Abstract
Griseofulvin, an antifungal agent, is a BCS class II drug slowly, erratically, and incompletely absorbed from the gastrointestinal tract in humans. The clinical failure of the conventional oral therapy of griseofulvin is most likely attributed to its poor solubility and appreciable inter- and intra-subject variation in bioavailability from different commercial products. Moreover, the conventional oral therapy is associated with numerous adverse effects and interactions with other drugs. The purpose of the study was to formulate a topical application of griseofulvin which would deliver the drug locally in a therapeutically effective concentration. Griseofulvin was solubilized in ethanol, d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS), and combinations of ethanol with varying amounts of TPGS; then, it was incorporated in the Carbopol (980 NF) base. The formulations were characterized and evaluated ex vivo using Laca mice skin, microbiologically against Microsporum gypseum and Microsporum canis and clinically in a small group of patients. The current study suggested that TPGS and ethanol synergistically enhanced the drug permeation and drug retention in the skin. The selected formulation F VII was found to be effective against M. gypseum and M. canis, non-sensitizing, histopathologically safe, stable at 4°C, 25°C, and 40°C with respect to percent drug content, permeation characteristics, pH, transparency, feel, viscosity, and clinically effective in a small group of subjects. The proposed topical formulation of griseofulvin may be an effective and convenient alternative to the currently available oral therapy for the treatment of superficial fungal infections.
Key words: griseofulvin, penetration enhancer, permeation, solubility, vitamin E-TPGS
INTRODUCTION
Topical administration possesses an advantage that drugs may be administered easily and conveniently to achieve a systemic or dermal, regional, or localized effect, as required. However, the absorption rate of topically applied drugs is generally much slower than that through the gastrointestinal tract, and achieving therapeutic levels of a particular drug is challenging. The barrier function of the stratum corneum (SC) of the skin is well established (1). Literature reports cite various approaches for enhancing skin permeation, which also involves the use of chemicals like surfactants and co-solvents (2). These materials either disrupt lipid structures or interact with the intracellular proteins of the SC, or both simultaneously, resulting in improved drug permeation through the skin (3). Non-ionic surfactants are more hydrophobic than ionic surfactants, possess a better capacity to dissolve water-insoluble drugs, and are less toxic to biological membranes (4). Tocopheryl polyethylene glycol succinate (TPGS) is a non-ionic water-soluble derivative of vitamin E having an HLB value of 13.2. Chemically, it is d-α-tocopheryl polyethylene glycol 1000 succinate possessing a hydrophilic (PEG) head and a lipophilic (phytyl) tail. The chemical properties of this distinctive compound have suggested its use as a solubilizer, an emulsifier, absorption/permeation enhancer (5), plasticizer (6), bioadhesive (6), and as a vehicle in lipid-based drug delivery formulations. The mechanism of action for increasing the bioavailability of poorly absorbed drugs employing TPGS is primarily due to its solubilising effect through micelle formation. It is a waxy solid at room temperature and forms micelles above its critical micelle concentration (0.02 wt.%) and continues to form low-viscosity solutions with water until a concentration of about 20 wt.% (7) (http://www.antareshealthproducts.com/about_tpgs/properties.html). When TPGS concentration is above 20 wt.%, high-viscosity liquid crystalline phases are formed which possess gel-like characteristics with a cloudy appearance (7). TPGS, with an excellent safety profile, has been used clinically to enhance the oral bioavailability of anti-HIV drugs amprenavir (8), tipranavir (9); anti-cancer drugs like cyclosporine A (10), paclitaxel (11, 12); fenofibrate (13); vancomycin hydrochloride (14); dermal retention of minoxidil (15) and estradiol (16). Various literature reports are available regarding the use of TPGS for solubility enhancement of poorly soluble drug entities, viz., nefidipine (17), levothyroxine (18), carbamazepine (19), and erythromycin (20). Griseofulvin is a hydrophobic antifungal drug and is used to treat dermatomycoses at an oral dose of 250 mg twice daily. The drug is currently available only as oral dosage forms like powder, tablets, capsules, and suspension. The oral route of administration of griseofulvin suffers from poor and erratic bioavailability. Also, the most common side effects of oral griseofulvin therapy are gastrointestinal disturbances, which are usually mild and reversible, but in some patients, blood dyscrasias, hepatotoxicity, and gynecomastia have been observed. To eliminate these side effects, to target the drug at the infection side, to reduce the duration of treatment, and to increase patient compliance, dermal application of griseofulvin seems to be an ideal route of administration. The aim was to engineer a formulation which would not only target the skin but also deliver the drug in a therapeutically effective concentration. Topical formulations of griseofulvin were prepared employing ethanol/TPGS/combinations of ethanol and TPGS. Ex vivo permeation studies were performed using Franz diffusion cell through Laca mice skin. The optimized formulation was evaluated for antifungal activity against Microsporum gypseum and Microsporum canis, histopathology, skin sensitivity, stability, and clinical efficacy in a small group of patients.
