Abstract
Polacrilin Potassium NF is a commonly used weak cation exchange resin disintegrant in pharmaceutical tablets. The objective of this research was to evaluate the effects of sorbed moisture on physical characteristics and disintegrant performance of four brands of Polacrilin Potassium NF. The disintegrants were stored in five different relative humidity chambers and their dynamic vapor adsorption–desorption analysis, effect of moisture on their compressibility, compactability, particle size, morphology, water uptake rate, and disintegration ability were studied. Moisture seemed to plasticize the disintegrants, reducing their yield pressures. However, certain optimum amount of moisture was found to be useful in increasing the compactablity of the tablets containing disintegrants. The tablets, however, lost their tensile strengths beyond this optimum moisture content. Moisture caused two brands of the disintegrants to swell; however, two other brands aggregated upon exposure to moisture. Swelling without aggregation increased the water uptake, and in turn the disintegrant performance. However, aggregation probably reduced the porosities of the disintegrants, reducing their water uptake rate and disintegrant performance. Different brands of Polacrilin Potassium NF differed in the abilities to withstand the effects of moisture on their functionality. Effect of moisture on disintegrant performance of Polacrilin Potassium NF needs to be considered before its use in tablets made by wet granulation.
KEY WORDS: disintegrant performance, effect of moisture, functionality, ion exchange resins, physical characterization, polacrilin potassium NF, sorbed water, tablet disintegrants
INTRODUCTION
Disintegrants are the pharmaceutical excipients whose action depends on their interaction with water and the changes brought about in their structure due to this interaction (1). They are capable of overcoming the cohesive strength of tablets introduced by particle–particle bonding due to compaction forces and binders (2,3). There are three accepted mechanisms by which tablet disintegrants are supposed to act: swelling, wicking (capillary action), and deformation recovery. “Swelling” of disintegrant particles is probably the most widely accepted mechanism of tablet disintegration. The disintegrant particles take up water, swell, and generate large swelling force which breaks the tablet apart. An example of a disintegrant that acts by swelling is sodium starch glycolate. Some disintegrants draw water into the porous network of the tablet by capillary action and the water thus taken up develops pressure which causes the tablet to disintegrate. This phenomenon is called “wicking” and the disintegrants that act by wicking swell little or may not swell at all, e.g., crospovidone. Sometimes, the disintegrant particles get deformed during compression, and these deformed particles get into their normal structure when they come in contact with the aqueous media. These disintegrants are said to act by “deformation recovery” (4).
Thus, a disintegrant assists in breakup of the solid dosage form when it comes in contact with water. However, the disintegrant can also absorb water present in the immediate environment and this may prove detrimental to the performance of the disintegrant. Moisture could potentially compromise the physical properties and functionality of the disintegrant.
Some of the factors which may affect the performance of disintegrants are particle size (5), molecular structure (6), compression force (7,8), method of incorporation in granulation (9,10), prior history of wetting (11), and compression (reworking) of the disintegrants (12,13). Hancock et al. (14) studied the effect of sorbed moisture on some pharmaceutical sugars by studying their dynamic vapor sorption. They also have discussed the effect of moisture on their physical characteristics such as compressibility, compactability, and glass transition and these methods would be useful in studying the effect of moisture on disintegrants as well. Moisture effects on disintegrants such as starch, sodium carboxymethyl cellulose and sodium starch glycolate (15,16), croscarmellose sodium, and crospovidone (16) have been studied extensively. Faroongsarng et al. (17) studied dynamic vapor sorption and thermal behavior of four disintegrants: microcrystalline cellulose, croscarmellose sodium, corn starch, and sodium starch glycolate. They observed that the transition, from the glassy to the rubbery state, of the polymers decreased their sorption properties, and they ascribed this to the sorption sites being in glassy state. Thibert et al. (18) studied the hydration of certain super disintegrants on exposure to moisture by environmental scanning electron microscopy. Arwidsson et al. (19) studied the effect of moisture sorption and glass transition temperature on compactability of microcrystalline cellulose alone and in presence of polyvinylpyrrolidone. Bravo-Osuna et al. (20) studied the water vapor sorption–desorption behavior of methyl methacrylate-starch co-polymers and the influence of variables such as the presence of hydrophobic components and the drying process on them.
