Abstract
Solid lipid nanoparticles (SLNs) of duloxetine hydrochloride (DLX) were prepared to circumvent the problems of DLX, which include acid labile nature, high first-pass metabolism, and high-dosing frequency. The DLX-SLNs were prepared by using two different techniques, viz. solvent diffusion method and ultrasound dispersion method, and evaluated for particle size, zeta potential, entrapment efficiency, physical characteristics, and chemical stability. Best results were obtained when SLNs were prepared by ultrasound dispersion method using glyceryl mono stearate as solid lipid and DLX in ratio of 1:20 and mixture of polysorbate 80 and poloxamer 188 as surfactant in concentration of 3%. The mean particle size of formulation and entrapment efficiency was 91.7 nm and 87%, respectively, and had excellent stability in acidic medium. Differential scanning calorimetry and X-ray diffraction data showed complete amorphization of DLX in lipid. In vitro drug release from SLNs was observed for 48 h and was in accordance with Higuchi kinetics. In vivo antidepressant activity was evaluated in mice by forced swim test. DLX-SLNs showed significant enhancement in antidepressant activity at 24 h when administered orally in comparison to drug solution. These results confirm the potential of SLNs in enhancing chemical stability and improving the efficacy of DLX via oral route. The SLN dispersion was converted into solid granules by adsorbing on colloidal silicon dioxide and characterized for particle size after redispersion, morphology, and flow properties. Results indicated that nanoparticles were successfully adsorbed on the carrier and released SLNs when dispersed in water.
Key words: acid labile, adsorption, duloxetine HCl, lymphatic absorption, solid lipid nanoparticles
INTRODUCTION
Duloxetine {(3S)-N-methyl-3-naphthalen-1-yloxy-3-thiophen-2-ylpropan-1-amine} (DLX) is a novel dual serotonin and norepinephrine reuptake inhibitor, approved for the treatment of major depressive disorders, diabetic peripheral neuropathic pain, fibromyalgia, generalized anxiety, and woman stress urinary incontinence. DLX has an edge over other existing antidepressants because of favorable pharmacodynamic features of DLX like dual inhibition, improved efficacy, tolerability, safety, faster recovery, fewer side effects, and low affinity for other neuronal receptors (1–3).
DLX is susceptible to hydrolytic degradation which is more pronounced at acidic pH (4). Marketed formulations of DLX have low and variable bioavailability ranging from 30% to 80% due to extensive metabolism in liver (www.ema.europa.eu/docs/en_GB/document…/WC500036776.pdf, accessed November 3, 2010). A steady-state plasma concentration of drug is desired for effective treatment of any disorder. Three times daily dose of DLX may cause fluctuation in the plasma concentration with reduced patient compliance due to conventional multiple daily dosing.
Lipid-based nanoparticulate systems such as microemulsions, lipid nanoparticles, and nanoemulsions have proved to be very successful in improving solubility of drug and absorption (5). Solid lipid nanoparticles (SLNs) are drug carriers possessing advantages with respect to stability, drug release profile, and biocompatibility. SLNs are composed of the matrix of biocompatible solid lipids like triglycerides, fatty alcohols, and waxes with generally regarded as safe status for oral, topical, and parenteral administration. The preparation and characterization methods are well documented and amenable to scale up (6). Several reports are available which demonstrate the utility of SLNs in enhancing oral bioavailability (7–10). These systems improve the oral bioavailability of actives by lymphatic transport and thus bypass the hepatic first-pass metabolism.
Few limitations of SLN dispersions include physical instability arising due to particle aggregation and gelation under accelerated storage conditions (11). This drawback can be overcome by conversion of SLN dispersion to dry powder. Solid oral formulations are the most acceptable, patient compliant, and robust dosage forms. Lyophilization has been the most common method to convert SLN dispersion to dry form (12). Other methods tried by researchers include spray drying (13), evaporative drying (14), and adsorption on inert carrier (15). Lyophilization and spray drying are exhaustive procedures which can have an impact on the properties of SLN crystals. Evaporative drying requires dedicated apparatus such as desiccators for controlled drying. Adsorption onto inert carrier has been successful for adsorption of lipid-based formulations (16, 17). This is a simple, less explored, and cost-effective procedure for immobilizing SLNs and converting into solid dry form.
The aim of the present study was to formulate acid stable lipid nanoparticulate granules of DLX, which can offer chemical protection to acid labile DLX, improve bioavailability, and sustain the release profile, thereby improving its efficacy in the above-mentioned therapeutic indications. Selection of appropriate solid lipid, surfactant, and method of preparation was performed on the basis of desired dosage form characteristics like stability, particle size, and entrapment of drug. The SLN system of DLX was characterized for acid protection and sustained release potential. The final optimized batch was evaluated by pharmacodynamic study on mice for antidepressant activity of DLX-loaded SLNs. The optimized SLN dispersion was converted into granules by adsorption onto carrier to impart physical stability to the lipidic system. These granules were evaluated for release of SLNs after reconstitution.
MATERIALS AND METHODS
Materials
Cithrol glyceryl monostearate (GMS) was obtained as gift sample from Croda, Singapore. Compritol 888 ATO (COM) and Precirol ATO 5 (PRE) were obtained as gift samples from Gatteffosse India Ltd, Mumbai, India. DLX was obtained as a gift sample from Shodhna Labs, Hyderabad, India. Solutol HS 15, Cremophor EL, and Poloxamer F68 were obtained as gift samples from BASF, Mumbai, India. Tween 80 was purchased from S. D. Fine Chem. Ltd., Mumbai, India. Aerosil 200 was obtained as gift sample from Evonik. All other chemicals and solvents were of analytical reagent grade and were used without further purification.
Screening of Lipids
The lipids screened for preparation of SLNs are given in Table I. For determination of solubility of drug in solid lipid, 20 mg of drug was transferred in a test tube maintained at temperature 5°C above the melting point of lipid. The solid lipid was added in increments of 50 mg till DLX was completely dissolved and amount of lipid required to solubilize DLX was determined.