MATERIALS
Ingredients
Griseofulvin was obtained as a gift sample from Wallace Pharmaceuticals Ltd., Mumbai, India. Speziol® TPGS Pharma was obtained as a gift sample from Cognis GmbH, Düsseldorf, Germany. Carbopol 980 NF was obtained as a gift sample from Lubrizol Advanced Materials India Private Limited, Mumbai, India. RPMI 1640 medium, with l-glutamine and without sodium bicarbonate, was obtained from Sigma Aldrich Corporation, Bangalore, India. Triple distilled water was used throughout the study. All other reagents and chemicals were of analytical grade.
Fungal Strains
M. gypseum (MTCC no. 2830) and M. canis (MTCC no. 2820) were procured from the Institute of Microbial Technology (IMTECH), Chandigarh, India.
Animals
Male Laca mice (4–5 weeks old, weighing 25–30 g) were obtained from Central Animal House, Panjab University, Chandigarh, India, and used for carrying out ex vivo permeation and histopathology studies. Ethical approval to perform the aforementioned studies in male Laca mice was obtained from Panjab University, Institutional Animal Ethics Committee, Chandigarh, India, and their guidelines were followed throughout the studies.
Clinical Studies on Human Subjects
Clinical studies were performed on human subjects suffering from Tinea infections of the skin. Ethical clearance to perform the clinical trials on human subjects under the supervision of Dr. Swami Dass Mehta at the Government Multi Speciality Hospital, Sector-16, Chandigarh, was obtained from the Director of Health and Family Welfare, Chandigarh Administration.
METHODS
Drug Solubility Studies
Solubility studies of griseofulvin were carried out in triple distilled water (TDW) and TPGS solutions (1–5%, w/v) using a conventional shake flask method (21). A required amount of TPGS was added to TDW preheated at 60–70°C. TPGS absorbed water immediately, forming a high-viscosity gel which was left for stirring at room temperature for 2 h to obtain a clear solution of TPGS. An excess amount of drug was added to the conical flask containing TDW/TPGS solutions and kept at 37 ± 1°C in a thermostatic water shaker bath for 48 h. Then, the contents were filtered through a 0.45-μm filter and the filtrate analyzed spectrophotometrically (UV 1601 spectrophotometer, Shimadzu, Japan) at λmax = 293 nm after appropriate dilution with ethanol. In order to check any interaction between griseofulvin and TPGS, spectrophotometric scanning was done.
Preparation of Griseofulvin Formulations
Carbopol 980 NF was soaked in water for 2 h and then dispersed by agitating at approximately 600 rpm with the aid of a mechanical stirrer to get a smooth dispersion. The stirring was stopped and the dispersion was allowed to stand so that any entrained air could escape. If any lumps of partially wetted Carbopol were present at this stage, the dispersion was discarded and a fresh batch was prepared. To this prepared dispersion, an aqueous solution of triethanolamine was added with slow-speed agitation. At this stage, griseofulvin in TPGS/ethanol/various combinations of ethanol and TPGS was incorporated into the prepared base. Ethanol in the preparation also served the purpose of a preservative; no additional preservative was added. Any entrapped air in the gel base was allowed to escape by keeping the formulations in vacuum overnight. Table I shows the list of ingredients used in different griseofulvin formulations.
Table I.
Composition of Various Griseofulvin (0.2%, w/w) Formulations
| Ingredients | Quantity (g), formulation code | |||||||
|---|---|---|---|---|---|---|---|---|
| F I | F II | F III | F IV | F V | F VI | F VII | F VIII | |
| Ethanol | − | − | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| TPGS | − | 0.5 | − | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
| Propylene glycol | − | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Carbopol 980 NF | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Triethanolamine | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Triple distilled water (q.s.) | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
TPGS tocopheryl polyethylene glycol succinate
Evaluation of Formulations
The formulations were evaluated for percent drug content, drug uniformity, pH, viscosity, and organoleptic characteristics such as feel tackiness, homogeneity, and transparency. Drug content was determined by taking 0.1 g of accurately weighed formulation which was diluted to 10 ml with methanol and analyzed spectrophotometrically at λmax = 293 nm. The pH of the formulations was determined using a pH meter (Labindia Pico+, Mumbai, India). The formulations were inspected visually for homogeneity, transparency, presence of any aggregates, smoothness, and spreadability. Viscosity of the formulations was determined at 25°C using a Brookfield viscometer RV II Pro+ (22).
