever sexually active | 355 | 77 | 105 | 70 | 123 | 78 | 127 | 83 | 0.10 | ||||
| Currently sexually active | 252 | 71 | 74 | 71 | 84 | 68 | 94 | 74 | 0.7 | ||||
| Past oral contraceptive use | 261 | 56 | 83 | 55 | 84 | 53 | 94 | 61 | 0.4 | ||||
Abbreviation: SD, standard deviation.
Of the 259 women in the BioCycle Study, 250 reported bleeding information in the daily diary: 30 reported bleeding for 1 cycle, and 220 reported information for 2 cycles for a total of 470 cycles in the overall cohort. By tertile of menstrual flow, there were 156 cycles for light, 160 cycles for medium, and 154 cycles for heavy menstrual blood loss.
Two-sided P values for continuous variables were calculated by repeated-measures analysis of variance and for categorical variables by Fisher’s exact test. All comparisons take repeated measures and correlations between cycles into account.
Body mass index: weight (kg)/height (m)2.
Bleeding patterns and anovulation
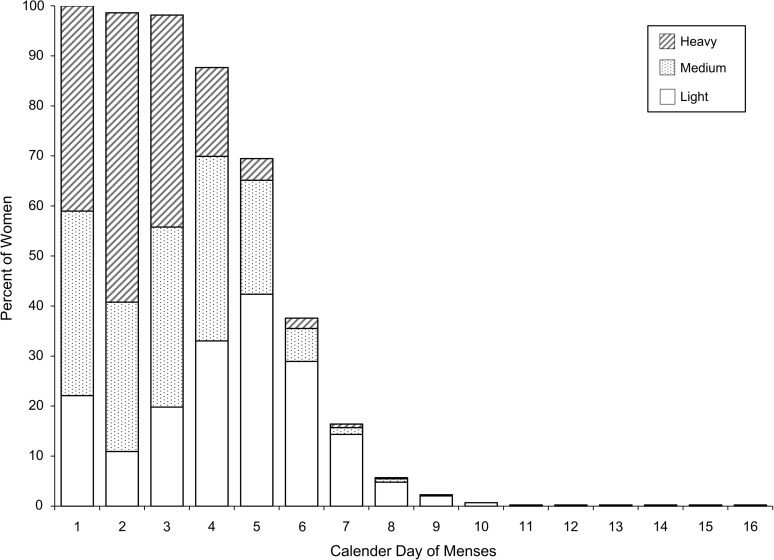
Women bled for a median of 5 days (mode: 5 days; range: 1–16), and the median number of light, medium, and heavy days was approximately 2 days each (Table 2). During most cycles, women reported the heaviest bleeding on the second day of menstruation (Figure 2).
Table 2.
Overall Bleeding Patterns and Bleeding Patterns Following an Ovulatory or Anovulatory Cycle Categorized by Volume of Menstrual Blood Loss, BioCycle Study, Buffalo, New York, 2005–2007
| No. of Cycles | Menstrual Blood Loss per Cyclea |
||||
| Overall, mL | Light (≤36.5 mL) | Medium (>36.5–≤72.5 mL) | Heavy (>72.5 mL) | ||
| All cycles | 470 | 45.5 (57.5) | 15.2 (16.4) | 54.4 (16.3) | 114.4 (51.0) |
| Following an anovulatory cycle | 23 | 29.6 (95.0)b | 6.8 (10.5) | N/A | 107.4 (60.8) |
| Following an ovulatory cycle | 212 | 47.2 (62.0)b | 16.1 (13.0) | 55.1 (17.5) | 113.0 (47) |
Abbreviations: IQR, interquartile range; N/A, not available (no women fell into this category of menstrual blood loss following an anovulatory cycle).
Results are presented as the geometric mean (IQR).
Menstrual blood loss following an ovulatory versus anovulatory cycle: P = 0.070.
Figure 2.
Percent of women categorized by light, medium, or heavy bleeding per calendar day of menses (470 cycles), BioCycle Study, Buffalo, New York, 2005–2007. Median bleeding days (menses length) was 5 (interquartile range, 2), and median light/medium/heavy days was 2.
Higher geometric mean blood loss (47.2 mL vs. 29.6 mL; P = 0.070) and longer menses length (5.4 days vs. 4.5 days; P = 0.025) were observed following ovulatory cycles, as compared with anovulatory cycles.