Table I.
Amount of Solid Lipid Required to Solubilize 20 mg of DLX and Percent Partitioning of DLX in Lipid vs Water
| Solid lipid | Amount (mg) | % Partitioning |
|---|---|---|
| Glyceryl monostearates | 400 | 92 |
| Glyceryl behenate | 700 | 33 |
| Glyceryl palmitostearate | 650 | 60 |
| Geleol | 450 | 60 |
| Gelucire 44/14 | 800 | – |
The percent partitioning of drug between lipid and water was evaluated as mentioned earlier (18). Briefly, 20 mg of DLX was dissolved in a mixture of molten lipid and 10 ml of hot distilled water, maintained at 5°C above the melting point of lipid. The amount of lipid used was as given in Table I. Both the phases were vortexed and then allowed to cool. Upon cooling, the lipid was separated by centrifugation at 10,000 rpm (Minispin, Eppendorf, Germany), and the drug content in the supernatant was analyzed spectrophotometrically at 288 nm. The percent partitioning was calculated as follows:
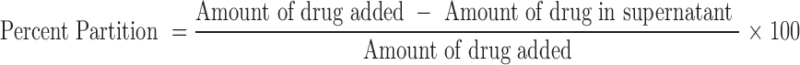 |
Preparation and Optimization of SLNs
DLX-SLNs were prepared by two different methods viz. solvent evaporation method and ultrasound dispersion method. In solvent evaporation method, DLX and solid lipid were dissolved in 1:1 mixture of acetone and ethanol (7 ml each) and heated to 70°C in a water bath. This organic phase was dispersed into aqueous phase containing surfactant at same the temperature under stirring. The organic phase was evaporated while the mixture was being stirred.
SLNs were also prepared using an ultrasound dispersion method. Solid lipids were melted at a temperature 5°C above their melting point, and drug was added to the molten lipid according to its solubility in the lipid. Aqueous solution of surfactant maintained at the same temperature was added to the lipid phase to form a coarse emulsion. The coarse emulsion was sonicated using a probe sonicator (Branson Ultrasonics Corporation, USA) at 30 W for 5 min to give a nanoemulsion which was cooled to room temperature to form SLNs.
Surfactants such as polyethylene glycol 15 stearate, polysorbate 80, polyoxyethylene castor oil, poloxamer 188, and polyvinyl alcohol (PVA) were screened. The effect of type and concentration of surfactant on particle size, polydispersity index (PI), and nanoparticulate stability at different pH was evaluated to select a suitable surfactant for the preparation of SLN by both the methods. The optimized formulations of both the batches were characterized further for physical stability.
Characterization of SLNs
Particle Size and Zeta Potential
SLNs prepared by both the methods were evaluated for particle size, PI, and zeta potential by using Beckman coulter—N5 submicron particle size analyzer and Brookhaven Zeta analyzer, respectively. All the samples were diluted appropriately, dispersed in double-distilled water, and analyzed at 25°C using disposable polystyrene cells.
Entrapment Efficiency
Entrapment efficiency (EE) was determined by separation of unentrapped drug using high speed centrifuge (Beckman coulter, USA). The SLNs were centrifuged at 25,000 rpm for 30 min, and the clear supernatant obtained was analyzed spectrophotometrically for drug content at 288 nm. The EE of DLX in SLNs was determined as per the equation:
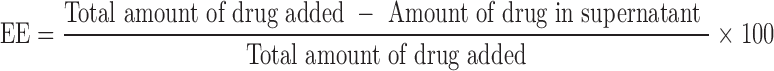 |
DSC and pXRD
DSC analysis was done using Mettler TA STARe System equipped with a DSC 822e cell at a heating rate of 10°C/min under nitrogen atmosphere. DSC thermograms of plain drug, blank SLNs, and DLX-SLNs were recorded.
X-ray powder diffraction (pXRD) patterns were recorded on a Philips PW 17291 powder X-ray diffractometer using Ni-filtered, Cu K_ radiation, a voltage of 40 kV, and a 25-mA current. The scanning rate used was 1° min−1 over the 10–40° 2è range. pXRD diffraction patterns were recorded for plain drug, blank SLNs, and DLX-SLNs.
HPLC Analysis of Drug
HPLC method was developed for analysis of DLX and to investigate possible degradation in SLN system. The HPLC system consisted of Jasco PU 2080 Plus Intelligent HPLC Pump, Jasco, Japan, equipped with HiQ Sil C18, 4.6 i.d. × 250 mm, 5 μm particle size column, and a Jasco UV 2075 Intelligent UV–VIS Detector, Jasco, Japan, with a 50-μL Rheodyne 7725 loop injector USA, managed by Jasco Borwin Chromatography software version 1.05. The mobile phase consisted of acetonitrile/10 mM phosphate buffer pH 4.5 in the ratio of 55:45. The flow rate was adjusted to 1.0 ml/min, and the detection wavelength was set at 288 nm which is the wavelength of maximum absorption for DLX.
In Vitro Release Study
In vitro release of DLX was evaluated using dialysis bag method (19). Five-milliliter DLX-SLN dispersion was transferred to dialysis bag (m.w. cutoff 12,000 Da, Himedia, India) and immersed in 500 ml phosphate buffer pH 6.8 maintained at 37°C. The medium was stirred at 50 rpm. An aliquot of 5 ml was removed at predetermined time points viz. 0.5, 1, 2, 6, 8, 12, 24, 36, and 48 h and analyzed by a validated HPLC method as mentioned in previously. All experiments were done in triplicate.
Evaluation of Chemical Stability in Aqueous and Acidic Media
Hydrolytic stability offered by SLNs was evaluated by placing DLX solution and DLX-SLNs for 1 month at 25°C and 40°C. After 1 month, samples were evaluated for drug content by HPLC method.
