Abstract
The purpose of this research was to formulate nanostructured lipid carriers (NLC) for the parenteral delivery of an anticancer drug, all-trans retinoic acid (ATRA). The ATRA was incorporated into NLC by the de novo emulsification method. The effect of the formulation factor, i.e., type and oil ratio, initial ATRA concentration on physicochemical properties was determined. The anticancer efficacy of ATRA-loaded NLC on HL-60 and HepG2 cells was also studied. NLC was formulated using a blend of solid lipids (cetyl palmitate) and liquid lipids (soybean oil (S), medium-chain triglyceride (M), S/oleic acid (O; 3:1) and M/O (3:1)) at a weight ratio of 1:1. ATRA-loaded NLC had an average size of less than 200 nm (141.80 to 172.95 nm) with a narrow PDI and negative zeta potential that was within an acceptable range for intravenous injection. The results indicated that oleic acid enhanced the ATRA-loading capacity of NLC. In vitro ATRA release was only approximately 4.06% to 4.34% for 48 h, and no significant difference in ATRA release rate from all NLC formulations in accordance with the composition of the oil phase. Moreover, no burst release of the drug was observed, indicating that NLC could prolong the release of ATRA. The initial drug concentration affected the photodegradation rate but did not affect the release rate. All ATRA-loaded NLC formulations exhibited the photoprotective property. The cytotoxicity results showed that all ATRA-loaded NLC had higher cytotoxicity than the free drug and HL-60 cells were more sensitive to ATRA than HepG2 cells.
Key words: all-trans retinoic acid, anticancer, nanostructured lipid carrier, oleic acid, parenteral delivery
INTRODUCTION
All-trans retinoic acid (ATRA) is a physiologically active form of a metabolic product of vitamin A. It is a poorly water soluble substance and is sensitive to light, heat, and air. Retinoids have been used as chemopreventive and anticancer agents because of their pleiotropic regulator function in cell differentiation, growth, proliferation, DNA repair, and apoptosis. ATRA can suppress the process of carcinogenesis both in vitro and in vivo, especially in the treatment of acute promyelocytic leukemia, Kaposi’s sarcoma, head and neck squamous cell carcinoma, ovarian carcinoma, bladder cancer, neuroblastoma, and liver cancer (1–3). Although oral administration of ATRA dosage form has been demonstrated to be effective against a range of cancers in clinical trials, an important drawback of using oral ATRA is its poor bioavailability. Therefore, plasma concentrations of orally administered ATRA are highly variable (4). There have been attempts to develop other routes of administration that might increase the therapeutic efficacy and reduce the side effects of the drug.
Parenteral formulations seem to be a good alternative for ATRA administration to increase the reliable potency and duration of ATRA’s activity in cancer patients. However, ATRA’s hydrophobicity is a limitation. To improve its aqueous solubility, attempts have been made to develop intravenous injectable formulations of ATRA using drug delivery systems such as cyclodextrin complexes (5), liposomes (6), niosomes (7), polymeric micelles (1,8), lipid emulsion (LE; 2,9), microemulsion (10), solid lipid nanoparticles (SLNs; 11), and nanostructured lipid carriers (NLC; 12). Currently, a parenteral liposomal encapsulated-ATRA formulation is being tested in a phase II clinical trial: ATRA-IV® (formerly known as ATRAGEN®, Antigenics Inc.; 6). The incorporation of ATRA into liposomes has significantly improved the potency and duration of ATRA’s activity while reducing the side effects associated with this drug. Most importantly, intravenously administered liposomal encapsulated ATRA could maintain stable plasma concentrations over a prolonged period after multiple dosing in preclinical and clinical models (6). Although liposomal encapsulated ATRA has advantages over the oral formulation, its capacity for drug loading and stability during storage are limited. For this reason, a new drug delivery system with a high solubilizing capacity for ATRA is needed. SLN and NLC are the most promising of the solubilizing approaches. In a previous study, we investigated the enhancement of ATRA loading in nanolipid emulsions and the improvement in the stability of the formulation using oleic acid (13). SLN have shown to be advantageous over liposomes and lipid emulsions in terms of physical stability, greater flexibility in modulating drug release, and protection of labile drugs from chemical, photochemical or oxidative degradation. Moreover, SLN have the advantages of solid polymeric particles, a solid matrix for controlled drug release, protection of incorporated drugs against chemical degradation, and slower in vivo metabolism (14). However, there are also limitations of the SLN system, such as limitations of drug loading due to the solubility of the drug in the solid lipid, the drug expulsion phenomenon (when lipid crystallizes to the stable β-form), and the particle concentration in the aqueous dispersions ranging from approximately 1% to a maximum of only 30%.
NLC were introduced at the end of the 1990s to overcome the potential limitations of SLN described above; the formation of lipid crystals affects drug loading, and the ongoing crystallization process towards a perfect crystal causes drug expulsion (11). NLC have been proposed as the SLN of a new generation; they comprise particles with a solid lipid matrix with an average diameter in the nanometer range. This carrier system can be used to overcome the observed limitations of conventional SLN, thus increasing the payload and preventing drug expulsion (15). For the production of NLC, very different lipid molecules are often mixed, i.e., blending solid lipids with liquid lipids. The resulting matrix of lipid particles shows a melting point depression compared to the original solid lipid. Hence, an increase in drug loading capacity can avoid/minimize potential expulsion of the active compounds during storage and can prevent a reduction in the water content of the particle suspension has a lower water content (16). The smart NLC as the new generation offer much more flexibility in drug loading, modulation of drug release and improved performance in producing final dosage forms, which include creams, tablets, capsules, and injectables. Many different drugs have been incorporated into NLC, but they are mainly used in dermal applications, and few investigations have focused on the parenteral route (13).
Therefore, the objective of this research was to develop an NLC formulation for the parenteral delivery of the anticancer drug, ATRA, with high loading and high stability. The formulation factor, i.e., type and oil ratio, initial ATRA concentration on physicochemical properties (particle size, size distribution, droplets surface charge, percentage yield, percentage drug release and photostability) were investigated. Moreover, the anticancer activity of ATRA and ATRA-loaded NLCs on human acute promyelocytic leukemia cells (HL-60) and human hepatoma cells (HepG2) was also studied.
MATERIALS AND METHODS
Materials
ATRA and butylated hydroxytoluene (BHT) were purchased from Sigma chemical company (St. Louis, MO, USA). Cetyl palmitate (Sabowax; CP) was purchased from SABO SpA (Levate, Italy). Soybean oil (S) and oleic acid (O) were purchased from Fluka Chemie AG (Seelze, Germany). Medium-chain triglyceride (M; Estasan® 3580) was obtained from Uniquema Asia Pacific (Kuala Lumpur, Malasia). Lecithin (Epikuron® 200, a soy phospholipid complex with a minimum of 92% phosphatidylcholine) was purchased from Degussa Bioactives GmbH (Freising, Germany). Polysorbate-80 was purchased from the NOF Corporation (Osaka, Japan). All other chemicals were commercially available and were of analytical and high-performance liquid chromatography (HPLC)-grade.