Incidence of spotting
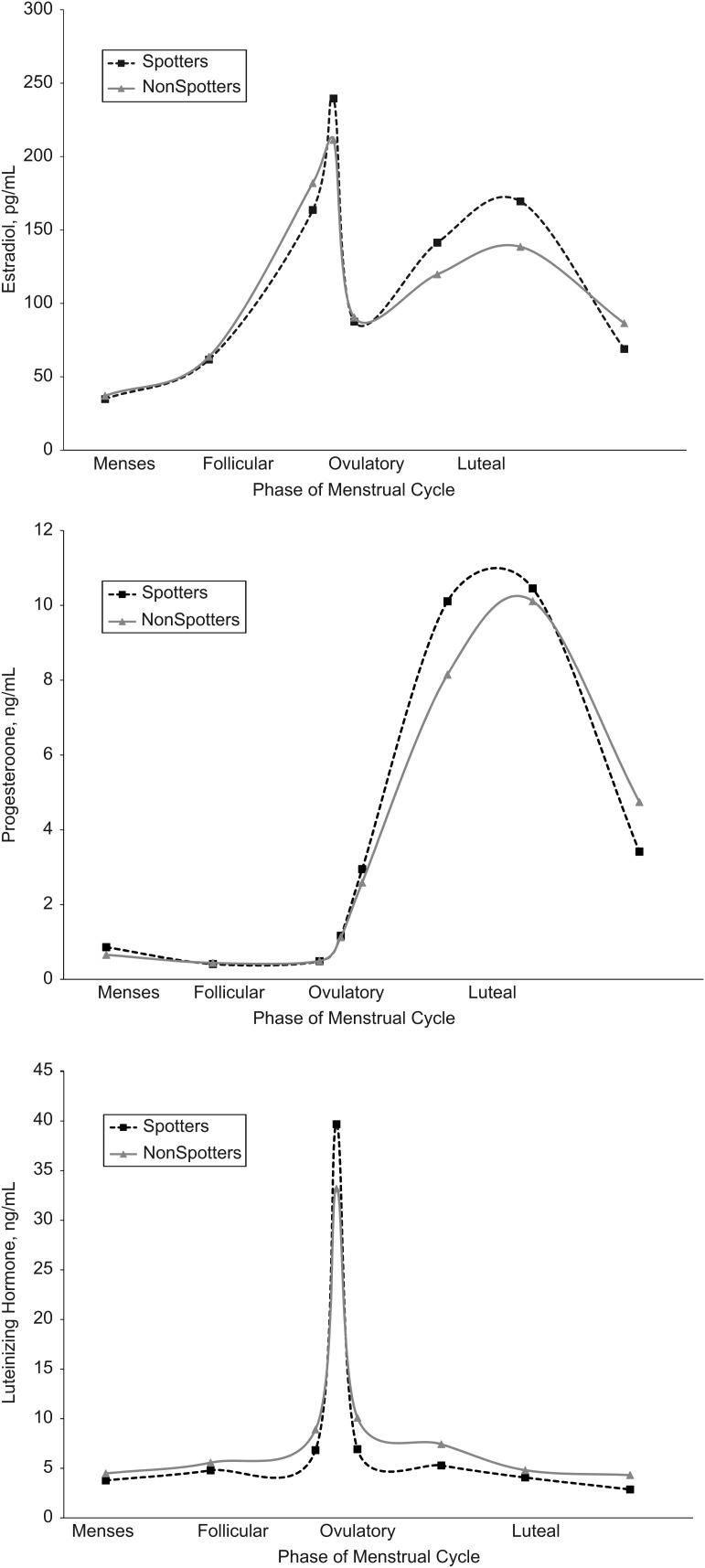
Of the 470 cycles with complete menstrual bleeding, only 13 episodes of midcycle bleeding (2.8% of cycles) were reported among 12 women (4.8%), with spotting episodes lasting 1 or 2 days (median: 1 day) (Table 3). Most cases involved a single incidence of spotting (11 of 12 women), with 1 woman experiencing 2 episodes across the 2 cycles under study. None of the women who experienced spotting were anovulatory, and the median menses and cycle lengths were not significantly different between spotting and nonspotting cycles. Higher estrogen levels around ovulation and during the luteal phase (P < 0.10), higher luteal progesterone levels (P < 0.05), and higher LH levels around ovulation (P < 0.05) were observed among women who reported spotting (Figure 3).