In order to check the DLX stability in acidic environment, DLX aqueous solution (0.5%–2 ml) and 2 ml of DLX-SLNs containing DLX equivalent to 0.5% were transferred to dialysis bag (m.w. cutoff 12,000 Da, Himedia, India) and immersed in 500 ml of 0.1 N HCl maintained at 37°C. The medium was stirred at 50 rpm. An aliquot of 5 ml was removed at predetermined time points viz. 0.5, 1, and 2 h and analyzed by HPLC method. No attempts were made to separate unentrapped drug from SLNs. The percent degradation of drug was calculated as
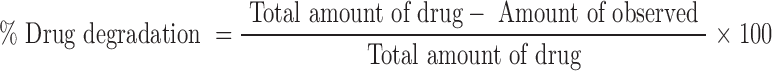 |
In Vivo Antidepressant Activity
In vivo efficacy was evaluated on male Swiss albino mice by forced swim test (20). The protocol for the study was approved by the Institutional Ethics Committee of Bombay College of Pharmacy, Mumbai, India, and the animal handling throughout the experiment was performed in accordance to the Committee for the Purpose of Control and Supervision of Experiments on Animals guidelines. Eighteen male Swiss albino mice, weighing between 25 and 30 g, were divided randomly in three groups (n = 6), viz. control group, DLX solution group, and formulation group consisting of DLX-SLNs. The dose for antidepressant activity was calculated as per Center for Drug Evaluation and Research guidelines using the formula based on body surface area (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm079266.pdf, Accessed April 16, 2010). The animal dose selected was 7.69 mg/kg body weight which corresponds to therapeutic human dose of 40 mg. The control group received 0.25 ml of saline solution. The DLX solution group received 7.69 mg/kg duloxetine, dissolved in distilled water. The formulation group received solid lipid nanoparticles (prepared by ultrasound dispersion method) equivalent to 7.69 mg/kg DLX. In the pre-test session, the mice were forced to swim for 10 min in an appropriate apparatus (25 cm id × 30 cm height) with water filled up to 25 cm height. On the next day, animals were again forced to swim for 6 min. A number of stops, i.e., number of times animal become immobile during stipulated time, were calculated in 6 min of swimming time. After the measurement, animals received the first dose of vehicle/drug solution/formulation and were returned to cages. After 22 h, the animals received second dose, following which the animals were forced to swim after 2, 6, and 24 h. A number of stops during 6 min of swimming period were recorded at every time point.
Statistical Analysis
Both inter-group and intra-group comparison were done by applying statistics (Sigma Plot 11) to the number of stops. Data were expressed as mean ± standard deviation, and the number of stops for the three groups was statistically assessed by paired t test and one-way analysis of variance followed by Bonferroni’s test and p value of <0.05 was considered as significant.
Preparation and Characterization of SLN Granules
The SLN dispersion was converted into dry granules by adsorbing on suitable adsorbent. In this method, the dispersion was placed in a bowl to which the adsorbent was added slowly and mixed vigorously to get the granular wet mass. The wet mass was dried in vacuum dryer for 4 h to obtain dry granules. These were then passed through a 500-μm sieve (B. S. S. 30 mesh) to get uniform-sized, free-flowing granules.
Different soluble as well as insoluble excipients were employed to prepare dried nanoparticles which gave good particle size in nanometer range after redispersion (data not shown). Best results with respect to particle size and PI were obtained by colloidal silicon dioxide, and hence, further evaluation was done using colloidal silicon dioxide as adsorbent. The SLN granules obtained were characterized for particle size, PI, flow properties, and morphology.
Particle Size and PI
Granules weighing 50 mg were dispersed in 10 ml double-distilled water and kept on horizontal shaker for 1 h. It was then filtered through coarse filter (Whatman filter no. 41), and the filtrate was analyzed for particle size by using Beckman coulter—N5 submicron particle size analyzer.
Morphology
The surface morphology, shape, and homogeneity were investigated using scanning electron microscope (SEM; JSM 6380, JEOL, Japan). The samples were sputter-coated with platinum under vacuum at room temperature before examination to render the surface of particles electroconductive.
Flow Properties
Flow property was evaluated by angle of repose by fixed height cone method. Accurately weighed powder was taken in a funnel maintained at 4 in. from the surface. The powder was allowed to flow through the funnel freely onto the surface. The height and diameter of the powder cone were measured, and angle of repose was calculated using equation:
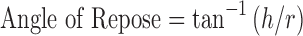 |
where h is height in centimeters of the powder cone and r the radius in centimeters of the powder cone.
RESULTS AND DISCUSSION
Screening of Lipids
Table I shows the amount of lipid required to solubilize DLX and percent partitioning of DLX in different lipids. DLX solubility and partitioning were found to be maximum in GMS, based on which GMS was selected for nanoparticulate formulations for further studies.
Formulation, Optimization, and Characterization of DLX-SLNs
Blank and DLX-loaded SLNs were prepared by two different method viz. solvent evaporation and ultrasound dispersion method. Processing parameters such as drug loading, lipid load, type and concentration of surfactant, particle size, and EE were optimized for both the methods in order to achieve higher EE with good chemical and physical stability to DLX-SLN system. Surfactant plays a very crucial role in formation and stabilization of nanoparticles in different environment. Based on safety and acceptability, profile surfactants like Solutol HS 15, Tween 80, Cremophor EL, Poloxamer 188, and PVA were screened for both the methods.