Polacrilin Potassium NF is partial potassium salt of a uni-functional low cross-linked carboxylic cation exchange resin prepared from methacrylic acid and divinyl benzene (21). It is superior to the traditional disintegrants and is effective at concentrations as low as 1–5% (22). It is highly hygroscopic, however, the effect of absorbed atmospheric moisture on its disintegrant performance is not known. From the earlier studies in our laboratory on the mechanism of action of polacrilin potassium, we have concluded that wicking appears to be its major mechanism of action (23). An understanding of the effect of adsorbed moisture on its physical properties along with the knowledge of its mechanisms of action would provide guidance on the proper storage, handling, and use of polacrilin potassium. No such data is currently available in the literature on the role of absorbed moisture on performance of Polacrilin Potassium NF as a disintegrant. The objective of this study was to expand our current understanding of the effect of moisture on physical characteristics and functionality of Polacrilin Potassium NF. Several tests were performed on four brands of Polacrilin Potassium NF samples stored in different relative humidities like their vapor sorption behavior (dynamic and static vapor sorption analysis), changes in particle size (particle size measurement by laser light scattering), morphological changes due to swelling and/or agglomeration (optical microscopy), changes in compressibility (Heckel analysis), compactability (tensile strength measurement), changes in crystallinity (X-ray crystallography), water uptake (water uptake measurements), and disintegrant performance (disintegration time) were studied.
EXPERIMENTAL
Materials
Four brands of Polacrilin Potassium NF were used in this study: Amberlite IRP 88® (Rohm and Haas Ltd, Philadelphia, PA19106, USA), Doshion P544DS™ (Doshion Ltd., Ahmedabad, India), Indion 294™ (Ion Exchange Ltd, Mumbai, India) and Tulsion 339™ (Thermax Ltd, Pune, India). Samples of all four materials were gifts from their respective manufacturers. Lactose, spray-dried NF, Dicalcium Phosphate Dihydrate USP, and Magnesium Stearate NF were gifts from Signet Chemicals, Mumbai, India. All other chemicals were of analytical reagent grade. The salts used to condition the humidity chambers, polyethylene glycol 4,000 (PEG 4,000) and all other chemicals were of analytical reagent grade.
Methods
Gravimetric Water Vapor Adsorption–Desorption Analyses
The dynamic water vapor adsorption–desorption analyses were performed using a Q5000SA Dynamic Vapor Sorption Analyzer (TA Instruments, New Castle, DE, USA). The initial sample size was 5–9 mg. The samples were analyzed under isothermal conditions. They were dried at 25°C until there was no weight change over 5 min. The sensitivity of the balance was 0.0001 mg. The temperature was kept constant at 25°C. The relative humidity (RH) was increased from 0% to 95% in increments of 5% and then was decreased down to 5% RH. The sample weight was recorded after the weight change had stabilized. The humidity was changed to the next step when the change of weight reached ±0.01 mg or after 30 min, whichever was later (25).
Equilibrium Moisture Content and Powder Sample Preparation
Initial moisture contents of the disintegrant powders were measured by loss on drying (LOD). Disintegrants (10 g) were accurately weighed and placed in pre-weighed Petri plates covered with perforated aluminum foil. The plates were placed in vacuum oven at 105°C for 6 h. They were allowed to cool over phosphorous pentoxide in a dessicator for an hour and then were accurately weighed. Four more replicates were done. The% LOD was calculated. The procedure was done in triplicate. % LOD reported was the mean of the three replicates along with the standard deviation.
To prepare the disintegrant powders having varying levels of moisture, the disintegrants were stored in humidity chambers containing saturated salt solutions at room temperature (19). The salts were chosen based on their ability to maintain constant humidity at 25 ± 2°C (Table I). The %RH achieved inside the humidity chambers was verified by means of a hygrometer kept in the glass chamber, which was visible from outside. Equilibrium moisture contents of the conditioned disintegrant samples were determined gravimetrically by measuring the equilibrium weight percent change of the powder based on its dry weight, which was calculated from the initial %LOD.
Table I.