Ex Vivo Drug Permeation and Skin Retention Studies
Permeation experiments were conducted employing an excised abdominal skin of Laca mice. The animals were killed with an overdose inhalation of chloroform. Hair on the dorsal side of the animals was removed with electric clipper in the direction of tail to head without damaging the skin. The shaven part of the skin was cut and the hypodermis, including blood vessels, removed using surgical blade no. 23. A preliminary wash of the skin was done with normal saline, and it was soaked overnight in saline and used subsequently. Experiments were run in Franz diffusion cells (PermeGear, Inc., PA, USA) having a receptor compartment volume of 30 ml and an available diffusion area of 3.14 cm2. Skin membranes were mounted with the stratum corneum side up. Test formulations equivalent to 1 mg of drug were applied to the skin surface non-occlusively. Phosphate buffer saline, pH 6.4, was used as the receptor medium. The cell contents were stirred by an externally driven magnetic bar and were maintained at temperature of 37 ± 1°C by circulating water through an external jacket of the cell. A 2-ml aliquot was periodically withdrawn at suitable time intervals from the sampling arm of the receptor chamber. Fresh diffusion medium was simultaneously replaced in the receptor chamber. The samples were analyzed spectrophotometrically at λmax = 296 nm.
At the end of the permeation experiments (24 h), the skin surface in the donor compartment was rinsed with ethanol to remove excess drug from the surface. The receptor medium was then replaced with 50% (v/v) ethanol. Receptor contents were stirred for the next 24 h, followed by spectrophotometric determination. The receptor solution (50% (v/v) ethanol in TDW) is reported to extract drug deposited in the skin, thus giving a measure of skin retention (23). Similar permeation and skin retention studies were performed using blank formulations (without drug), and the absorbance values were subtracted from the test formulations to account for the effect of excipients. The cumulative amount permeated per unit area (micrograms per square centimeter), flux (micrograms per hour per square centimeter), and skin retention (micrograms per square centimeter) were calculated. Each experiment was conducted in triplicate.
Statistical Analysis
All the data were statistically analyzed by one-way analysis of variance followed by Dunnett’s method. Results were quoted as significant where P < 0.05.
Antifungal Studies
Antifungal tests were performed against M. gypseum and M. canis using a broth microdilution method (24). The tests were performed in RPMI 1640 medium, with l-glutamine and without sodium bicarbonate buffered at pH 7.0. The stock solution of drug was prepared in 100% dimethyl sulfoxide at a concentration of 1,600 μg/ml. Serial drug dilutions were performed beginning 100 times at test concentration followed by a 1:50 dilution in RPMI medium to yield twice the final concentration required for testing.
Inoculum Preparation
Stock inoculum suspensions of the fungi were prepared from 7- to 15-day-old cultures grown on potato dextrose agar at 28°C. Mature colonies were covered with approximately 10 ml of sterile saline (0.9%) by scraping the surface with the tip of a Pasteur pipette. The resulting mixture of conidia and hyphal fragments was withdrawn and transferred to sterile tubes. Heavy particles were allowed to settle for 15–20 min at room temperature; the upper suspension was mixed with a vortex mixer for 15 s. The turbidity of the supernatants was measured spectrophotometrically at a wavelength of 530 nm, and transmission was adjusted to 65–70%. Each suspension was diluted 1:50 in RPMI 1640 to obtain the final test inoculum twice.
Test Procedure
The tests were performed in sterile, round-bottomed, 96-well microplates. Aliquots of 100 μl of the drug dilution were inoculated into the wells with a multichannel pipette. When the susceptibility test was performed, 100 μl of the diluted inoculum suspensions was added to each well to bring the drug dilutions to the final test concentrations. Test concentrations for drug ranged from 0.0039 to 16 μg/ml. Growth and sterility control wells were also included for the tested fungal strains. The microplate contents were incubated at 28°C and were read visually with the aid of an inverted reading mirror after 4 days of incubation. The minimum inhibitory concentration was defined as the lowest concentration showing 100% growth inhibition.
Histopathological Examination
Male Laca mice weighing around 25–30 g were used for histopathology. The hair on the dorsal side of the animals was removed with an electric clipper in the direction of tail to head without damaging the skin. One mouse was kept as the control (untreated). The selected formulation, F VII, was applied uniformly on the dorsal region and kept in contact for 4 h. After that, the animals were killed by the cervical dislocation method and the exposed dorsal surface cut. Then, each specimen was fixed in 10% buffered formalin, embedded in paraffin, and microtoned. The sections were stained with hematoxylin and eosin. Finally, the specimens were observed under a high-power light microscope (25).
Skin Sensitivity Studies
Modified Draize patch test was used to access the skin irritancy potential of the developed topical formulations. All the ethical and moral norms were followed during the procedure. Twenty healthy human volunteers (age, 20–27 years) of either sex were selected for the study. Formulation FVII was applied on the inner upper arm (hair-free skin) of volunteers by uniform spreading within an area of 4 cm2. The formulation was applied three times a day for 3 days consecutively (26). The skin was observed for any visible change such as erythema and burning/ itching sensation (27).
Stability Studies
Formulation F VII was subjected to stability studies in screw-capped glass tubes at three different temperatures (4°C, 25°C, and 40°C) and evaluated periodically for percent drug content, pH, transparency, feel, and viscosity for 3 months.