Table 3.
Cycle Characteristics by Women Who Experienced Spotting, BioCycle Study, Buffalo, New York, 2005–2007a
| Cycles |
Women |
Median Bleeding Length, days (IQR) | Median Cycle Length, days (IQR) | |||
| No. | % | No. | % | |||
| Total cohort | 470 | 100 | 250 | 100 | 5 (2) | 28 (5) |
| Spotters | 13 | 2.8 | 12 | 4.8 | 6 (2)b | 27 (7)c |
| Nonspotters | 457 | 97.2 | 238 | 95.2 | 5 (1) | 28 (4) |
Abbreviation: IQR, interquartile range.
Women with a midcycle bleeding episode were defined as “spotters.”
Spotting cycles versus nonspotting cycles: P = 0.6.
Spotting cycles vs. nonspotting cycles: P = 0.9.
Figure 3.
Mean concentrations of estradiol (top), progesterone (middle), and luteinizing hormone (bottom) across the menstrual cycle by spotting status (defined as a midcycle bleeding episode), BioCycle Study, Buffalo, New York, 2005–2007.
Menstrual hormones
Increased concentrations of FSH and progesterone throughout the cycle were associated with increased blood loss (Table 4). In the fully adjusted model, for every log-unit (mIU/mL) increase in FSH (we observed an average change of 0.8 log-units over the menstrual cycle), menstrual blood loss increased by approximately 22% (β = 0.202, 95% confidence interval (CI): 0.130, 0.274). We further observed that this effect was modified by age, such that increases in FSH were associated only with increased blood loss among women less than 30 years of age (<30 years: β = 0.311, 95% CI: 0.222, 0.401; ≥30 years: β = −0.002, 95% CI: −0.121, 0.117). Similarly, for every 1 log-unit (pg/mL) increase in progesterone (an increase of about 2 log-units was observed between the follicular and luteal phases), menstrual blood loss increased by approximately 6% (β = 0.057, 95% CI: 0.023, 0.092). The progesterone effect was not modified by age. No associations were observed between estrogen or LH concentrations and menstrual blood loss, and no other effect modification was found. Increases in FSH were also significantly associated with increased length of menstrual bleeding (Table 4). Specifically, for every log-unit increase in FSH, menses length increased by 3% (survival time ratio = 1.03, 95% CI: 1.01, 1.05). No other associations were observed between hormones and menses length. In addition, similar inferences were observed when menstrual blood loss was treated as a categorical variable, when analysis was restricted to ovulatory cycles, and when hormones were analyzed at specific cycle phases (follicular phase for estrogen, luteal phase for progesterone, follicular phase and ovulation for FSH, and ovulation for LH) (data not shown).
Table 4.
Associations Between Serum Concentrations of Menstrual Hormones and Volume and Length of Menstrual Bleeding, BioCycle Study, Buffalo, New York, 2005–2007a
| Log Hormone and Model | Menstrual Blood Loss, mL |
Menses Length, days |
||
| β | 95% CI | β | 95% CI | |
| Estrogen, pg/mL | ||||
| Model 1 | 0.013 | −0.047, 0.074 | −0.013 | −0.028, 0.003 |
| Model 2 | 0.001 | −0.058, 0.061 | −0.014 | −0.029, 0.001* |
| Model 3 | −0.025 | −0.087, 0.037 | −0.003 | −0.019, 0.012 |
| Progesterone, ng/mL | ||||
| Model 1 | 0.030 | −0.004, 0.064* | 0.0001 | −0.009, 0.009 |
| Model 2 | 0.034 | 0.0005, 0.067** | 0.0008 | −0.008, 0.009 |
| Model 3 | 0.057 | 0.023, 0.092** | 0.0004 | −0.008, 0.009 |
| FSH, mIU/mL | ||||
| Model 1 | 0.124 | 0.045, 0.203** | 0.030 | 0.010, 0.050** |
| Model 2 | 0.083 | 0.003, 0.162** | 0.029 | 0.009, 0.049** |
| Model 3 | 0.202 | 0.130, 0.274*** | 0.028 | 0.008, 0.048** |
| LH, ng/mL | ||||
| Model 1 | 0.036 | −0.019, 0.090 | 0.016 | 0.001, 0.030** |
| Model 2 | 0.048 | −0.006, 0.102* | 0.015 | 0.001, 0.029** |
| Model 3 | 0.040 | −0.019, 0.099 | −0.007 | −0.023, 0.009 |
Abbreviations: β, effect estimate; CI, confidence interval; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
*P < 0.10; **P < 0.05; ***P < 0.0001.