List of Salts and the Relative Humidity Achieved by Their Saturated Solutions
| Saturated salt solution | Target relative percent humidity at 25°C |
|---|---|
| LiCl | 11.3 |
| MgCl2 | 33 |
| Mg(NO3)2, 6H2O | 53 |
| NaCl | 75.3 |
| KNO3 | 94 |
Particle Size Measurement
The volume median diameters of disintegrants stored at different relative humidities were measured using laser diffraction technique (Mastersizer Micro, Malvern Instruments, PA, USA). The dry powder sampling accessory was not available, and the particle size was measured by suspension method using cyclohexane which is a solvent that does not cause polacrilin potassium to swell. The powders were dispersed in 500 ml of cyclohexane in the large volume dispersing unit of the Malvern Mastersizer. The instrument was connected to the circulating cell to achieve an obscuration of 10% to 30%. The stirrer speed was set to 2,200 rpm. The volume median diameters were recorded as an average of six measurements. The nonswelling of the disintegrants in this medium was confirmed by comparing the volume median diameters measured by wet method using cyclohexane and the volume median diameters measured using the dry powder dispersion method performed on another instrument (Mastersizer 2,000, Malvern Instruments, PA, USA) located in another laboratory (formulation and development laboratory, Dr Reddy’s Labs, Hyderabad, India).
Heckel Analyses
Disintegrant powders (250 mg) exposed to different RHs were compressed to compacts on a KBr press (model no. M-15, Technosearch Instruments, Mumbai, India) at 25, 50, 75, 100, 125, and 150 MPa pressure using a 8-mm die at dwell time of 30 s. The out of die thickness and diameters were measured using a micrometer. This was used to calculate the densities of the tablets.
The true densities of powders were determined in triplicate by means of helium displacement using a Helium Densitometer (Ultrapycnometer 1,000, Quantachrome Instruments, Boynton Beach, FL, USA). The true volume of the sample was calculated by determining the volume of the helium displaced by the sample during the test. The true density was then calculated from the weight of the sample. The relative density, D, was calculated from the density of the tablet and the true density of the powder. Graph was plotted for the natural log of 1/1-D versus compression pressure. The reciprocal of the slope of the regression line gave the yield pressures of the disintegrants.
Tensile Strength
A precision dial-type hardness tester (Progressive Instruments Co. Ltd., Mumbai, India) was used to measure the diametral breaking force of the disintegrant tablets. Tablets were compressed on a single station of a rotary tablet press (Remek Minipress, Karnavati Instruments, Ahmedabad, India) using 7 mm diameter flat-faced punches. Tablets were compressed at 600 kgf. The following formula was used for calculating the tensile strength of the tablets from the breaking force (24):
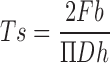 |
1 |
Where, Ts = tensile strength, Fb = breaking force, D = diameter, and h = thickness of the tablet. Tensile strength was reported as the mean of three readings along with the standard deviation.
X-ray Powder Diffraction
X-ray powder diffraction (XRPD) studies were performed on disintegrants equilibrated at 11.3% and 75.3% RH at room temperature by using an Xpert pro X-ray diffractometer (PanAnalytical B.V., Almelo, Netherlands). The source of radiation was Cu Kα (λ = 1.54060 Å) obtained at 40 mA and 45 kV. A nickel beta filter was used to eliminate K-ß lines with a divergent (1°), sollar slit (0.02 rad), and anti-scatter (1/2°) slits. The diffractometer was also equipped with a 2θ-compensating slit, which was calibrated with a silicon disc. Solid-state detector (X’Celerator) was used to obtain XRD data. The sample was placed in a rectangular sample holder. The data was processed using X’pert data collector (LTU) diffraction software. The following scan conditions were used: scanning started at 50(2θ), step size of 0.0170 (2θ), scan step time: 20 s.
Microscopic Examination
All the photomicrographs were recorded using a microscope (model CX32, Olympus Corporation, Tokyo, Japan) fitted with a video camera connected to a computer. The disintegrant samples were mounted on a glass slide covered with a coverslip. The microscope field was displayed on the monitor, and particle size measurements were made using Magnus Pro 3.0 software (Magnus Analytics, New Delhi, India) using a digital pad and mouse. The horizontal bisecting diameter (Martin’s diameter) was determined for 20 particles, and then mean value reported. To evaluate the size and shape changes of the particles in presence of water, distilled water was introduced onto the microscope slide using a 200-μl micropipette. As water was introduced, it spread under the coverslip due to capillary action.
Water Uptake Rates of Disintegrants Exposed to Moisture
In order to investigate liquid uptake measurement of disintegrant powders, a modified gravimetric liquid uptake measurement apparatus described by Brezeczko (26) was used .To determine the liquid uptake, 400 mg of disintegrant conditioned at 11.3% RH was carefully placed into the glass filter in the sample holder, and the weight loss from the measuring vessel was monitored every 2 s for 15 min at room temperature. Each disintegrant was tested in triplicate for uptake of distilled water. The same procedure was repeated with disintegrants exposed to 75.3% RH.