Clinical Studies
A randomized controlled trial was performed on ten immunocompetent adults. The presence of dermatophytes was confirmed by microscopy. Each patient signed an informed consent to participate in the study. The patients were made aware about the requirements of the study, viz., not to take any oral antifungal drug nor apply any topical antifungal/steroidal formulation during the treatment time period. The patients were advised to apply the selected formulation, F VII, topically twice a day. The time period required for complete cure for each patient was observed. Complete cure is defined as the achievement of both clinical and mycological cures. Clinical parameters like erythema, scaling, itching, edema, and size of lesions were also observed for the evaluation of treatment efficacy. These parameters were determined before treatment and at weekly intervals during the treatment.
RESULTS AND DISCUSSION
The results of spectrophotometric analysis revealed no interaction between griseofulvin and TPGS (Fig. 1a, b). The aqueous solubility of griseofulvin in the presence of various concentrations of TPGS ranging from 1% to 5% (w/w) was enhanced when compared with drug solubility in TDW (Table II). This may be attributed to the formation of multimers of TPGS which not only facilitated the drug encapsulation in micelles but also decreased the surface tension of the system, assisting enhancement of the solubility of the drug.
Fig. 1.
Spectrophotometric scan of griseofulvin in ethanol (a) and griseofulvin and TPGS solution in ethanol (b)
Table II.
Solubility Data of Griseofulvin in TDW and TPGS Solutions
| Media | Solubility (mg/ml, n = 3) |
|---|---|
| Triple distilled water | 0.054 ± 0.023 |
| 1% (w/w) TPGS solution | 0.384 ± 0.016 |
| 2% (w/w) TPGS solution | 0.601 ± 0.014 |
| 3% (w/w) TPGS solution | 0.852 ± 0.070 |
| 4% (w/w) TPGS solution | 1.133 ± 0.060 |
| 5% (w/w) TPGS solution | 1.266 ± 0.072 |
The drug content of griseofulvin formulations was found to be 98.95 ± 0.7%. The results of drug content uniformity test for all the formulations indicated that the drug was properly and uniformly dispersed. The pH value of formulations was observed to be 6.5 ± 0.1. The formulations were transparent, homogenous and smooth, easily spreadable, and non-dripping in nature. The viscosity of the formulations varied between 25.6 and 30.1 Pa s.
Ex Vivo Permeation Studies Through Mice Skin
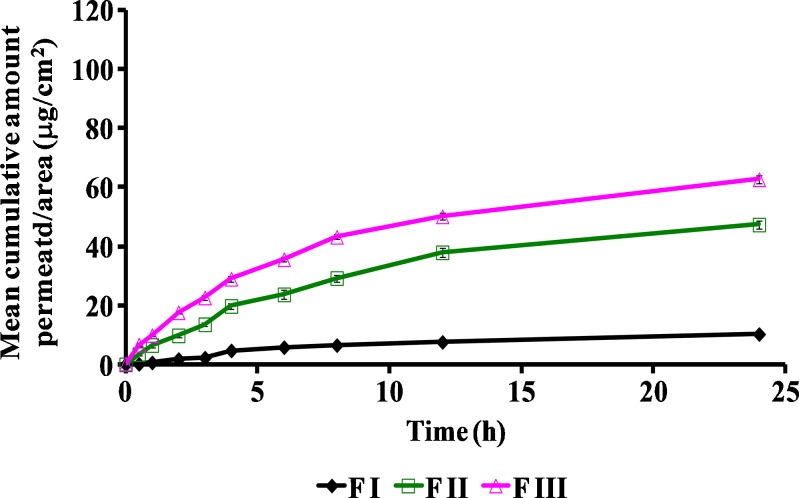
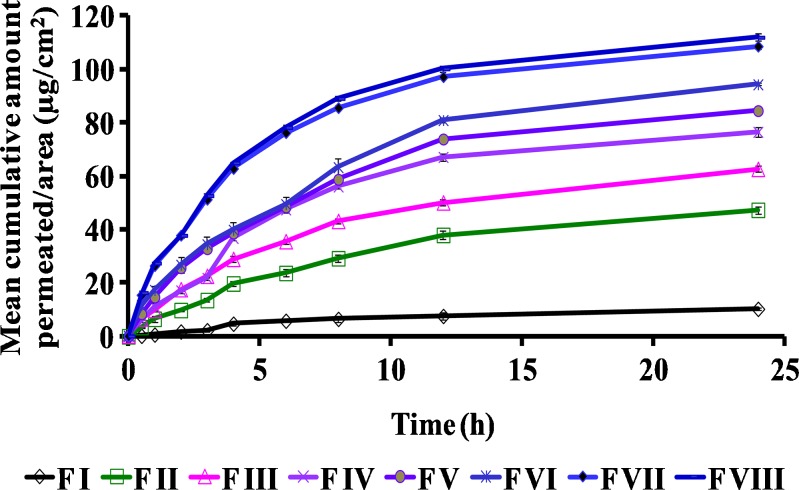