Model 1: unadjusted. Model 2: adjusted for age, race, and body mass index. Model 3: adjusted for age, race, body mass index, and other reproductive hormones through the use of inverse-probability-of-exposure weights. Models for menstrual blood loss were obtained by using weighted linear mixed models and for menses length by using weighted parametric survival analysis models.
DISCUSSION
This study empirically evaluated menstrual bleeding patterns and their associations with hormone levels among healthy young women not trying to conceive and with self-reported regular menstrual cycles. Women bled for a median of 5 days during menstruation, with the first 3 days typically reported as heavier bleeding. Anovulatory cycles were followed by decreased blood loss and shorter menses length, while higher FSH and progesterone levels were associated with heavier menstrual bleeding. In addition, only a small number of women (4.8%) exhibited spotting, and these women had higher peak LH levels and higher levels of estrogen and progesterone during the luteal phase. The observed associations among blood loss, spotting, hormone levels, and anovulation are useful for defining bleeding patterns among regularly menstruating women. Given that hormones affect menstrual bleeding in the following cycle, this paper highlights the importance and utility of capturing bleeding patterns following hormone assessment to fully characterize a given ovarian and menstrual cycle.
The association between lighter bleeding and anovulation has been noted in several observational studies among perimenopausal women (35, 36). In a small study that measured blood loss by using the hemoglobin content of menstrual fluid, menstrual blood loss after ovulatory cycles was greater than blood loss after anovulatory cycles (mid-reproductive age group: 30 mL vs. 21 mL, n = 21; late-menopausal transition group: 64 mL vs. 12 mL, n = 23). The observed median menstrual blood loss after ovulatory cycles was larger than the blood loss reported in our study (geometric mean: 47 mL), although the median blood loss after an anovulatory cycle was smaller than what we observed (geometric mean: 30 mL). This difference could be a result of the late-menopausal status of the women as compared with the premenopausal women in our study. Another study reported that women (aged 42–52 years) with heavy menstruation were more likely to have had an ovulatory cycle compared with anovulatory (19).
Only one study to our knowledge has evaluated the association between menstrual blood loss and sex hormones. Van Voorhis et al. (19) observed an association between elevated baseline FSH levels and heavy menses in women approaching menopause, while we found increased FSH levels associated with heavier bleeding only among the younger women in our study. The observed association between progesterone levels and menstrual blood loss is consistent with results from previous studies of ovulation and bleeding patterns and with our knowledge of changes in the endometrium following ovulation, although prior studies did not evaluate this association directly. As previously mentioned, after ovulation, a rise in progesterone levels stimulates secretory changes in the endometrium that could be attributed to ovulation itself (3). The endometrium achieves full maturity after these morphologic changes and may be thicker. It is possible that these postovulatory changes in the endometrium could result in a higher amount of menstrual blood loss than if the woman was anovulatory, thus giving insight into the biologic mechanism of the effect of ovulation and progesterone on menstrual bleeding. Interestingly, orally or intrauterine-administered progestins (i.e., levonorgestrel) are often prescribed to lower menstrual blood loss (37) and, like many other oral contraceptives, work to prevent pregnancy by inhibiting ovulation. Thus, the decreased blood loss in response to exogenous progesterone is likely due to inhibition of ovulation, which is consistent with our findings. In addition, the influence of FSH on menstrual bleeding could be rooted in the mechanism that threshold levels of FSH cause an LH surge, resulting in ovulation (3). Thus, hormones associated with ovulation and subsequent changes to the endometrium caused by ovulation could be associated with the amount of menstrual bleeding. Increasing levels of FSH were not associated with higher blood loss among older women, possibly because as women age, follicular resistance to FSH increases and, thus, higher levels are needed to initiate feedback mechanisms and compensate for follicular resistance (38). Future studies are needed to clarify the role of endogenous hormones, especially those associated with ovulation, in the amount and duration of menstrual bleeding.