Preparation of ATRA-Loaded NLCs
ATRA-loaded NLCs were prepared by the de novo emulsification method. The composition of the oil phase was ATRA, a mixture of liquid lipid and solid lipid (S/CP, M/CP, SO/CP, MO/CP), lecithin and BHT, whereas the aqueous phase was composed of polysorbate-80 and distilled water. After separately preheating the oil phase and aqueous phase to 70°C, the aqueous phase was transferred to the oil phase and stirred with a magnetic stirrer at 1,400 rpm for 1 min. To reduce the droplet size to the nanometer range, these mixtures were placed in 25°C sonicator bath and sonicated five times for 5 min with an interval of 10 s at 40% amplitude by a probe-type ultrasonicator (Sonics & Materials, CT, USA). The pH of the formulations was subsequently adjusted to 8 ± 0.1 with 1 N NaOH, then filtered through a 0.45-μm membrane filter to remove precipitated ATRA. The formulations were stored in light-protected and sealed containers at 4°C. The sterilization method used in this study was sterilization with membrane filtration (through a 0.22-μm membrane filter).
Incorporation Efficiency
The concentrations of ATRA incorporated into NLC were directly determined by HPLC after appropriate dilution with isopropyl alcohol (IPA). All samples were measured in triplicate. The percentage yield or incorporated efficiency of ATRA in NLC and ATRA content were calculated as presented in Eqs. 1–2, respectively.
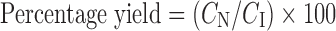 |
1 |
Where CN is the concentration of ATRA measured in the NLC; CI is the initial concentration of ATRA added in the NLC.
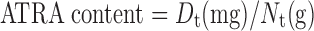 |
2 |
Where Dt is the total amount of ATRA in NLC and Nt is the total amount of NLC.
HPLC Assay
ATRA was assayed using HPLC method (7). The HPLC system (Thermo Separation Products, Mountain View, CA, USA) consisted of a mobile phase delivery pump (P-1500), UV detector (UV-1000) and integrator (I-1000). The C18 reverse-phase column (Symmetry®, Waters, Milford, MA, USA) with dimensions of 5 μm, 150 × 0.5 mm was used. The mobile phase was composed of an 84.5:15.0:0.5% (v/v/v) mixture of acetonitrile/water/glacial acetic acid, pH 4.2, and detected at 342 nm. The injection volume was 20 μL and the flow rate was 1.5 mL/min. Validation for ATRA analysis, i.e., the system suitability, accuracy, precision, and linearity, was performed. The quantitative determination of ATRA in the tested samples was obtained from the calibration curve, which gave good linearity at a range of 0.01–100 μg/mL (R2 = 0.9997).
Particle Size and Charge
NLC droplet size, polydispersity index (PDI), and zeta potential of ATRA-loaded NLC were determined at 25°C by photon correlation spectroscopy using a Zetasizer Nano ZS. Prior to measurement, 1 μL of NLC was diluted with 1 mL distilled water that was filtered through a 0.22-μm membrane filter. All samples were measured in triplicate.
Formulation Stability Study
Photostability Study
To investigate the effects of NLC on photodegradation protection over the time, samples were placed in a 1-mL tightly closed glass tube and were irradiated up to 6 h using a UV lamp (wavelength of 320–400 nm) placed 50 cm from the samples. Eighty-microliter aliquots were taken from each sample at set time intervals (0.5, 1, 2, 4, and 6 h) and diluted with IPA. The amounts of intact ATRA were analyzed by HPLC. For comparison, an isopropyl alcohol solution of ATRA in the same concentration was investigated by the same procedures as the NLC.
Short- and Long-Term Stability Study
ATRA-loaded NLCs were stored at 4°C for 56 days. For the accelerated condition, the formulations were autoclaved at 121°C for 15 min (Autoclave: Tattnauer; Germany). Both the physical and chemical stability of ATRA were evaluated. The physical stability was determined by visual observation, such as drug crystallization and phase separation. The microscopic assessment was carried out using an optical BX51 microscope with a polarized filter (Olympus, Japan). The chemical stability was determined by measuring drug content on days 0, 14, 28, 42, and 56 by HPLC assay.
In Vitro Drug Release Study
The in vitro release studies of NLC were operated using a dialysis bag (MWCO 6000–8000, CelluSep®, Membrane Filtration Products, USA). Fifty milliliters of a 10:15:75% (v/v) mixture of ethanol/polysorbate-80/phosphate buffer, pH 7.4, was used as the receptor medium and for the establishment of sink conditions (13). One milliliter of the NLC was placed in the dialysis bag, and the dialysis bag was placed in a double-jacket beaker with an external constant temperature circulation water bath under constant stirring at 37 ± 0.5°C and protected from light. All experiments were carried out with protect from light. At time intervals of 1, 2, 4, 8, 12, 24, and 48 h, 3-mL aliquots of the medium were withdrawn, and the same volume of fresh medium was added. The content of ATRA in the sampling solutions was assayed by HPLC. All experiments were performed in triplicate.
Anticancer Activity of ATRA
The investigation of the anticancer activity of ATRA and ATRA-loaded NLC was based on the inhibition of proliferation in two different types of cancer cells, human acute promyelocytic leukemia cells (HL-60 cells) and human hepatocellular carcinoma (HepG2) cells, by MTT assay (10–12). HL-60 cells and HepG2 cells were routinely cultured in Iscove’s modified Dulbecco’s medium (IMDM) and minimum essential medium (MEM) supplemented with 10% fetal bovine serum, 1% non-essential amino acid, and 1% glutamax® in a humidified atmosphere (5% CO2, 95% air, 37°C). The cells were seeded in 96-well plates at 2 × 104 and 5 × 104 cells/well densities for the HL-60 and HepG2 cells, respectively. After 4-h (HL-60) or overnight (HepG2) incubation, varying concentrations of free ATRA or ATRA-loaded NLC formulations was added to the wells after dilution with culture medium. Free ATRA was prepared by dissolving in DMSO and then dilution with culture medium to obtain varying concentrations of ATRA. The cells were also separately treated with DMSO dilutions as a control. After 4-day (HL-60) or overnight (HepG2) incubation under 5% CO2 at 37°C, the medium was removed, and 20 μL of MTT (3-(4,5-dimethy-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide, 5 mg/mL) in serum-free medium was added to each well. The cells were incubated for 4 h to allow the formation of formazan; the medium was then removed, and 100 μL of DMSO was added to dissolve the formazan crystals. The absorbance of formazan was measured at 550 nm using a microplate reader (SpectraMax®M2, Molecular Devices; USA). Each experiment was performed in triplicate. The relative cell viability was calculated using the following Eq. 3 (11,12).
 |
3 |
Where OD550 (sample) represents the absorbance from the wells treated with samples, and OD550 (control) represents the absorbance from the wells treated with medium alone.