The permeation profiles of various compositions of griseofulvin are shown in Figs. 2 and 3. The aqueous suspension (F I) of the drug exhibited only 10.28 ± 1.25 μg/cm2 permeation in 24 h. TPGS dispersion (F II) showed 47.35 ± 1.43 μg/cm2 of drug permeation in 24 h, which is almost five times more as compared with the aqueous suspension. This may be attributed to the surfactant-like properties of TPGS which could have enhanced the drug permeation. Formulation F III containing ethanol exhibited 62.69 ± 1.25 μg/cm2 of drug permeation, which is nearly six times higher as compared with F I. This may be attributed to the presence of ethanol in the formulation which was used to dissolve the drug completely before incorporation into the polymer base. Formulation F IV (containing 1% (w/w) TPGS and ethanol) and F V (containing 2% (w/w) TPGS and ethanol) revealed mean cumulative permeations of 76.58 ± 1.64 and 84.52 ± 0.90 μg/cm2 in 24 h, which were significantly higher than F I and F II but not significantly different from F III at P < 0.05 (Fig. 2). Formulation F VI (containing 3% (w/w) of TPGS and ethanol) exhibited 94.35 ± 0.46 μg/cm2 of the mean cumulative permeation/area in 24 h, which was significantly higher than F III at P < 0.05 (Fig. 3). When further higher concentrations of TPGS were investigated in formulation F VII (4% (w/w) TPGS and ethanol) and F VIII (containing 5% (w/w) TPGS and ethanol), mean cumulative amounts of 108.65 ± 0.96 and 111.98 ± 1.36 μg/cm2 of drug permeated respectively in 24 h, which were significantly higher (nearly twofold) as compared with F III at P < 0.05 (Fig. 3). The penetration/permeation enhancers are known to modify the skin barrier function, thereby improving the drug permeation. Ethanol and TPGS used in the present study not only served the purpose of solubilizing the drug but also acted as permeation enhancers by altering the interfacial barrier function of the stratum corneum to decrease resistance to drug permeation.
Fig. 2.
Comparison of ex vivo permeation profiles of topical griseofulvin formulations F I, F II, and F III through mouse skin
Fig. 3.
Comparison of ex vivo permeation profiles of different topical formulations of griseofulvin (F I to F VIII) through mouse skin
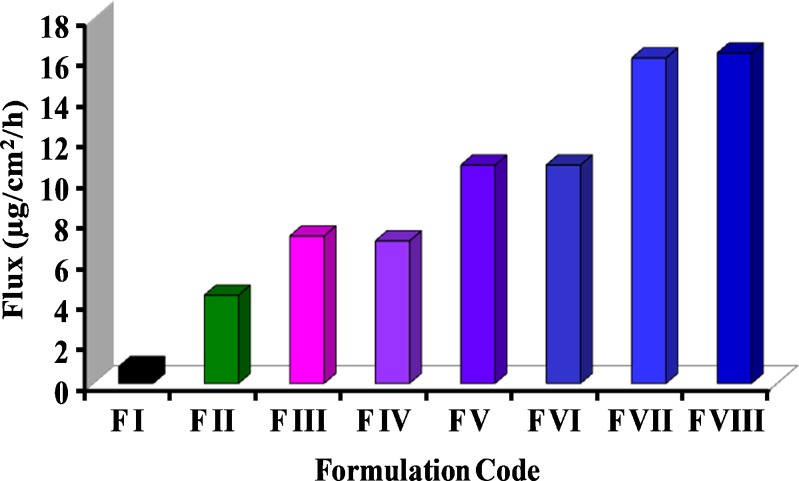
The rate of permeation (flux) enhanced from 0.83 ± 0.23 μg cm−2 h−1 for F I (aqueous suspension) to 16.3 ± 0.17 μg cm−2 h−1 for F VIII (containing 5% TPGS and ethanol; Fig. 4). Formulations F II (containing 5% TPGS) and F III (containing ethanol) enhanced the flux values to 4.35 ± 0.16 and 7.27 ± 0.11 μg cm−2 h−1, respectively. The incorporation of ethanol with varying concentrations of TPGS in formulations F IV, V, VI, VII, and VIII further increased the flux from 7.27 ± 0.11 μg cm−2 h−1 (F III) to 16.29 ± 0.17 μg cm−2 h−1 (F VIII), which was significantly higher (two times more) than F III at P < 0.05 (Fig. 3). It may be concluded that ethanol and TPGS synergistically enhanced the flux of drug through mouse skin by favorably modifying the interfacial interaction and drug partition phenomena.
Fig. 4.