The incidence of spotting observed in this study was much lower than that reported in previous research. This could be due in part to differences in study population characteristics and differences in data collection. Women in the BioCycle Study self-reported as having regular menstrual cycles and, thus, women who had spotting may have considered themselves “irregular” and not enrolled in the study. A previous study on time to pregnancy observed that 8% of women aged 18–40 years who were trying to conceive experienced spotting during the first recorded cycle (n = 74) (39). Demographic and bleeding characteristics were similar to those of the women in the BioCycle Study, except that women in the time-to-pregnancy study joined the study with the intention of becoming pregnant. An additional study observed that 13.3% of women aged 21–40 years reported midcycle bleeding over the past year on the basis of a single reproductive questionnaire (n = 3,941) (9). The analysis was restricted to women not taking oral contraceptives at the time of the questionnaire, but it did not specify use during the past 12 months. Thus, intermenstrual bleeding could have been more prevalent in this cohort because spotting in the past year reflected breakthrough bleeding caused by oral contraceptives. Further research is needed to determine the incidence of midcycle bleeding among healthy women.
Our work exhibited several strengths over those of previous studies. First, we used validated questionnaires to objectively measure the volume of menstrual blood flow among regularly menstruating women. Second, temporality was preserved as we compared levels of sex hormones in a given cycle with menstrual blood loss and menses length in the following cycle. Also, this is the first study to evaluate menses length through survival analysis, with time to end of menstruation as the outcome, which provides a novel approach for evaluating time in this study. Overall, daily diary compliance was high, strengthening the quality of collected data. Further, intensive monitoring of a large number of young, ethnically diverse women throughout 2 menstrual cycles, with multiple clinic visits timed with fertility monitors, was a significant improvement over monitoring in previous studies (36). The prospective design and exclusion criteria at baseline of the BioCycle Study strengthen the ability to draw inference, having reduced the potential for bias from known risk factors for anovulation and abnormal uterine function or structure.
Nevertheless, the study faced several limitations, including the absence of daily transvaginal ultrasounds to confirm ovulation. In addition, women were followed for only 2 menstrual cycles. Only a very small number of women experienced midcycle bleeding (n = 12), which limited our comparisons of the hormonal profile between spotters and nonspotters. The lack of episodes with midcycle bleeding could be an issue of missing data or a true low incidence of spotting in this population despite high compliance with filling out the daily diary. We did observe that women with incomplete data were younger on average than those who consistently recorded bleeding information (23 vs. 28 years) and were less likely to have used oral contraceptives and to have children. In a sensitivity analysis, we used multiple imputation to address missing data for menstrual blood loss and menses length. Assuming that the women with missing bleeding data were missing at random (i.e., missing data depends on observed covariates but not on the unobserved data), the results obtained after imputation were similar to the results obtained with the complete case analysis (Web Table 1, posted on the Journal’s website (http://aje.oxfordjournals.org/)).
In conclusion, increased duration and volume of menstrual bleeding were associated with increased FSH concentrations and ovulatory cycles among healthy women of reproductive age. Our findings provide a baseline for women and medical professionals when considering abnormalities in bleeding patterns in regularly menstruating women and suggest that detailed characterizations of bleeding patterns may provide noninvasive markers for endocrine status in a given cycle, which could be associated with other outcomes (11, 12, 40). Further studies are needed to confirm these findings. Given that, biologically, hormones affect menstruation in the following cycle, future studies should collect menstrual cycle information through the end of bleeding in the next cycle to allow for more complete comparisons between sex hormones and menstrual blood loss.
Acknowledgments
Author affiliations: Epidemiology Branch, Division of Epidemiology, Statistics, and Prevention Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Rockville, Maryland (Sonya S. Dasharathy, Sunni L. Mumford, Anna Z. Pollack, Neil J. Perkins, Donald R. Mattison, Enrique F. Schisterman); and Department of Social and Preventive Medicine, University at Buffalo, The State University of New York, Buffalo, New York (Jean Wactawski-Wende).
This study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
The authors are indebted to all the investigators and staff at the Epidemiology Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the University at Buffalo for their respective roles in the study, their dedication and effort, and assistance in study implementation. The authors would like to thank the BioCycle Working Group for their helpful suggestions.