Statistical Analysis
All values were expressed as the mean value ± standard deviation. Statistical significance of differences was examined using one-way analysis of variance followed by a least significant difference post hoc test. The significance level was set at p < 0.05.
RESULTS AND DISCUSSIONS
Preparation of ATRA-Loaded NLCs
Our previous study showed that ATRA-loaded lipid emulsions (ATRA-LEs) consisting of 30% medium-chain triglyceride (M) as an oil phase and 1.2% lecithin as an emulsifier combined with 8% polysorbate-80 as a co-emulsifier produced good stability (7). Moreover, lipid emulsion formulations of oil mixtures (S/O and M/O = 3:1) could form a lipid emulsion, and oleic acid enhanced the loading capacity of ATRA in such lipid emulsions (12). Therefore, these formulations were selected in the present study with the addition of solid lipid (CP). The oil phases were composed of a liquid lipid blend with CP at a weight ratio of 1:1 as S/CP (1:1), M/CP (1:1), S/O/CP (3:1:4) and M/O/CP (3:1:4). The ATRA-loaded NLCs formulations are shown in Table I. Probe sonication was used to reduce the emulsion droplets to achieve sizes in the nanometer range. The obtained ATRA-loaded NLCs formulations existed as a milky emulsion with an off-white to pale-yellowish color.
Table I.
Composition of ATRA-Loaded NLC Formulations
| Composition | Formulations | |||
|---|---|---|---|---|
| S/CP | M/CP | S/O/CP | M/O/CP | |
| ATRA (mg/g of NLCs) | 1,3,5,7,9 | 1,3,5,7,9 | 1,3,5,7,9 | 1,3,5,7,9 |
| Oil phase (%) | ||||
| Soybean oil (S) | 15 | – | 11.25 | – |
| Medium chain triglyceride (M) | – | 15 | – | 11.25 |
| Oleic acid (O) | – | – | 3.75 | 3.75 |
| Cetyl palmitate (CP) | 15 | 15 | 15 | 15 |
| Lecithin (%) | 1.2 | 1.2 | 1.2 | 1.2 |
| BHT (%) | 0.002 | 0.002 | 0.002 | 0.002 |
| Polysorbate-80 (%) | 8 | 8 | 8 | 8 |
| Distilled water qs. | 100 | 100 | 100 | 100 |
Incorporation Efficiency
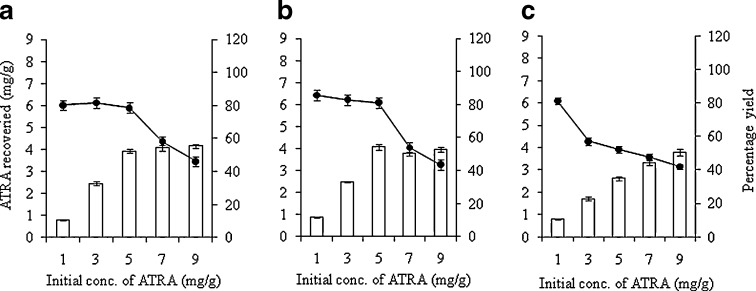
To study the amount of ATRA loaded in the NLC formulation, the initial addition of ATRA was varied (1, 3, 5, 7, and 9 mg/g of NLC). The physical properties, ATRA content and incorporation efficiency of the ATRA-loaded NLC were examined using different types of oil phases. To investigate the influence of the ratios of liquid lipid (M)/solid lipid (CP) in the oil phase on the incorporation efficiency of the ATRA-loaded NLC, the mixture of M and CP were varied to be 5:1, 1:1, and 1:5. As the solid lipid (CP) concentration in the oil matrix increased, the incorporation efficiency of ATRA decreased. The results shown in Fig. 1 revealed that the ATRA incorporation efficiency loaded at an M/CP ratio of 5:1, 1:1, and 1:5 was higher than 80% with ≤5, ≤5, and ≤1 mg/g of initial ATRA, respectively. High drug loading in NLC and a good physical appearance of the NLC were the major objectives of this study. Therefore, liquid lipids with an equal ratio of solid lipid (1:1) were chosen as the oil phase for future formulation and characterization.
Fig. 1.
Effect of initial ATRA concentration on (open square) the ATRA content and (filled circle) the percentage incorporation efficiency of ATRA incorporated in NLC formulations composed with different ratios of liquid lipid (medium-chain triglyceride, M)/solid lipid (cetyl palmitate, CP); a M/CP = 5:1, b M/CP = 1:1, c M/CP = 1:5
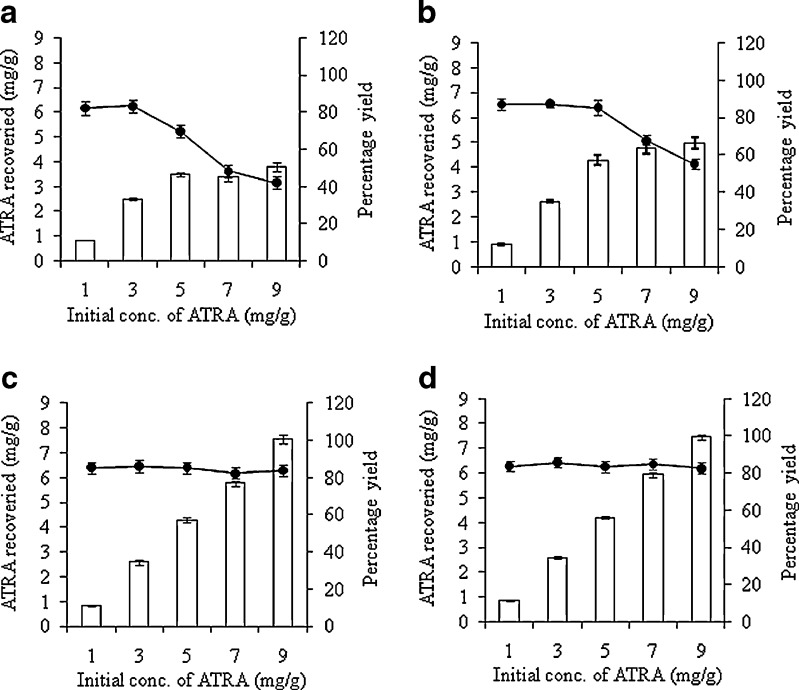
In all formulations (Table I), as the initial ATRA concentration increased from 1 to 9 mg/g, the incorporation efficiency of ATRA decreased. The incorporation efficiencies of ATRA-loaded NLC composed of S/CP, M/CP, S/O/CP, and M/O/CP were higher than 80% with ≤3, ≤5, ≤9, and ≤9 mg/g of initial ATRA, respectively (Fig. 2). The highest loading capacity of ATRA in S/CP, M/CP, S/O/CP, and M/O/CP NLC formulations was 3.80 ± 0.16, 5.44 ± 0.08, 7.55 ± 0.19, and 7.63 ± 0.16 mg/g, respectively. These results demonstrated that NLC formulations consisting of S/O/CP and M/O/CP gave the highest ATRA loading at initial concentrations of ATRA of 9 mg/g. These results indicated that oleic acid influenced the ATRA-loading capacity in the same way as the lipid emulsions. However, regardless of the type of oil, the loading capacity of ATRA into NLC formulations was higher than that into lipid emulsions. The highest loading capacity of the S/O and M/O (3:1) lipid emulsion formulations were 5.13 ± 0.10 mg/g and 4.75 ± 0.04 mg/g when the initial concentrations of ATRA were 7 and 5 mg/g, respectively (12). Although it is not clear how CP increased the ATRA-loading in NLC, it might be possible that NLC was composed of solidified lipid, which could give higher viscosity within the lipid droplet of NLC and thus less mobility of incorporated ATRA or less migration of ATRA from the lipid particles. These results demonstrated that NLCs formulation have advantages in ATRA-loading capacity over lipid emulsions.