Comparison of flux of griseofulvin from different topical formulations (F I to F VIII) through mouse skin
The skin deposition of drug with F I (aqueous suspension) was observed to be 0.65 ± 0.15 μg/cm2 only. This may be ascribed to the hydrophobic nature of the drug because the drug, being insoluble, did not penetrate the skin. Formulation F II (TPGS dispersion) resulted in skin deposition of 15.49 ± 0.49 μg/cm2 of drug. This may be credited to the surfactant-like properties of TPGS which enhanced the drug solubility and drug penetration through the formation of micelles. F III (containing drug solubilized in ethanol) not only increased the rate of skin penetration (flux) of drug but also led to an increased retention of drug in skin (12.23 ± 0.51 μg/cm2). The formulations containing ethanol and varying proportions of TPGS (F IV, V, VI, VII, and VIII) led to drug retention in the skin ranging from 22.88 ± 1.11 to 36.77 ± 1.13 μg/cm2, which was almost twofold higher as compared with F II and two to threefold higher as compared with F III (Fig. 5). The skin deposition study advocates that the therapeutically effective concentration of griseofulvin (14–46 μg/cm3) could be achieved with the formulations containing a combination of ethanol and TPGS.
Fig. 5.
Comparison of skin retention of griseofulvin from different topical formulations (F I to F VIII) through mouse skin
Antifungal Studies
The broth microdilution method revealed that the drug concentration of 0.5 μg/ml resulted in 100% inhibition of M. gypseum and M. canis. Also, the ex vivo permeation studies demonstrated that the formulations F VII and F VIII led not only to sufficient skin permeation but also significantly high skin concentrations of griseofulvin amounting to 35.57 ± 1.18 and 36.77 ± 1.13 μg/cm3, respectively. These findings are further supported by literature reports which divulge that griseofulvin concentrations of 14–46 μg/cm3 in the skin are expected to inhibit fungal growth (28). Therefore, on the basis of ex vivo permeation studies and antifungal studies, it can be deciphered that formulations F VII (containing ethanol and 4% (w/w) TPGS) and F VIII (containing ethanol and 5% (w/w) TPGS), when applied topically, may be effective against dermatophytes.
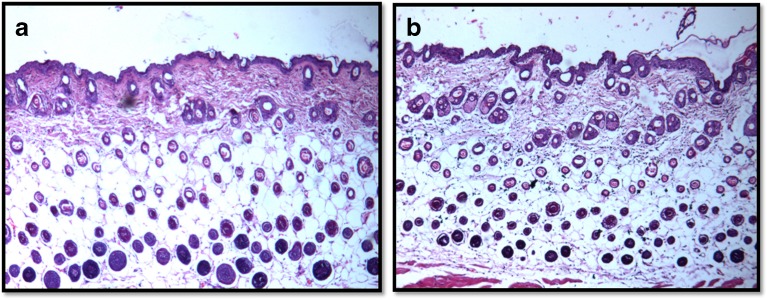
Histopathological Studies
The aim of these studies was to establish the dermal tolerance of the selected formulation. The microscopic appearance of the mouse skin without treatment (control) and after treatment with the selected formulation, F VII, revealed no anatomical and pathological changes (Fig. 6). Thus, the results established the safety of the prepared formulation on mouse skin.
Fig. 6.
Histological photographs of mice skin after 4 h treatment with control (no treatment) (a) and F VII (ethanol and 4% (w/w) TPGS) (b)
Skin Sensitivity Studies
Skin sensitivity test was done by applying the selected formulation, F VII, to the skin using an open patch test on 20 healthy human volunteers for 24 h. There was no sign of any redness, irritation, or swelling. The results of this study suggested that the formulation was safe on normal human skin.
Stability Studies
The selected formulation, F VII, was subjected to stability studies at three different temperatures (4°C, 25°C, and 40°C) for 6 weeks. The formulations were evaluated for drug content, pH, and macroscopic characteristics like transparency, feel, smoothness, and spreadability; no significant change was found in these characteristics. This shows that the prepared formulation is stable at the studied temperatures.
Clinical Studies
Ten patients in the age group of 16–68 years completed the trial successfully. The duration of complete cure varied from 2 to 6 weeks (Table III). This can be explained on the basis of skin surface area involved, site of infection, compliance of the patient toward treatment, and personal hygiene. The non-occluded body sites like fingers and hands exhibited faster complete cure rate as compared with the occluded body sites like the groin region. All patients reported symptomatic relief from burning and itching (commonly associated symptoms with fungal disease) within a day or two after the initiation of treatment. Moreover, a good feel of the preparation reported by all the patients may be due to the emollient action of TPGS and the cooling effect due to the presence of ethanol in the formulation. The infection confined to a single body site as in case of patient 1 was cured in 2 weeks. In the case of patient 2, although the site of infection was the buttock region, still, complete cure was achieved in 2 weeks. Patient 3 suffered infections in the groin region, legs, and buttocks. The infection on the legs and the buttock region was completely cured in 2 weeks, but the infection in the groin region required a further treatment of 2 weeks for complete clinical cure. Although the site of infection in the case of patient 5 was a non-occluded region, right hand, still, the duration of treatment was 5 weeks. This long duration of treatment may be ascribed to the occupation of the patient, which slowed down the recovery from infection. The duration of treatment for the rest of the five patients varied between 5 and 6 weeks. This may be explained on the basis of site of infection. All these patients suffered a generalized infection in the occluded region, i.e., groin, hence the longer duration of treatment.