Solvent Evaporation Method
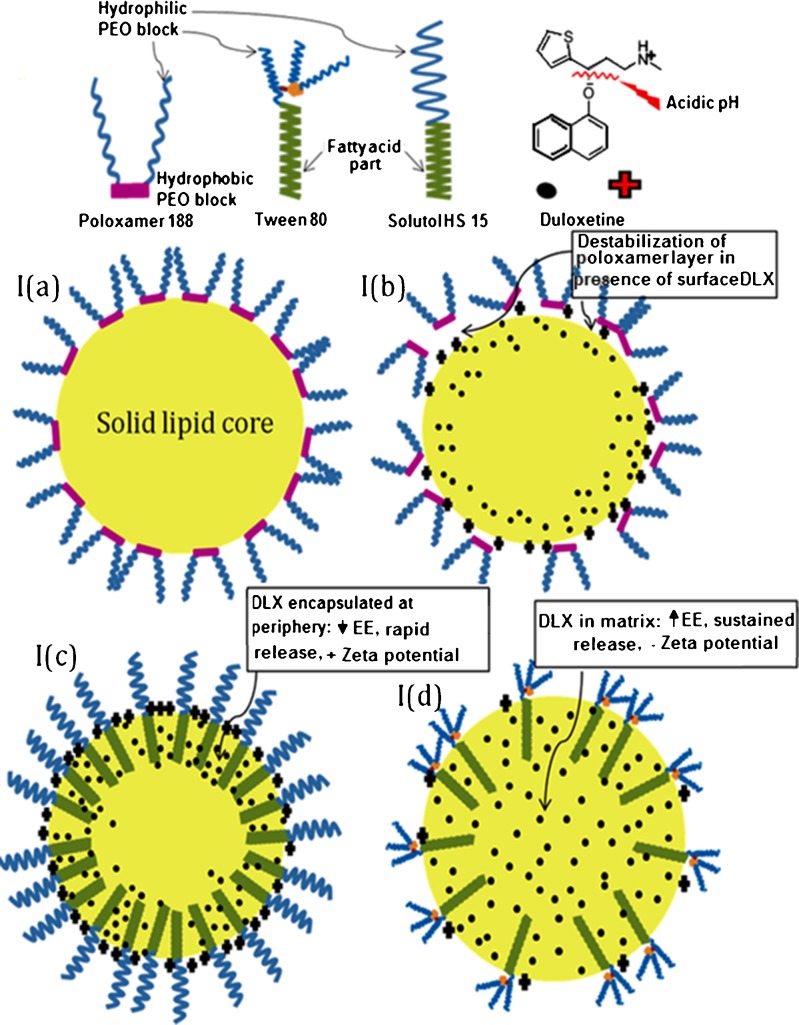
Particle size was optimized by varying parameters like aqueous phase volume, concentration and type of surfactant, lipid load, and drug load. Volume of organic phase and volume of aqueous phase were successfully reduced with simultaneously increase in lipid and drug load (data not shown). Blank SLNs prepared by solvent evaporation showed particle size ~250 and 450 nm with poloxamer 188 (2%) and PVA (2%), respectively, while the same composition with DLX loading showed particle size above 600 nm (Table II). SLNs prepared by using low concentration 0.5% or 1% were unstable and got converted into gel within few minutes which could be explained by concentration-dependent stabilization by poloxamer 188. The polypropylene oxide (PPO) portion of the poloxamer anchors to the hydrophobic surface of particles, leaving the polyethylene oxide (PEO) chains to protrude into the surrounding aqueous media (Fig. 1). Li et al. suggested that a low surface concentration allows a nonextended PEO chain conformation, which gives rise to more unstable particles (21). Nevertheless, if the poloxamer surface concentration is increased, crowded surfactant surface exhibits a more extended PEO layer which favors the steric stabilization. However, when DLX-SLNs were prepared by same method, micron-sized particles were obtained. Drug-loaded SLNs exhibited positive surface charge because of incorporation and adsorption of cationic drug on the surface of nanoparticles. Schematic representation of drug-incorporated SLNs was given in Fig. 1a. PPO block of poloxamer 188 might not have easy access to highly cationic surface, and hence, there could have been no adsorption of poloxamer on nanoparticles. Hence, poloxamer and PVA failed in stabilizing DLX-loaded SLN system Fig. 1b.
Table II.
Comparison of Particle Size of DLX-SLN Prepared by Different Surfactant and Methods
| Surfactant | Particle size (nm)/PI | |
|---|---|---|
| Solvent evaporation method | Ultrasound dispersion method | |
| Poloxamer 188 | 665/1.6 | – |
| PVA | 417.2/0.508 | – |
| Cremophor EL | 167.0/0.6 | 265.6/0.663 |
| Tween 80 | 145.3/0.686 | 103.0/0.249 |
| Solutol HS 15 | 133.6/0.256 | 131.3/0.536 |
| Tween 80 + Poloxamer 188 | – | 91.7/0.330 |
PI polydispersity index, EE entrapment efficiency
Fig. 1.
Schematic representation of SLN system and orientation of surfactant and DLX. a Blank (no-drug) SLN prepared using poloxamer 188 (solvent evaporation method). b DLX-loaded SLN prepared using poloxamer 188 (solvent evaporation method). c DLX-loaded SLN prepared using Solutol HS 15 (solvent evaporation method). d DLX-loaded SLN prepared using Tween 80 and Poloxamer 188 bland (ultrasound dispersion method)
Blank SLNs prepared with Tween 80, Solutol HS 15, and Cremophor EL gave particle size ~150 nm, and more interestingly, DLX-SLNs showed particle size similar to blank SLNs (Table II). Solutol HS 15 was selected as surfactant based on particle size, PI, and stability of nanoparticulate system. Figure 1c represents DLX-loaded SLN stabilized by Solutol HS 15. Unlike poloxamer or PVA which provide stabilization by adsorption, fatty acid-based surfactants offer stability due to fatty acid chain incorporated into lipid matrix and PEG chain protruding outside in aqueous phase. Particle size increased with increase in drug load at same surfactant concentration. Also, EE and particle size were reduced with increase in surfactant concentration. However, when drug load was increased to 22.5 mg, the particle size and EE reduced drastically to 74.5 nm and 17.53%, respectively. This system was also found to possess good colloidal stability. This could be due to adsorption of unentrapped drug on the lipidic surface of nanoparticles which in turn developed high positive charge on particle surface. This high charge causes electrostatic repulsion among particles and reduces particle aggregation (22).