Fig. 2.
Effect of initial ATRA concentration on (open square) the ATRA content and (filled circle) the percentage incorporation efficiency of ATRA incorporated in NLC formulations composed with different oil phases; a S/CP (1:1), b M/CP (1:1), c S/O/CP (3:1:4) and d M/O/CP (3:1:4)
Particle Size and Charge
The mean particle size and surface charge are important parameters for predicting the physical stability of colloidal dispersion systems (17). In the preliminary stability test, all ATRA-loaded NLC were kept at 4°C for 7 days, and good stability formulations (no ATRA crystal) were chosen for future characterization. All selected NLC formulations had average sizes less than 200 nm (141.80–172.95 nm) with a narrow PDI and negative zeta potential, which was within an acceptable range for intravenous injection. The zeta potential values of NLC formulated with S/CP and M/CP was approximately −32.23 to −34.92 mV. NLC formulated with S/O/CP and M/O/CP gave a higher negative charge of −55.23 to −57.38 mV (Table II). NLC formulated with oleic acid exhibited a higher negative charge compared with those without oleic acid. These results indicated that oleic acid affected the incorporation efficiency and stability of ATRA-loaded NLC by improving the negative surface charge of lipid droplets. The previous study showed that the addition of oleic acid to parenteral fat emulsions improve the stability of the emulsion (18).
Table II.
Particle Size and Zeta Potential of ATRA-Loaded NLCs
| Formulations | Size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|
| S/CP-ATRA 1 mg/g | 168.10 ± 1.21 | 0.13 ± 0.02 | −32.23 ± 2.06 |
| S/CP-ATRA 3 mg/g | 170.73 ± 1.50 | 0.14 ± 0.03 | −32.42 ± 2.87 |
| M/CP-ATRA 1 mg/g | 151.03 ± 2.44 | 0.15 ± 0.03 | −34.53 ± 1.21 |
| M/CP-ATRA 3 mg/g | 151.65 ± 2.42 | 0.17 ± 0.05 | −34.92 ± 3.87 |
| M/CP-ATRA 5 mg/g | 153.17 ± 1.19 | 0.16 ± 0.03 | −33.90 ± 2.25 |
| S/O/CP-ATRA 1 mg/g | 162.68 ± 0.75 | 0.11 ± 0.04 | −57.02 ± 1.55 |
| S/O/CP-ATRA 3 mg/g | 167.10 ± 3.01 | 0.13 ± 0.04 | −57.38 ± 4.39 |
| S/O/CP-ATRA 5 mg/g | 169.50 ± 1.55 | 0.12 ± 0.03 | −56.63 ± 1.44 |
| S/O/CP-ATRA 7 mg/g | 170.80 ± 4.67 | 0.14 ± 0.03 | −55.93 ± 1.04 |
| S/O/CP-ATRA 9 mg/g | 172.95 ± 2.14 | 0.13 ± 0.04 | −56.33 ± 1.96 |
| M/O/CP-ATRA 1 mg/g | 141.80 ± 1.36 | 0.14 ± 0.03 | −55.43 ± 2.64 |
| M/O/CP-ATRA 3 mg/g | 143.72 ± 2.12 | 0.13 ± 0.04 | −55.45 ± 1.30 |
| M/O/CP-ATRA 5 mg/g | 145.93 ± 0.92 | 0.13 ± 0.01 | −55.80 ± 1.48 |
| M/O/CP-ATRA 7 mg/g | 148.13 ± 0.79 | 0.16 ± 0.02 | −55.48 ± 1.70 |
| M/O/CP-ATRA 9 mg/g | 152.60 ± 1.86 | 0.14 ± 0.03 | −55.23 ± 1.56 |
Formulation Stability Study
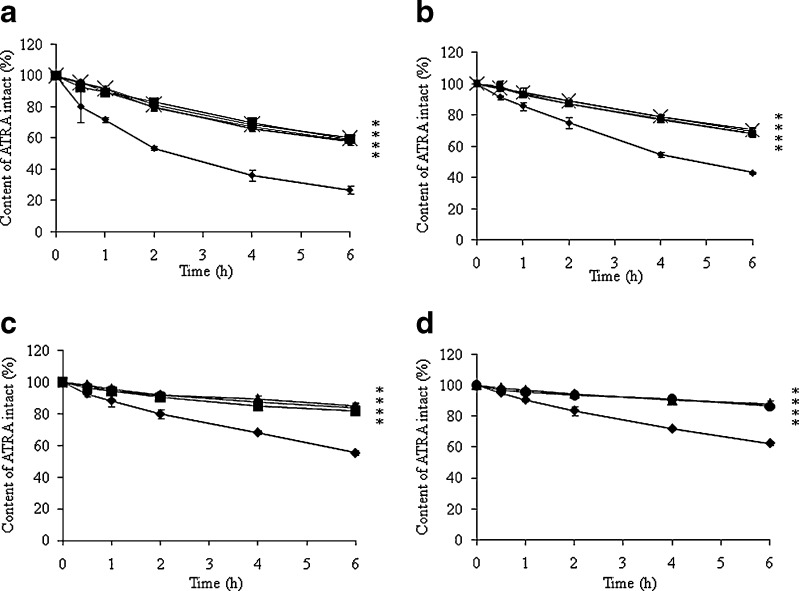
ATRA undergoes degradation when exposed to light (19,20). Therefore, the development of formulations characterized by high photoprotection of ATRA is important. We determined that samples stored at 25°C in the absence of UVA were chemical stable for months. Figure 3 shows the photodegradation of ATRA-loaded NLCs with different oil phases and initial ATRA concentrations at 25 ± 0.5°C in the presence of UVA light for 6 h compared with ATRA in IPA. The results revealed that the intact ATRA content significantly decreased with an increase of ATRA concentration. The ATRA content in all formulations of ATRA-loaded NLCs was significantly higher than that in IPA solutions. ATRA-loaded NLC using different oil phases was not significantly different in terms of intact ATRA content. The determination of the kinetics of photodegradation of ATRA in IPA and in NLC was performed by plotting the concentration of the drug remaining versus time (zero-order kinetic) and the log of the concentration of the drug versus time (first-order kinetic). The regression coefficients (R2) were obtained. The best fit observed indicated the reaction order. The rate constant of photodegradation (k0 or k1) of ATRA was determined from the slopes of the linear correlation. It was found that most of ATRA photodegradation followed first-order kinetics. The