Table III.
Results of Clinical Investigations using Griseofulvin Formulation F VII
| Patient no. | Age/sex | Body part/area affected | Duration of treatment until complete cure (weeks) |
|---|---|---|---|
| 1 | 68/M | Right hand index finger | 2 |
| 2 | 65/F | Buttock region | 2 |
| 3 | 56/M | Groin region, legs, buttock region | 4 |
| 4 | 52/F | Right hand | 6 |
| 5 | 16/M | Groin region | 5 |
| 6 | 46/M | Groin region | 5 |
| 7 | 39/F | Groin region | 5 |
| 8 | 23/M | Groin region, toenails | 6 |
| 9 | 35/F | Waistline, groin | 6 |
| 10 | 31/M | Groin region, right foot little toe | 6 |
From the present study, it can be deciphered that the topical route of administration of griseofulvin was successful in achieving clinical cure. The oral dosage for griseofulvin is 250 mg twice a day, which unnecessarily burdens the system and also leads to side effects. The topical route of delivery for griseofulvin could be a better alternative to the current existing oral therapy which normally ranges from 4 to 8 weeks or even 1 year depending on the site of infection. Therefore, dermal application would not only reduce the “body burden” of drug but also prevent adverse effects associated with oral therapy and increase patient compliance.
CONCLUSION
The prepared topical formulations of griseofulvin containing TPGS were observed to be safe, stable, and therapeutically efficacious in the treatment of superficial fungal infections of the skin. These preliminary investigations on mouse skin and clinical investigations reveal the therapeutic potential of these topical formulations which may serve as an effective alternative to the currently available oral therapy.
ACKNOWLEDGMENTS
The authors gratefully acknowledge CSIR, New Delhi, for providing financial assistance. Gift samples of griseofulvin supplied by Wallace Pharmaceuticals Ltd., Mumbai, India, Speziol® TPGS Pharma provided by Cognis GmbH, Düsseldorf, Germany, and Carbopol 980 NF from Lubrizol Advanced Materials India Private Limited, Mumbai, India, are gratefully acknowledged. Ethical clearance provided by the Director of Health and Family Welfare, Chandigarh Administration, for conducting clinical trials on human subjects is also gratefully acknowledged.
Declaration of Interest
The authors report no declaration of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1.Harding CR. The stratum corneum: structure and function in health and disease. Dermatol Ther. 2004;17:6–15. doi: 10.1111/j.1396-0296.2004.04S1001.x. [DOI] [PubMed] [Google Scholar]
- 2.Franz TJ, Tojo K, Shah KR, Kydonieus AF. Transdermal delivery. In: Kydonieus AF, editor. Treatise of controlled drug delivery. New York: Marcel Dekker; 1992. pp. 341–421. [Google Scholar]
- 3.Shin S, Cho C, Oh I. Effects of non-ionic surfactants as permeation enhancers towards piroxicam from the poloxamer gel through rat skins. Int J Pharm. 2001;222:199–203. doi: 10.1016/S0378-5173(01)00699-8. [DOI] [PubMed] [Google Scholar]
- 4.Bogman K, Erne-Brand KF, Alsenz J, Drewe J. The role of surfactants in the reversal of active transportmediated by multidrug resistance proteins. J Pharm Sci. 2003;92:1250–61. doi: 10.1002/jps.10395. [DOI] [PubMed] [Google Scholar]
- 5.Ismailos G, Peppas C, Macheras P. Enhancement of cyclosporine A solubility by d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) Eur J Pharm Sci. 1994;1:269–71. doi: 10.1016/0928-0987(94)90021-3. [DOI] [Google Scholar]
- 6.Repka MA, McGinity JW. Influence of vitamin E TPGS on properties of hydrophilic films produced by hot melt extrusion. Int J Pharm. 2000;202:63–70. doi: 10.1016/S0378-5173(00)00418-X. [DOI] [PubMed] [Google Scholar]
- 7.PCI-102B Eastman Vitamin E TPGS NF, Applications and Properties, p. 1–24.
- 8.Brouwers J, Tack J, Lammert F, Augustijns P. Intraluminal drug and formulation behavior and integration in in vitro permeability estimation: a case study with amprenavir. J Pharm Sci. 2006;95:372–83. doi: 10.1002/jps.20553. [DOI] [PubMed] [Google Scholar]
- 9.Drug encyclopedia > tipranavir–vitamin E TPGS 100 mg/mL Oral Soln. https://members.kaiserpermanente.org.