Results have been published in abstract form for the 24th Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research, Montreal, Canada, June 20–21, 2011.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
- LOD
limit of detection
References
- 1.Rebar RW. Evaluation of amenorrhea, anovulation, and abnormal bleeding. Chap 4. In: Rebar RW, editor. Female Reproductive Endocrinology. South Dartmouth, MA: MDText.com, Inc; 2010. ( http://www.endotext.org/female/female4/femaleframe4.htm) [Google Scholar]
- 2.Chiazze L, Jr, Brayer FT, Macisco JJ, Jr, et al. The length and variability of the human menstrual cycle. JAMA. 1968;203(6):377–380. [PubMed] [Google Scholar]
- 3.Nussey S, Whitehead S. Endocrinology: An Integrated Approach. Oxford, United Kingdom: BIOS Scientific Publishers, Limited; 2001. [PubMed] [Google Scholar]
- 4.Garland M, Hunter DJ, Colditz GA, et al. Menstrual cycle characteristics and history of ovulatory infertility in relation to breast cancer risk in a large cohort of US women. Am J Epidemiol. 1998;147(7):636–643. doi: 10.1093/oxfordjournals.aje.a009504. [DOI] [PubMed] [Google Scholar]
- 5.Jensen TK, Scheike T, Keiding N, et al. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology. 1999;10(4):422–428. doi: 10.1097/00001648-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 6.La Vecchia C, Decarli A, Franceschi S, et al. Menstrual and reproductive factors and the risk of myocardial infarction in women under fifty-five years of age. Am J Obstet Gynecol. 1987;157(5):1108–1112. doi: 10.1016/s0002-9378(87)80271-5. [DOI] [PubMed] [Google Scholar]
- 7.Michels-Blanck H, Byers T, Mokdad AH, et al. Menstrual patterns and breast cancer mortality in a large U.S. cohort. Epidemiology. 1996;7(5):543–546. [PubMed] [Google Scholar]
- 8.Parazzini F, La Vecchia C, Negri E, et al. Menstrual factors and the risk of epithelial ovarian cancer. J Clin Epidemiol. 1989;42(5):443–448. doi: 10.1016/0895-4356(89)90134-0. [DOI] [PubMed] [Google Scholar]
- 9.Rowland AS, Baird DD, Long S, et al. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13(6):668–674. doi: 10.1097/00001648-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Small CM, Manatunga AK, Klein M, et al. Menstrual cycle characteristics: associations with fertility and spontaneous abortion. Epidemiology. 2006;17(1):52–60. doi: 10.1097/01.ede.0000190540.95748.e6. [DOI] [PubMed] [Google Scholar]
- 11.Solomon CG, Hu FB, Dunaif A, et al. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA. 2001;286(19):2421–2426. doi: 10.1001/jama.286.19.2421. [DOI] [PubMed] [Google Scholar]
- 12.Solomon CG, Hu FB, Dunaif A, et al. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002;87(5):2013–2017. doi: 10.1210/jcem.87.5.8471. [DOI] [PubMed] [Google Scholar]
- 13.Tavani A, Ricci E, La Vecchia C, et al. Influence of menstrual and reproductive factors on ovarian cancer risk in women with and without family history of breast or ovarian cancer. Int J Epidemiol. 2000;29(5):799–802. doi: 10.1093/ije/29.5.799. [DOI] [PubMed] [Google Scholar]
- 14.Whelan EA, Sandler DP, Root JL, et al. Menstrual cycle patterns and risk of breast cancer. Am J Epidemiol. 1994;140(12):1081–1090. doi: 10.1093/oxfordjournals.aje.a117208. [DOI] [PubMed] [Google Scholar]
- 15.Belsey EM, Machin D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development, and Research Training in Human Reproduction. Contraception. 1986;34(3):253–260. doi: 10.1016/0010-7824(86)90006-5. [DOI] [PubMed] [Google Scholar]
- 16.Belsey EM. The association between vaginal bleeding patterns and reasons for discontinuation of contraceptive use. Contraception. 1988;38(2):207–225. doi: 10.1016/0010-7824(88)90039-x. [DOI] [PubMed] [Google Scholar]
- 17.Cole LA, Ladner DG, Byrn FW. The normal variabilities of the menstrual cycle. Fertil Steril. 2009;91(2):522–527. doi: 10.1016/j.fertnstert.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 18.Hale GE, Manconi F, Luscombe G, et al. Quantitative measurements of menstrual blood loss in ovulatory and anovulatory cycles in middle- and late-reproductive age and the menopausal transition. Obstet Gynecol. 2010;115(2 pt 1):249–256. doi: 10.1097/AOG.0b013e3181ca4b3a. [DOI] [PubMed] [Google Scholar]