Ultrasound Dispersion Method
In ultrasound dispersion method, up to 5% of lipid was loaded without adversely affecting the particles size and EE, which favored drug loading and the feasibility of converting it into dry powdered unit dosage form. However, stability problems were evident in systems stabilized by Tween 80, Cremophor EL, or Solutol HS 15. The batches prepared by above surfactants aggregated within 2–3 days and later on were converted into gel-like structure which was due to characteristic crystalline behavior of solid lipids and lack of surfactant to cover newly formed surface due to crystalline modification of lipids. Steric stabilizer poloxamer 188 (1%) used in combination with Tween 80 (2%) gave particle size less than 100 nm with good stability (Fig. 1d).
Particle Size and Entrapment Efficiency
Two formulations F1 and F2, prepared by solvent evaporation method and ultrasound dispersion method respectively, were characterized further. Their compositions are given in Table III. DLX-SLNs prepared with ultrasound dispersion method gave smaller particle size and higher EE in comparison to SLNs prepared by solvent evaporation method (Table III). SLNs prepared by solvent evaporation underwent gelation within 1 month, but SLNs prepared by ultrasound dispersion were stable for longer period of time. This could be due to better association of surfactant with lipid particles in ultrasound dispersion method. Although total amount of surfactant in proportion with amount of lipid used in solvent evaporation method was four to five times higher in comparison to ultrasound dispersion method, SLNs prepared by ultrasound dispersion were more stable because of good association and use of blend of two different types of surfactants. Aqueous volume was higher in solvent evaporation method; hence, surfactant might be dispersed in water rather than associated with lipid and could not completely cover the surface of nanoparticles.
Table III.
Optimized Formulae of DLX-SLN by Solvent Evaporation and Ultrasound Dispersion Method
| Optimized batches | Solvent evaporation method F1 | Ultrasound dispersion method F2 |
|---|---|---|
| Formula of optimized batch | ||
| Solid lipid | GMS 300 mg | GMS 200 mg |
| Drug | DLX 15 mg | DLX 10 mg |
| Surfactant | Solutol HS 15 (2%) | Tween 80 (2%) + Poloxamer 188 (1%) |
| Batch volume | 35 ml | 5 ml |
| Particle size/PI (nm) | 246 nm/0.431 | 91.7 nm/0.33 |
| Entrapment efficiency | 67.5% | 87% |
| Zeta potential | ||
| Blank SLN | −32.20 mv | −22.97 mv |
| DLX-SLN | +24.11 mv | −8.62 mv |
Effect of Drug and Surfactant on Zeta Potential and Stability
There was a significant difference observed in the zeta potential of solid lipid nanoparticles prepared with and without loading DLX. Zeta potential of formulae without drug was more negative compared to drug-loaded formulae (Table III). DLX has a cationic moiety so adsorption of drug on surface shifts the zeta potential toward positive side. In F1, a significant change in zeta potential was observed between blank SLNs (−32.20 mv) and DLX-loaded SLNs (+24.11 mv) in comparison to F2. The probable reason behind difference in the shift of zeta potential between batches prepared by two methods could be entrapment of drug, availability of drug for adsorption on surface, as well surfactant on the surface.
DLX entrapment in F1, i.e., SLN prepared by solvent evaporation method was 67%, which was lower as compared to F2, prepared by ultrasound dispersion method (87%). In F1, more amount of free DLX is available on surface for adsorption, leading to highly positive zeta potential. Moreover, adsorption of poloxamer 188 on the nanoparticulate surface, in F2, would have been responsible for less change in surface charge in comparison to F1.
Surfactant for final formulations was selected on the basis of required specification like particle size and PI of nanoparticles, chemical stability of drug, and physical stability of the formulation. Thus, ultrasound method was found to give better results with respect to physical stability, and hence, formulation F2 was selected for further characterization.
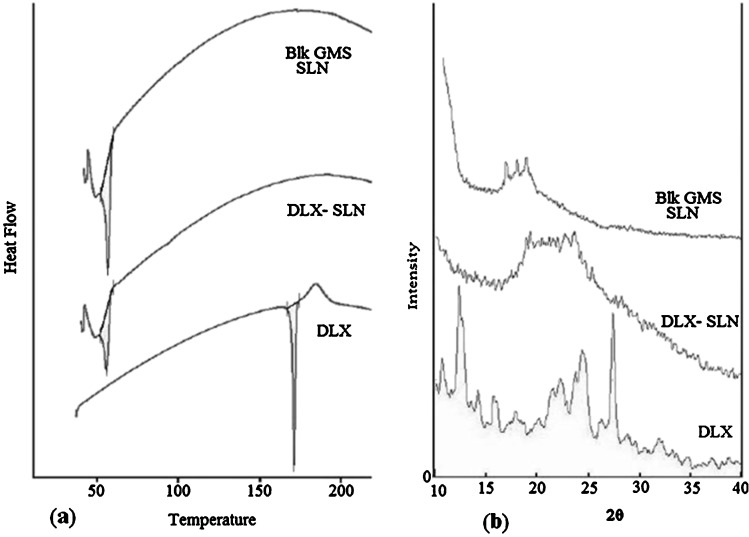
DSC and pXRD Analysis
The DSC thermograms and pXRD peaks of duloxetine HCl, blank SLNs, and DLX-SLNs are shown in Fig. 2. The DSC curve of DLX showed a melting endotherm at 171.48°C. The peak corresponding to the melting of DLX was disappeared in the thermograms of DLX-SLN formulation. Absence of melting endotherm in the thermograms of DLX-SLNs indicated complete solubilization of DLX inside the lipid matrix of the GMS.
Fig. 2.