- 10.Wempe MF, Wright C, Little JL, Lightner JW, Large SE, Caflisch GB, et al. Inhibiting efflux with novel non-ionic surfactants: rational design based on vitamin E TPGS. Int J Pharm. 2009;370:93–102. doi: 10.1016/j.ijpharm.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Joshi R, Sands H, Rubinfeld J. Methods for administration of paclitaxel. US Patent 6,828,346, to SuperGen, Inc., Dubin, CA 2003.
- 12.Khandavilli S, Panchagnula R. Nanoemulsions as versatile formulations for paclitaxel delivery. J Invest Dermatol. 2007;127:154–62. doi: 10.1038/sj.jid.5700485. [DOI] [PubMed] [Google Scholar]
- 13.Bhise SD. Ternary solid dispersions of fenofibrate with Poloxamer 188 and TPGS for enhancement of solubility and bioavailability. Int J Res Pharm Biomed Sci. 2011;2:583–95. [Google Scholar]
- 14.Prasad YVR, Puthli SP, Eaimtrakarn S, Ishida M, Yoshikawa Y, Shibata N, Takada K. Enhanced intestinal absorption of vancomycin with Labrasol and d-α-tocopheryl PEG 1000 succinate in rats. Int J Pharm. 2003;250:181–90. doi: 10.1016/S0378-5173(02)00544-6. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Sheu M, Wu A, Lin K, Ho H. Simultaneous effects of tocopheryl polyethylene glycol succinate (TPGS) on local hair growth promotion and systemic absorption of topically applied minoxidil in a mouse model. Int J Pharm. 2005;306:91–8. doi: 10.1016/j.ijpharm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Sheu M-T, Chen Shin-Yi, Chen L-C, Ho Hsiu-O. Influence of micelle solubilization by tocopheryl polyethylene glycol succinate (TPGS) on solubility enhancement and percutaneous penetration of estradiol. Journal of Controlled Release. 2003;88:355–68. doi: 10.1016/S0168-3659(02)00492-3. [DOI] [PubMed] [Google Scholar]
- 17.Rajebahadur M, Zia H, Nues A, Lee C. Mechanistic study of solubility enhancement of nifedipine using vitamin E TPGS or solutol HS-15. Drug Deliv. 2006;13:201–6. doi: 10.1080/10717540500316094. [DOI] [PubMed] [Google Scholar]
- 18.Pabla D, Hossein Z. Development and characterization of a levothyroxine sodium formulation: solubility enhancement of the drug. 2008 AAPS Annual Meeting and Exposition Online Itinerary, 15–20 Nov 2008.
- 19.Charkoftaki G, Dokoumetzidis A, Valsami G, Macheras P. Supersaturated dissolution data and their interpretation: the TPGS-carbamazepine model case. J Pharm Pharmacol. 2011;63:352–61. doi: 10.1111/j.2042-7158.2010.01226.x. [DOI] [PubMed] [Google Scholar]
- 20.Fromm MF, Kim RB. Drug transporters: handbook of experimental pharmacology 201. Berlin: Springer; 2011. [Google Scholar]
- 21.Bakka E, Comer JEA, Takács-Novák K. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J Pharm Bio Anal. 2007;22:335–41. doi: 10.1016/j.jpba.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Nokhodchi A, Nazemiyeh H, Ghafourian T, Hassan-Zadeh D, Valizadeh H, Bahary LAS. The effect of glycyrrihizin on the release rate and skin permeation of diclofenac sodium from topical formulations. II Farmaco. 2002;57:883–8. doi: 10.1016/S0014-827X(02)01298-3. [DOI] [PubMed] [Google Scholar]
- 23.El Maghraby GM, Williams AC, Barry BW. Skin delivery of 5-fluorouracil from ultradeformable and standard liposomes in vitro. J Pharm Pharmacol. 2001;53:1069–77. doi: 10.1211/0022357011776450. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Torres B, Carrillo AJ, Matin E, Del Palacio A, Moore MK, Valverde A, et al. In vitro activities of 10 antifungal drugs against 508 dermatophyte strains. Antimicrob Agents Chemother. 2001;45:2524–8. doi: 10.1128/AAC.45.9.2524-2528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godkar PB, Godkar DP. Textbook of medical laboratory technology. Mumbai: Bhalani Publishing House; 2003. [Google Scholar]
- 26.Fisher AA. Sensitivity testing. In: Balsam MS, Sagarin E, editors. Cosmetics—science and technology. New York: Wiley Interscience; 1974. pp. 283–310. [Google Scholar]
- 27.Vogel HG. Drug discovery and evaluation: pharmacological assays. 3rd ed. New York: Springer-Verlag Berlin Heidelberg; 2006.
- 28.Ritschel WA, Hussain AS. In vitro skin penetration of griseofulvin in rat and human skin from an ointment dosage form. Arzneim Forsch. 1988;38:1622–30. [PubMed] [Google Scholar]