photodegradation rate constant (k) and the corresponding half-life (t1/2 = 0.693/k) of ATRA and ATRA-loaded NLCs at 25 ± 0.5°C under UVA exposure for 6 h are summarized in Table III. Results obtained from this photostability study led us to suppose that the better photoprotection of ATRA loaded in NLC is the consequence of the higher inclusion value of this drug in NLC. The relationships between the photodegradation rate and drug concentration have been well documented (21–23). These results were consistent with a previous study that showed that an ATRA-loaded lipid emulsion improved ATRA photostability independent of the type of oil phase used (12).
Fig. 3.
Effect of UVA exposure on the chemical stability of ATRA-loaded NLCs with different oil phases; (ex symbol) S/CP, (filled square) M/CP, (filled upright triangle) S/O/CP, (filled circle) M/O/CP compared with (filled diamond) ATRA in an isopropyl alcohol solution (IPA) with different initial ATRA concentrations; a 1 mg/g, b 3 mg/g, c 5 mg/g and d 7 mg/g. * significantly different (p ≤ 0.05) when compared with ATRA in IPA
Table III.
Photodegradation Rate and Half-Life (t 1/2) of ATRA in Isopropyl Alcohol (IPA) Solution and ATRA-Loaded NLCs at 25 ± 0.5°C in the Presence of UV A for 6 h (N = 3)
| Formulation | Photodegradation rate (×10−3 h−1) | Half-life (h) |
|---|---|---|
| ATRA in IPA solution | ||
| ATRA 1 mg/g | 92.8 | 7.5 |
| ATRA 3 mg/g | 61.5 | 11.3 |
| ATRA 5 mg/g | 41.2 | 16.8 |
| ATRA in NLCs | ||
| S/CP-ATRA 1 mg/g | 37.6 | 18.4 |
| S/CP-ATRA 3 mg/g | 25.6 | 27.1 |
| M/CP-ATRA 1 mg/g | 36.7 | 18.9 |
| M/CP-ATRA 3 mg/g | 28.4 | 24.4 |
| M/CP-ATRA 5 mg/g | 13.9 | 49.7 |
| S/O/CP-ATRA 1 mg/g | 39.8 | 17.4 |
| S/O/CP-ATRA 3 mg/g | 27.7 | 25.0 |
| S/O/CP-ATRA 5 mg/g | 11.3 | 61.5 |
| S/O/CP-ATRA 7 mg/g | 9.18 | 75.5 |
| S/O/CP-ATRA 9 mg/g | 9.4 | 74.0 |
| M/O/CP-ATRA 1 mg/g | 40.9 | 16.9 |
| M/O/CP-ATRA 3 mg/g | 26.8 | 25.8 |
| M/O/CP-ATRA 5 mg/g | 12.6 | 55.2 |
| M/O/CP-ATRA 7 mg/g | 9.4 | 73.8 |
| M/O/CP-ATRA 9 mg/g | 9.9 | 69.4 |
In the long-term stability study, ATRA-loaded NLCs were kept at 4°C for 56 days. In the accelerated condition, the formulations were autoclaved at 121°C for 15 min. The physical (visual observation) and chemical stability of the formulations are presented in Table IV. No ATRA crystals were found in freshly prepared NLCs under polarized light microscopy. After being kept at 4°C for 56 days, the crystallization of ATRA was found only in the S/O/CP-ATRA 7 and 9 mg/g and M/O/CP-ATRA 9 mg/g formulations, and there was no phase separation in any formulations. There was no significant pH change compared to the initial formulations; all were within a range of 7.95–8.0. The percentage retained for all ATRA-loaded NLC formulations was more than 90%. The chemical stability, as shown in terms of percentage of intact ATRA content in NLCs, was slightly decreased during storage. However, the percentage retained was more than 90% for all preparations. The degradation rate of ATRA kept at 4°C was not significantly different among the formulations. This revealed that the degradation of ATRA is independent of oil phase type. At the accelerated condition, the percentage yield of ATRA-loaded NLCs greatly decreased after being autoclaved at 121°C. Approximately 68.86–73.77% was retained. The mean particle size of the NLCs was drastically increased to approximately 341–497 nm, indicating that the mixed film at the oil–water interface was altered by exposure to pressure and temperature during the sterilization process. However, Levy and Benita have reported no change in mean droplet size distribution for an injectable diazepam submicron-emulsion after 15 min of autoclaving at 121°C (24). It might be noted that the effect of autoclaving on droplets size must be considered case by case. Because the parenteral formulations must be sterilized, non-heat sterilization of these ATRA-loaded NLCs should be employed by filtration through 0.22-μm membrane filters.
Table IV.