DSC and XRD data. a Comparative thermograms of plain DLX, blank SLNs, and DLX-loaded SLNs; b comparative thermograms of plain DLX, blank SLNs, and DLX-loaded SLNs
The pXRD pattern of DLX shows principal peaks at angles of 12.5° and 27.6°. The principal peak of DLX was absent in DLX-loaded SLN formulations. Furthermore, the principal peak of the lipid did not shift but had a reduced intensity. These changes can be attributed to the incorporation of DLX between parts of the crystal lattice of the lipid, leading to a change in the crystallinity of the DLX-loaded SLNs. XRD diffractogram of blank and drug-loaded GMS SLN showed decrease in the crystallinity of GMS in the presence of drug. These values complement the DSC data and clearly indicate the possible change in crystallinity of the lipid after DLX incorporation and formulation as nanoparticles.
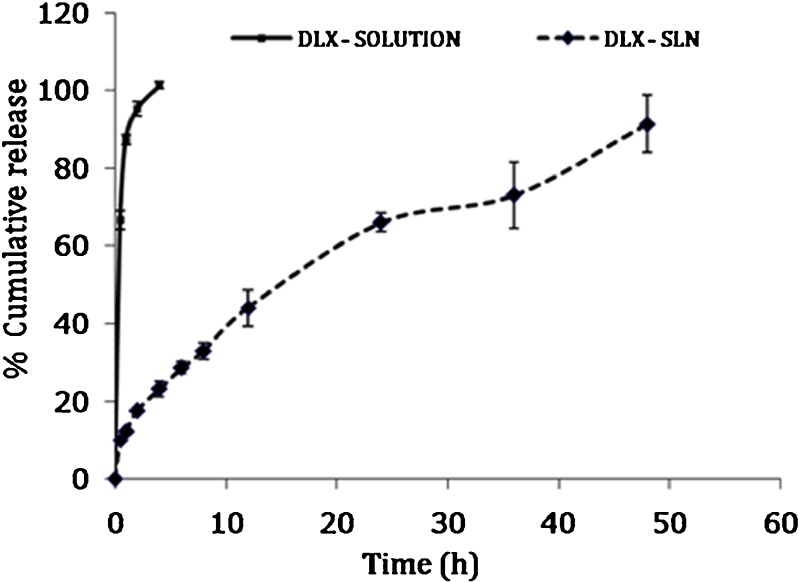
In Vitro Release Study
The in vitro release of duloxetine from the DLX solution and formulation F2 in phosphate-buffered saline (pH 6.8) at 37°C are shown in Fig. 3. The results indicated that the release of DLX from DLX solution was very quick. Approximately 87.28% of the drug was released within 1 h, and complete drug release was observed within 4 h. On the contrary, the release of DLX from SLNs was slow, and 12.1% was released within 1 h followed by a slower and continuous release reaching 91.33% in 48 h. Less burst release indicated that DLX was uniformly dispersed into the lipid matrix. Drug release data from the DLX-SLNs were found to fit Higuchi kinetics according to the equation, Q = 13.065 t1/2–1.523 (r2 = 0.9929).
Fig. 3.
Comparison of release profile of drug solution and SLN. Plain drug solution (solid line), DLX-loaded SLNs (dashed line)
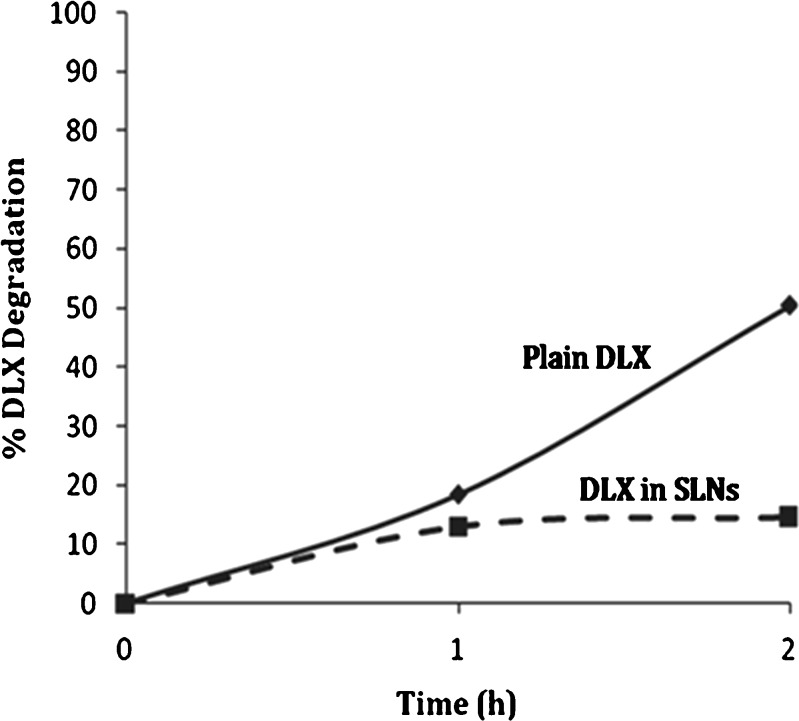
Evaluation of Chemical Stability of DLX in Aqueous and Acidic Media
There was no significant hydrolytic degradation observed in samples stored at 25°C. DLX solution stored at 40°C showed evidence of degradation (≈3%) within a month, while DLX-SLNs (formulation F2) stored at same temperature showed no sign of degradation of DLX. Acid degradation studies demonstrated significantly lower degradation in DLX-SLNs (formulation F2) in comparison to drug solution. The study indicated that almost half of the DLX degraded within 2 h in solution of pure DLX powder in HCl. In case of DLX-loaded SLN, 14.61% degradation was observed within 2 h (Fig. 4). The entrapment efficiency of DLX-loaded SLN was around 87%. Hence, 7–8% of drug degradation can be attributed to free drug present in the formulation which was exposed to HCl. This is evident from the first hour reading which indicates degradation of free drug initially. The remainder of degradation was due to slow drug released from SLN matrix at the end of 2 h. Hence, degradation was substantially lower than solution of pure DLX powder. This indicated that significant protection was offered by SLN matrix to DLX in acidic conditions.