- 19.Van Voorhis BJ, Santoro N, Harlow S, et al. The relationship of bleeding patterns to daily reproductive hormones in women approaching menopause. Obstet Gynecol. 2008;112(1):101–108. doi: 10.1097/AOG.0b013e31817d452b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livingstone M, Fraser IS. Mechanisms of abnormal uterine bleeding. Hum Reprod Update. 2002;8(1):60–67. doi: 10.1093/humupd/8.1.60. [DOI] [PubMed] [Google Scholar]
- 21.Kolstad HA, Bonde JP, Hjøllund NH, et al. Menstrual cycle pattern and fertility: a prospective follow-up study of pregnancy and early embryonal loss in 295 couples who were planning their first pregnancy. Fertil Steril. 1999;71(3):490–496. doi: 10.1016/s0015-0282(98)00474-9. [DOI] [PubMed] [Google Scholar]
- 22.Jabbour HN, Kelly RW, Fraser HM, et al. Endocrine regulation of menstruation. Endocr Rev. 2006;27(1):17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- 23.Maybin JA, Critchley HO, Jabbour HN. Inflammatory pathways in endometrial disorders. Mol Cell Endocrinol. 2011;335(1):42–51. doi: 10.1016/j.mce.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Wactawski-Wende J, Schisterman EF, Hovey KM, et al. BioCycle Study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23(2):171–184. doi: 10.1111/j.1365-3016.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harlow SD, Lin X, Ho MJ. Analysis of menstrual diary data across the reproductive life span: applicability of the bipartite model approach and the importance of within-woman variance. J Clin Epidemiol. 2000;53(7):722–733. doi: 10.1016/s0895-4356(99)00202-4. [DOI] [PubMed] [Google Scholar]
- 26.Belsey EM, Farley TM. The analysis of menstrual bleeding patterns: a review. Contraception. 1988;38(2):129–156. doi: 10.1016/0010-7824(88)90035-2. [DOI] [PubMed] [Google Scholar]
- 27.Wyatt KM, Dimmock PW, Walker TJ, et al. Determination of total menstrual blood loss. Fertil Steril. 2001;76(1):125–131. doi: 10.1016/s0015-0282(01)01847-7. [DOI] [PubMed] [Google Scholar]
- 28.Gaskins AJ, Mumford SL, Zhang C, et al. Effect of daily fiber intake on reproductive function: the BioCycle Study. Am J Clin Nutr. 2009;90(4):1061–1069. doi: 10.3945/ajcn.2009.27990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig CL, Marshall AL, Sjöström M, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 30.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Harlow SD, Matanoski GM. The association between weight, physical activity, and stress and variation in the length of the menstrual cycle. Am J Epidemiol. 1991;133(1):38–49. doi: 10.1093/oxfordjournals.aje.a115800. [DOI] [PubMed] [Google Scholar]
- 33.Harlow SD, Campbell BC. Host factors that influence the duration of menstrual bleeding. Epidemiology. 1994;5(3):352–355. doi: 10.1097/00001648-199405000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Harlow SD, Campbell B. Ethnic differences in the duration and amount of menstrual bleeding during the postmenarcheal period. Am J Epidemiol. 1996;144(10):980–988. doi: 10.1093/oxfordjournals.aje.a008868. [DOI] [PubMed] [Google Scholar]
- 35.Bayer SR, DeCherney AH. Clinical manifestations and treatment of dysfunctional uterine bleeding. JAMA. 1993;269(14):1823–1828. [PubMed] [Google Scholar]
- 36.Howards PP, Schisterman EF, Wactawski-Wende J, et al. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol. 2009;169(1):105–112. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116(3):625–632. doi: 10.1097/AOG.0b013e3181ec622b. [DOI] [PubMed] [Google Scholar]
- 38.Hale GE, Zhao X, Hughes CL, et al. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92(8):3060–3067. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 39.Mikolajczyk RT, Louis GM, Cooney MA, et al. Characteristics of prospectively measured vaginal bleeding among women trying to conceive. Paediatr Perinat Epidemiol. 2010;24(1):24–30. doi: 10.1111/j.1365-3016.2009.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang ET, Cirillo PM, Vittinghoff E, et al. Menstrual irregularity and cardiovascular mortality. J Clin Endocrinol Metab. 2011;96(1):E114–E118. doi: 10.1210/jc.2010-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]