The Physical (Visual Observation) and Chemical Stability of ATRA-loaded NLCs Following Autoclave and Storage at 4°C for 56 Days
| Formulation | Visual observation | % ATRA content | |||||
|---|---|---|---|---|---|---|---|
| Autoclave | Kept at 4°C for (days) | ||||||
| ATRA crystallization | Phase separation | 14 | 28 | 42 | 56 | ||
| S/CP-ATRA 1 mg/g | – | No | 69.07 | 98.86 | 95.97 | 93.56 | 92.48 |
| S/CP-ATRA 3 mg/g | – | No | 70.24 | 98.76 | 96.18 | 92.55 | 91.27 |
| M/CP-ATRA 1 mg/g | – | No | 70.74 | 99.23 | 96.16 | 94.08 | 92.03 |
| M/CP-ATRA 3 mg/g | – | No | 70.41 | 99.08 | 96.58 | 94.56 | 92.79 |
| M/CP-ATRA 5 mg/g | – | No | 69.45 | 98.89 | 95.03 | 93.17 | 92.01 |
| S/O/CP-ATRA 1 mg/g | – | No | 71.50 | 98.55 | 96.19 | 94.99 | 93.45 |
| S/O/CP-ATRA 3 mg/g | – | No | 70.01 | 99.08 | 97.51 | 95.69 | 92.96 |
| S/O/CP-ATRA 5 mg/g | – | No | 72.16 | 98.89 | 97.23 | 95.93 | 93.96 |
| S/O/CP-ATRA 7 mg/g | + | No | 71.19 | 98.90 | 97.12 | 95.43 | 92.43 |
| S/O/CP-ATRA 9 mg/g | + | No | 68.86 | 98.53 | 96.10 | 95.10 | 92.33 |
| M/O/CP-ATRA 1 mg/g | – | No | 73.77 | 98.97 | 97.04 | 95.04 | 92.70 |
| M/O/CP-ATRA 3 mg/g | – | No | 70.56 | 99.23 | 97.29 | 94.55 | 92.75 |
| M/O/CP-ATRA 5 mg/g | – | No | 73.59 | 99.02 | 96.85 | 95.20 | 92.11 |
| M/O/CP-ATRA 7 mg/g | – | No | 69.28 | 99.05 | 96.28 | 94.98 | 92.83 |
| M/O/CP-ATRA 9 mg/g | + | No | 69.85 | 98.88 | 96.01 | 94.25 | 92.47 |
In Vitro Release of ATRA-Loaded NLCs
The effect of NLCs on the release of ATRA was investigated in vitro by determining the drug release across a dialysis bag. The in vitro release of ATRA from the ATRA-loaded NLC system was approximately 4.06–4.34% at 48 h (Table V). There was no significant difference in the ATRA release rate from all NLCs in accordance with the composition of the oil phase. In comparison, the ATRA release from NLCs was less than from a lipid emulsion (approximately 1.60–11.38% at 48 h) in a previous report (12). Moreover, in lipid emulsions, it was found that the release rate of ATRA depended greatly on the type of oil phase. This finding could be due to the presence of a solid lipid, CP; the melted blend of solid lipid and liquid lipid increased the viscosity of the particles, leading to a slower release rate according to the Strokes–Einstein law (25). All of these results support that ATRA incorporated into both lipid emulsion and NLCs is preferable to the use of lipid nanoparticle delivery systems. Moreover, no burst in drug release was observed, indicating that a prolonged release of ATRA from the lipid nanoparticles delivery systems was possible.
Table V.
Cumulative ATRA Released of ATRA-Loaded NLCs at 37 ± 0.5°C
| Formulations | % Cumulative drug released at | |||
|---|---|---|---|---|
| 2 h | 8 h | 24 h | 48 h | |
| ATRA (control) | 0.0009 ± 0.0001 | 0.0034 ± 0.0004 | 0.0149 ± 0.001 | 0.0281 ± 0.003 |
| S/CP-ATRA 1 mg/g | 0.32 ± 0.03 | 0.93 ± 0.08 | 2.57 ± 0.15 | 4.16 ± 0.29 |
| S/CP-ATRA 3 mg/g | 0.26 ± 0.04 | 0.95 ± 0.11 | 2.54 ± 0.10 | 4.31 ± 0.16 |
| M/CP-ATRA 1 mg/g | 0.25 ± 0.01 | 1.01 ± 0.06 | 2.44 ± 0.17 | 4.06 ± 0.22 |
| M/CP-ATRA 3 mg/g | 0.30 ± 0.05 | 1.09 ± 0.10 | 2.65 ± 0.10 | 4.34 ± 0.15 |
| M/CP-ATRA 5 mg/g | 0.26 ± 0.02 | 0.95 ± 0.14 | 2.64 ± 0.15 | 4.09 ± 0.19 |
| S/O/CP-ATRA 1 mg/g | 0.25 ± 0.01 | 1.00 ± 0.00 | 2.54 ± 0.13 | 4.15 ± 0.14 |
| S/O/CP-ATRA 3 mg/g | 0.26 ± 0.01 | 0.99 ± 0.07 | 2.54 ± 0.21 | 4.25 ± 0.13 |
| S/O/CP-ATRA 5 mg/g | 0.27 ± 0.01 | 1.06 ± 0.13 | 2.42 ± 0.12 | 4.10 ± 0.11 |
| S/O/CP-ATRA 7 mg/g | 0.24 ± 0.02 | 0.98 ± 0.17 | 2.54 ± 0.15 | 4.08 ± 0.17 |
| S/O/CP-ATRA 9 mg/g | 0.27 ± 0.02 | 1.05 ± 0.08 | 2.58 ± 0.16 | 4.12 ± 0.17 |
| M/O/CP-ATRA 1 mg/g | 0.26 ± 0.01 | 0.95 ± 0.10 | 2.38 ± 0.16 | 4.09 ± 0.18 |
| M/O/CP-ATRA 3 mg/g | 0.27 ± 0.02 | 1.00 ± 0.02 | 2.57 ± 0.19 | 4.24 ± 0.08 |
| M/O/CP-ATRA 5 mg/g | 0.24 ± 0.02 | 0.93 ± 0.08 | 2.51 ± 0.02 | 4.15 ± 0.13 |
| M/O/CP-ATRA 7 mg/g | 0.25 ± 0.01 | 1.01 ± 0.13 | 2.59 ± 0.11 | 4.15 ± 0.14 |
| M/O/CP-ATRA 9 mg/g | 0.25 ± 0.02 | 1.04 ± 0.08 | 2.53 ± 0.08 | 4.14 ± 0.12 |
Anticancer Activity of ATRA
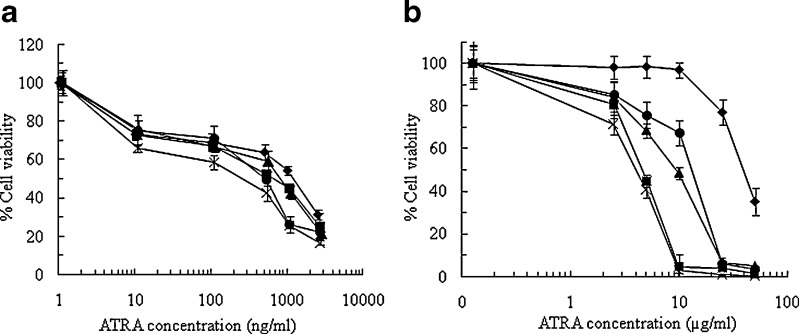