Fig. 4.
Comparison of percent DLX degradation in HCl. Plain drug solution (solid line), DLX-loaded SLNs (dashed line)
In Vivo Antidepressant Activity
In vivo efficacy evaluation of drug solution and nanoparticulate formulation was done by forced swim test in mice. Rodents, when forced to swim in a restricted space from which they cannot escape, are induced to a characteristic behavior of immobility. This behavior reflects a state of despair which can be reduced by antidepressant drugs. Antidepressant drugs reduce immobility time as well as number of times animal stops swimming.
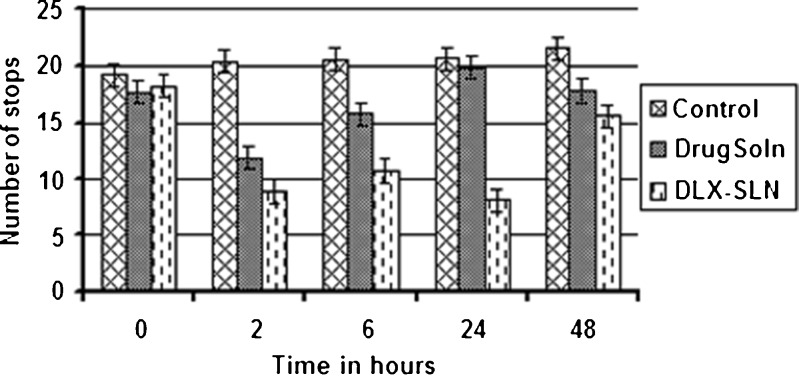
The results of forced swim test are shown in Fig. 5. There was a reduction in number of stops in mice treated with DLX-SLNs (formulation F2) as well as DLX solution. However, at every time point, DLX-SLNs showed more reduction in number of stops in comparison to DLX solution suggesting superior antidepressant activity. At 2 h post-dose, both drug solution- and formulation-treated animal groups showed significant difference in number of stops (p < 0.01) as compared to control group. Animals treated with drug solution showed 38.08% reduction in number of stops while formulation group showed 50.08% reduction in number of stops. At 6 h, animals treated with drug solution did not show antidepressant activity, and formulation group showed 33% less number of stops but did not show any statistically significant difference when compared with control. Initial higher activity could be due to quick availability of unentrapped DLX as well as initial DLX release which can be sufficient enough to block norepinephrine and serotonin reuptake. At 24 h, a number of stops for animals treated with drug solution came back to that of the initial value, but formulation group showed significant difference compare to both control and solution group (Fig. 5). Formulation group at 24 h maintained reduction in the number of stops up to 50%. Antidepressant activity at 24 h clearly indicates the sustained release and bioavailability enhancing potential of SLNs. In vitro release studies also showed sustained and continuous release of DLX from SLNs up to 48 h. The improved efficacy of formulation can be attributed to the sustained release observed in vitro.
Fig. 5.
Number of stops at different time intervals in swim test.  Control,
Control,  Drug solution,
Drug solution,  DLX-SLN
DLX-SLN
Formulation and Characterization of DLX-SLN Granules
The formula of dry granules of DLX-SLN is given in Table IV. The particle size, PI, and angle of repose of DLX-SLN granules are given in Table V. An increase in particle size and PI was observed when dried SLNs were resuspended. Particle size increased by 1.5 times, and the PI observed was three times the initial. Adsorption on neusilin particles was attempted by Chakraborty et. al. (15) where the particle size and PI of resuspended SLNs was less than the initial one. This difference in results could be due to difference in procedure in preparation of adsorbed SLNs. The authors have also attributed this observation to saturation of adsorbent. The particle size and PI observed by redispersion of SLN powders obtained using lyophilization and spray drying processes were close to that of initial SLNs. This can be due to high amount of carrier used in spray drying process which also increases the bulk of the formulations. Evaporative drying caused temperature-dependent increase in particle size and PI of resuspended SLNs (13, 14).
Table IV.
Formula of Dry Granules of DLX-SLN
| Ingredient | Quantity (mg) |
|---|---|
| DLX | 10 |
| Lipid | 200 |
| Surfactant | 6 |
| Colloidal silicon dioxide | 200 |
Table V.
Characterization of SLN Granules
| Parameter | Results |
|---|---|
| Particle size | 162.7 (96.3) |
| Polydispersity index | 0.638 (0.245) |
| Angle of repose | 29o |
Figures in parenthesis indicate initial particle size and PI
Figure 6 depicts SEM of SLN granules. The solid lipid nanoparticles are seen as tiny spherical structures. It is evident that SLNs were in nanosize shown by spherical particles adhering to surface of adsorbents. The picture also revealed the agglomeration of individual particles.
Fig. 6.
SEM of the adsorbed SLNs. The arrows indicate the SLNs adsorbed on inert carrier
Conclusion
Screening and selection of appropriate lipid and surfactant is very essential in designing SLN system with desired particle size and EE and also for maintaining integrity of SLN for longer period of time. Detailed screening of surfactant indicated that nanoparticles prepared with blend of chemically diverse surfactants exhibited desired particle size, distribution, and good stability. Formulation F2 prepared by ultrasound dispersion method proved to be better with respect to particle size and encapsulation efficiency as compared to formulation F1 prepared by solvent evaporation technique. SLNs proved to be a good system to sustain the release of DLX as well as lipid matrix gave good protection to encapsulated drug in acidic environment. In vivo efficacy of DLX-SLNs showed enhanced antidepressant activity in animals which is indicative of enhanced bioavailability. Adsorption of SLNs on carrier is cost-effective method in conversion of SLN dispersions into dry granules. Conversion of SLN dispersion into dried granules yielded granules with good flow and satisfactory particle size. Such systems can be further investigated to aid in commercialization of SLNs.
Acknowledgments
The authors are thankful to Shodhna Labs, Hyderabad, India, for providing gift sample of duloxetine hydrochloride and All India Council of Technical Education for funding the research work.
References
- 1.Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, Shaw JL, Thompson L, Nelson DL, Hemrick-Luecke SK, Wong DT. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25:871–80. doi: 10.1016/S0893-133X(01)00298-6. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Goldberg MJ, Cerimele BJ. Pharmacokinetics and safety of duloxetine, a dual-serotonin and norepinephrine reuptake inhibitor. J Clin Pharmacol. 2000;40:161–7. doi: 10.1177/00912700022008810. [DOI] [PubMed] [Google Scholar]
- 3.Turcotte JE, Debonnel G, de Montigny C, Hebert C, Blier P. Assessment of the serotonin and norepinephrine reuptake blocking properties of duloxetine in healthy subjects. Neuropsychopharmacology. 2001;24:511–21. doi: 10.1016/S0893-133X(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 4.Sinha VR, Kumria R, Bhinge JR. Stress degradation studies on duloxetine hydrochloride and development of an RP-HPLC method for its determination in capsule formulation. J Chromatogr Sci. 2009;47:589–93. doi: 10.1093/chromsci/47.7.589. [DOI] [PubMed] [Google Scholar]
- 5.Hauss DJ. Oral lipid-based formulations. Adv Drug Deliv Rev. 2007;59:667–76. doi: 10.1016/j.addr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–77. doi: 10.1016/S0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 7.Kumar VV, Chandrasekar D, Ramakrishna S, Kishan V, Rao YM, Diwan PV. Development and evaluation of nitrendipine loaded solid lipid nanoparticles: influence of wax and glyceride lipids on plasma pharmacokinetics. Int J Pharm. 2007;335:167–75. doi: 10.1016/j.ijpharm.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Luo Y, Chen D, Ren L, Zhao X, Qin J. Solid lipid nanoparticles for enhancing vinpocetine’s oral bioavailability. J Control Release. 2006;114:53–9. doi: 10.1016/j.jconrel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Muller RH, Runge S, Ravelli V, Mehnert W, Thunemann AF, Souto EB. Oral bioavailability of cyclosporine: solid lipid nanoparticles (SLN) versus drug nanocrystals. Int J Pharm. 2006;317:82–9. doi: 10.1016/j.ijpharm.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 10.Suresh G, Manjunath K, Venkateswarlu V, Satyanarayana V. Preparation, characterization, and in vitro and in vivo evaluation of lovastatin solid lipid nanoparticles. AAPS PharmSciTech. 2007;8:24. doi: 10.1208/pt0801024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunjes H, Westesen K, Koch MHJ. Crystallization tendency and polymorphic transitions in triglyceride nanoparticles. Int J Pharm. 1996;129:159–73. doi: 10.1016/0378-5173(95)04286-5. [DOI] [Google Scholar]
- 12.Zimmermann E, Muller RH, Mader K. Influence of different parameters on reconstitution of lyophilized SLN. Int J Pharm. 2000;196:211–3. doi: 10.1016/S0378-5173(99)00424-X. [DOI] [PubMed] [Google Scholar]
- 13.Freitas C, Mullera RH. Spray-drying of solid lipid nanoparticles (SLN TM) Eur J Pharm Biopharm. 1998;46:145–51. doi: 10.1016/S0939-6411(97)00172-0. [DOI] [PubMed] [Google Scholar]
- 14.Cavalli R, Gasco MR, Barresi AA, Rovero G. Evaporative drying of aqueous dispersions of solid lipid nanoparticles. Drug Dev Ind Pharm. 2001;27:919–24. doi: 10.1081/DDC-100107672. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborty S, Shukla D, Vuddanda PR, Mishra B, Singh S. Utilization of adsorption technique in the development of oral delivery system of lipid based nanoparticles. Colloids Surf B Biointerfaces. 2010;81:563–9. doi: 10.1016/j.colsurfb.2010.07.058. [DOI] [PubMed] [Google Scholar]
- 16.Dixit RP, Nagarsenker MS. Dry adsorbed emulsion of simvastatin: optimization and in vivo advantage. Pharm Dev Technol. 2007;12:495–504. doi: 10.1080/10837450701557246. [DOI] [PubMed] [Google Scholar]
- 17.Dixit RP, Nagarsenker MS. Self-nanoemulsifying granules of ezetimibe: design, optimization and evaluation. Eur J Pharm Sci. 2008;35:183–92. doi: 10.1016/j.ejps.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Venkateswarlu V, Manjunath K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J Control Release. 2004;95:627–38. doi: 10.1016/j.jconrel.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Yang SC, Lu LF, Cai Y, Zhu JB, Liang BW, Yang CZ. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J Control Release. 1999;59:299–307. doi: 10.1016/S0168-3659(99)00007-3. [DOI] [PubMed] [Google Scholar]
- 20.Ciulla L, Menezes HS, Bueno BB, Schuh A, Alves RJ, Abegg MP. Antidepressant behavioral effects of duloxetine and fluoxetine in the rat forced swimming test. Acta Cir Bras. 2007;22:351–4. doi: 10.1590/S0102-86502007000500005. [DOI] [PubMed] [Google Scholar]
- 21.Li JT, Caldwell KD, Rapoport N. Surface properties of pluronic-coated polymeric colloids. Langmuir. 1994;10:4475–82. doi: 10.1021/la00024a016. [DOI] [Google Scholar]
- 22.Han F, Li S, Yin R, Liu H, Xu L. Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: nanostructured lipid carriers. Colloids Surf A Physicochem Eng Asp. 2008;315:210–6. doi: 10.1016/j.colsurfa.2007.08.005. [DOI] [Google Scholar]