To study whether the anticancer efficacy of ATRA was retained after processing, the antiproliferative effects of ATRA on human carcinoma cell lines, acute promyelocytic leukemia cells (HL-60) and human hepatoma cells (HepG2), were investigated by MTT assay. This study is just the first preliminary study regarding the anticancer activity of these formulations. ATRA-loaded NLC formulations (S/CP, M/CP, S/O/CP, M/O/CP) were examined. The survival curves of HL-60 cells (Fig. 4a) and HepG2 cells (Fig. 4b) after exposure to ATRA, either in solution or in NLCs, showed that the growth inhibitory effects of ATRA were significantly different between free ATRA and ATRA-loaded NLC. The activity of ATRA-loaded NLC on HL-60 and HepG2 cells was greater than that of free ATRA. ATRA could inhibit the proliferation of HL-60 and HepG2 cell lines in a dose-dependent manner in the range of 1–2,500 ng/mL and 0.1–50 μg/mL, respectively. The results revealed that the HL-60 cells were more sensitive to ATRA than the HepG2 cells. Table VI showed the amount of ATRA required to achieve 50% of growth inhibition (IC50) of ATRA-loaded NLC, and ATRA solution on the two cell lines. The IC50 of ATRA-loaded NLCs was much lower than ATRA solution. NLCs enhanced the cytotoxicity of ATRA approximately 2.06–2.78-fold on HL-60 cell and 45–137-fold on HepG2 compared with ATRA solution. It could be observed that ATRA-loaded NLC, particularly in formulation with oleic acid, could significantly decrease the viability of HepG2 cells. This result indicated that the type of oil phase used in this study affected the cell viability. The increase of ATRA anticancer activity, or at least unchanged in anticancer activity, compared to the free drug solution has already been reported. Lim et al. studied the antiproliferative effects of an SLN powder formulation tested on HL-60, MCF-7 and KB. They found that the antiproliferative effect in cells was not significantly different between the SLN powder formulation and free ATRA (11). Kawakami and coworkers reported that the incorporation of ATRA into cationic liposomes composed of DOTAP/cholesterol showed much higher toxicity effects on A594 cells and induced apoptosis activity compared with the free drug or ATRA incorporated in DSPC/cholesterol liposomes (26). Hwang and coworkers found that ATRA loaded in a phospholipid-based microemulsion provided similar growth inhibitory effects on HL-60 and MCF-7 cell lines to that free ATRA (12). The result is also in agreement with a previous study showing that ATRA incorporated into Nanodisks, disk-shaped phospholipid bilayers whose edge is stabilized by the association of apolipoprotein molecules, mediated time-dependent inhibition of cultured HepG2 cell growth more effectively than free ATRA (27). Cancers associated with defects in the retinoic acid pathway respond well to ATRA therapy. These include hepatocellular carcinoma and acute myeloid leukemias (28). Clinical studies have shown a significant decrease in the occurrence of both primary and secondary malignancies in patients with hepatocellular carcinoma when treated with ATRA, encouraging further development of drug delivery methods (29). Therefore, it is verified that the production process of these nanolipid carriers did not change the anticancer activity of ATRA and may also increase its effectiveness. The enhanced activity of NLCs associated ATRA may be related to the mode of entry of ATRA-loaded NLCs into the cell.
Fig. 4.
Cytotoxicity of ATRA-loaded NLCs composed of different oil phases; (ex symbol) S/CP, (filled square) M/CP, (filled upright triangle) S/O/CP, (filled circle) M/O/CP compared with (filled diamond) ATRA in dimethylsulfoxide (DMSO) in a HL-60 cells and b HepG2 cells
Table VI.
Inhibitory Concentration of ATRA Producing 50% of Cell Inhibition (IC50) in HL-60 Cells and HepG2 Cells
| Formulation | IC50 | |
|---|---|---|
| HL-60 (ng/ml) | HepG2 (μg/ml) | |
| ATRA solution | 921.37 | 342.94 |
| S/CP-ATRA 3 mg/g | 445.31 | 5.86 |
| M/CP-ATRA 3 mg/g | 331.08 | 7.67 |
| S/O/CP-ATRA 3 mg/g | 389.00 | 2.93 |
| M/O/CP-ATRA 3 mg/g | 371.04 | 2.48 |
CONCLUSION
In the present study, ATRA-loaded NLC were successfully prepared by an ultrasonication technique. These NLC contained 30% oil (S/CP, M/CP, S/O/CP or M/O/CP), 1.2% lecithin, 0.002% BHT and 8% polysorbate-80. The use of oleic acid in the NLC resulted in a higher loading capacity. All ATRA-loaded NLC formulations exhibited a photoprotective property and higher anticancer activity than the free drug on human carcinoma cell lines. Our study demonstrates that NLC may provide an alternative parenteral formulation of ATRA.
ACKNOWLEDGMENTS
The authors would like to thank National Nanotechnology Center (Nanotec), and Newcharoen Pharmaceutical Limited Partnership for their help and kindly providing the facilities. The authors are grateful to Thailand Graduate Institute of Science and Technology Scholarship (TGIST) and the Commission of Higher Education (Thailand), the Thailand Research Funds through the Golden Jubilee Ph.D. Program (Grant No. PHD/0141/2552) for financial support.
REFERENCES
- 1.Ozpolat B, Berestein GL. Pharmacokinetics of intravenously administered liposomal all-trans-retinoic acid (ATRA) and orally administered ATRA in healthy volunteers. J Pharm Pharm Sci. 2003;6:292–301. [PubMed] [Google Scholar]
- 2.Lin HS, Chean CS, Ng YY, Chan SY, Ho PC. 2-hydroxypropyl-beta-cyclodextrin increases aqueous solubility and photostability of all-trans-retinoic acid. J Clin Pharm Ther. 2000;25:265–269. doi: 10.1046/j.1365-2710.2000.00285.x. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu K, Tamagawa K, Takahashi N, Takayama K, Maitani Y. Stability and antitumor effects of all-trans retinoic acid-loaded liposomes contained sterylglucoside mixture. Int J Pharm. 2003;258:45–53. doi: 10.1016/S0378-5173(03)00151-0. [DOI] [PubMed] [Google Scholar]
- 4.Manconi M, Sinico C, Valenti D, Loy G, Fadda AM. Niosomes as carriers for tretinoin. I. Preparation and properties. Int J Pharm. 2002;234:237–248. doi: 10.1016/S0378-5173(01)00971-1. [DOI] [PubMed] [Google Scholar]
- 5.Zuccari G, Carosio R, Fini A, Montaldo PG, Orienti I. Modified polyvinyl alcohol for encapsulation of all-trans-retinoic acid in polymeric micelles. J Controlled Release. 2005;103:369–380. doi: 10.1016/j.jconrel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Opanasopit P, Ngawhirunpat T, Rojanarata T, Choochottiros C, Chirachanchai S. N-phthaloylchitosan-g-mPEG design for all-trans retinoic acid-loaded polymeric micelles. Eur J Pharm Sci. 2007;30:424–431. doi: 10.1016/j.ejps.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Chinsriwongkul A, Opanasopit P, Ngawhirunpat T, Chareansriwilaiwat N, Sila-On W, Ruktanonchai U. Physicochemical properties of lipid emulsions formulated with high-load all-trans retinoic acid. PDA J Pharm Sci Technol. 2007;61:461–471. [PubMed] [Google Scholar]
- 8.Su J, Zhang N, Ho PC. Evaluation of the pharmacokinetics of all-trans-retinoic acid (ATRA) in Wistar rats after intravenous administration of ATRA loaded into tributyrin submicron emulsion and its cellular activity on caco-2 and HepG2 cell lines. J Pharm Sci. 2008;97:2844–2853. doi: 10.1002/jps.21193. [DOI] [PubMed] [Google Scholar]
- 9.Gu MY, Wang ZW, Yu HX, Li GZ. Microemulsion of Tween-80/n-butylalcohol/H2O system and its entrapment efficiency of ATRA. J Dispersion Sci Technol. 2006;27:949–954. doi: 10.1080/01932690600766827. [DOI] [Google Scholar]
- 10.Lim SJ, Lee MK, Kim CK. Altered chemical and biological activities of all-trans retinoic acid incorporated in solid lipid nanoparticle powders. J Controlled Release. 2004;100:53–61. doi: 10.1016/j.jconrel.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Hwang SR, Lim SJ, Park JS, Kim CK. Phospholipid-based microemulsion formulation of all-trans-retinoic acid for parenteral administration. Int J Pharm. 2004;276:175–183. doi: 10.1016/j.ijpharm.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Chinsriwongkul A, Opanasopit P, Ngawhirunpat T, Rojanarata T, Sila-on W, Ruktanonchai U. Oleic acid enhances all-trans retinoic acid load in nano-lipid emulsions. PDA J Pharm Sci Technol. 2010;64:113–123. [PubMed] [Google Scholar]
- 13.Joshi MD, Müller RH. Lipid nanoparticles for parenteral delivery of actives. Eur J Pharm Sci. 2009;71:161–172. doi: 10.1016/j.ejpb.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Mehnert W, Mäder K. Solid lipid nanoparticles production, characterization and applications. Adv Drug Delivery Rev. 2001;47:165–196. doi: 10.1016/S0169-409X(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 15.Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Delivery Rev. 2002;54:S131–S155. doi: 10.1016/S0169-409X(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 16.Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366:170–184. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Lee MK, Lim SJ, Kim CK. Preparation, characterization and in vitro cytotoxicity of paclitaxel-loaded sterically stabilized solid lipid nanoparticles. Open Biomater J. 2007;28:2137–2146. doi: 10.1016/j.biomaterials.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Washington C, Davis SS. Ageing effects in parenteral fat emulsions: the role of fatty acids. Int J Pharm. 1987;39:33–37. doi: 10.1016/0378-5173(87)90195-5. [DOI] [Google Scholar]
- 19.Brisaert MG, Everaerts I, Plaizier-Vercammen JA. Chemical stability of tretinoin in dermatological preparations. Pharm Acta Helv. 1995;70:161–166. doi: 10.1016/0031-6865(95)00016-3. [DOI] [Google Scholar]
- 20.Carlotti ME, Rossatto V, Gallarate M. Vitamin A and vitamin A palmitate stability over time and under UVA and UVB irradiation. Int J Pharm. 2002;240:85–94. doi: 10.1016/S0378-5173(02)00128-X. [DOI] [PubMed] [Google Scholar]
- 21.Tan X, Meltzer N, Lindenbaum S. Determination of the kinetics of degradation of 13-cis retinoic acid and all-trans retinoic acid in solution. J Pharm Biomed Anal. 1993;11:817–822. doi: 10.1016/0731-7085(93)80074-B. [DOI] [PubMed] [Google Scholar]
- 22.Ioele G, Cione E, Risoli A, Genchi G, Ragno G. Accelerated photostability study of tretinoin and isotretinoin in liposome formulations. Int J Pharm. 2005;293:251–260. doi: 10.1016/j.ijpharm.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Caddeo C, Manconi M, Valenti D, Pini E, Sinico C. Photostability and solubility improvement of β-cyclodextrin-included tretinoin. J Inclusion Phenom Macrocyclic Chem. 2007;59:293–300. doi: 10.1007/s10847-007-9326-z. [DOI] [Google Scholar]
- 24.Teeranachaideekul V, Souto EB, Junyaprasert VB, Müller RH. Cetyl palmitate-based NLC for topical delivery of Coenzyme Q10—development, physicochemical characterization and in vitro release studies. Eur J Pharm Biopharm. 2008;67:141–148. doi: 10.1016/j.ejpb.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Levy MY, Benita S. Short- and long-term stability assessment of a new injectable diazepam submicron emulsion. J Parenter Sci Technol. 1991;45:101–107. [PubMed] [Google Scholar]
- 26.Kawakami S, Suzuki S, Yamashita F, Hashida M. Induction of apoptosis in A549 human lung cancer cells by all-trans retinoic acid incorporated in DOTAP/cholesterol liposomes. J Controlled Release. 2006;110:514–521. doi: 10.1016/j.jconrel.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Edmond KA, Nguyen TS, Ryan RO. All-trans-retinoic acid nanodisks. Int J Pharm. 2007;339:246–50. [DOI] [PMC free article] [PubMed]
- 28.Sun S, Lotan R. Retinoids and their receptors in cancer development and chemoprevention. Crit Rev Oncol Hematol. 2002;41:41–55. [DOI] [PubMed]
- 29.Arce F, Gätjens-Boniche O, Vargas E, Valverde B, Díaz C. Apoptotic events induced by naturally occurring retinoids ATRA and 13-cis retinoic acid on human hepatoma cell lines Hep3B and HepG2. Cancer Lett. 2005;229:271–81. [DOI] [PubMed]