Disintegrant Performance
Preparation of Tablets Containing Standard Soluble/Non Soluble Diluents
Blends containing dibasic calcium phosphate (DCP) or anhydrous lactose and 2% disintegrant stored at 11.3% or 75.3% RH were prepared. The blends were mixed manually in a small container by handshaking for 10 min. Tablets were made individually on a single station of a 10-station rotary tablet compression machine (Remek Minipress, Karnavati Instruments, Ahmedabad, India) at 600 kgf. The punches and dies were lightly lubricated with 2% suspension of magnesium stearate in acetone before filling with the powder bed. Six tablets were prepared for each blend.
Preparation of Tablets Containing Slow Disintegrating Tablet Matrices
The tablets were formulated according to the formula given in Table II. The blends were mixed manually in a small container by handshaking for 10 min. Tablets were made individually on a single station of a 10-station rotary tablet compression machine (Remek Minipress, Karnavati Instruments, Ahmedabad, India) at 600 kgf. For each blend, six tablets were prepared. Disintegration tests were performed according to the USP procedure at 37.5°C temperature in distilled water without discs.
Table II.
Formula of Tablets Containing Slow-Disintegrating Matrix
| Ingredient | Quantity (mg) |
|---|---|
| Disintegrant | 09 |
| Magnesium stearate | 03 |
| PEG 4,000 | 47 |
| Dicalcium phosphate dihydrate | 141 |
RESULTS AND DISCUSSION
Gravimetric Water Vapor Adsorption–Desorption Analyses
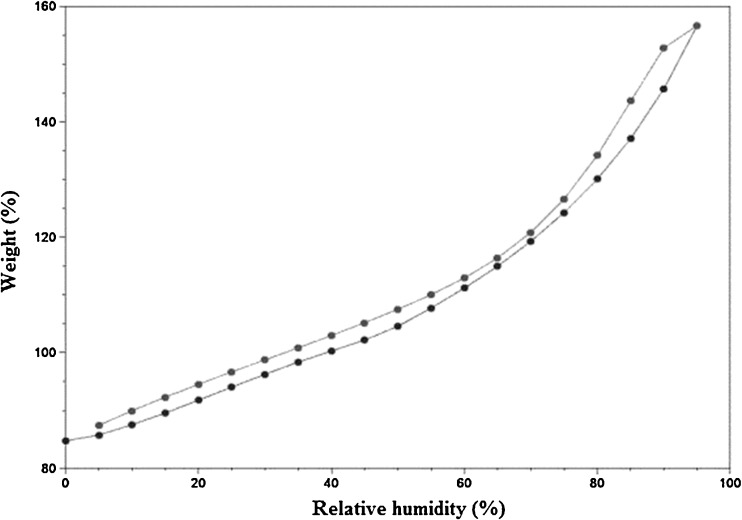
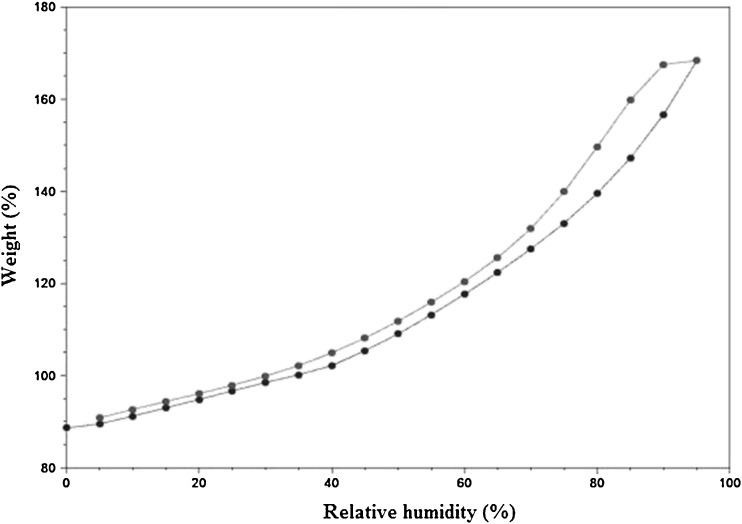
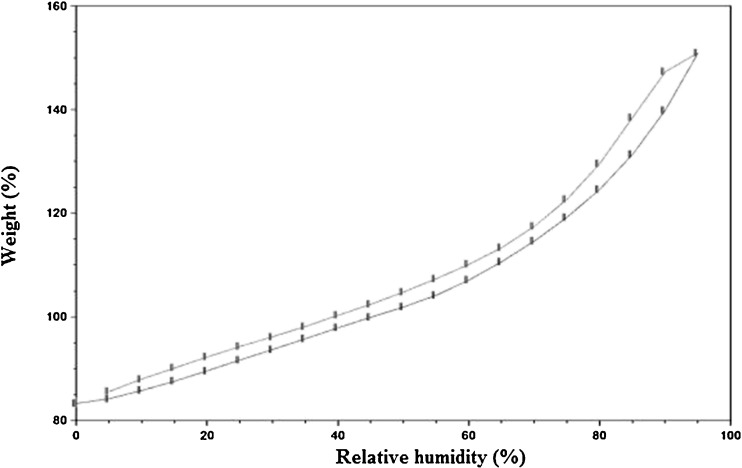
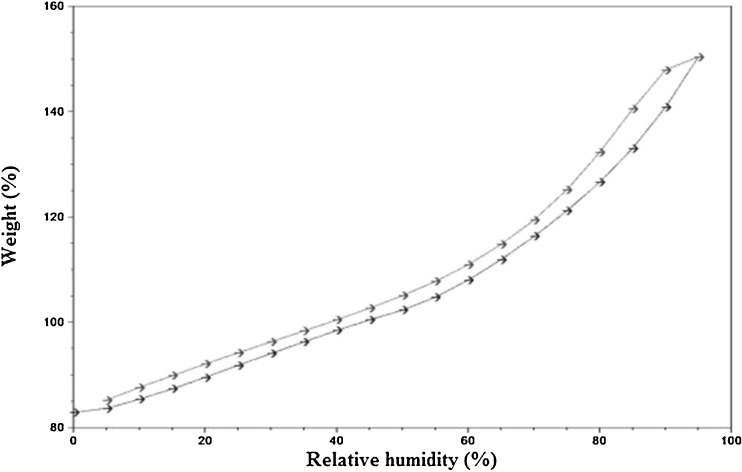
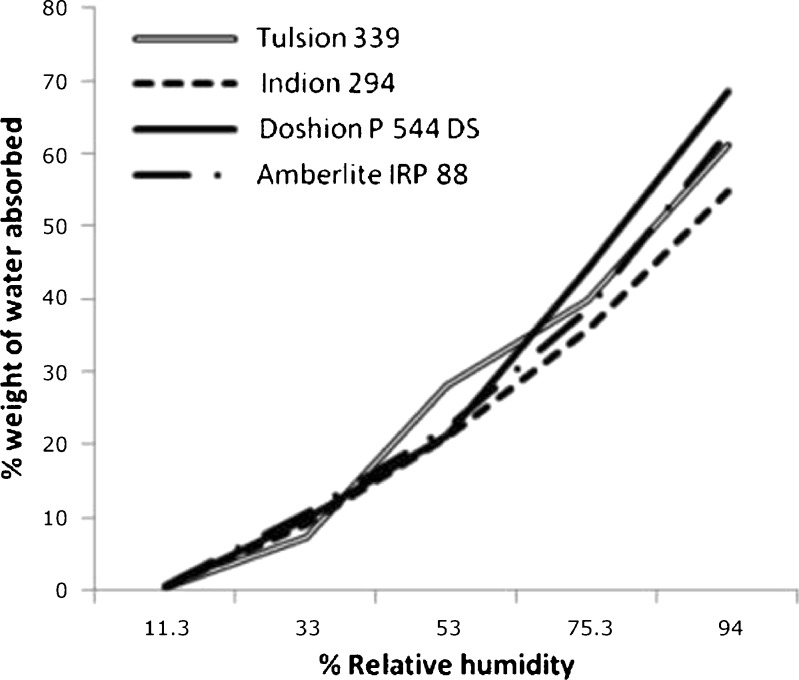
The water vapor adsorption–desorption curves for the four brands of disintegrants are shown in Figs. 1, 2, 3, and 4. All the disintegrants were hygroscopic, as they absorbed water vapors more than their own weight, particularly above 40% RH. The adsorption–desorption profiles exhibited a slight hysteresis, which is common with many pharmaceutical materials. During desorption phase, the RH was only reduced down to 5%, but the profiles showed that the weight change was approaching 0%. The moisture sorption–desorption profiles of all the four brands were difficult to classify. None of the profiles exhibited the knee bend-shaped curves, as seen in type II isotherms. The isotherms appeared to be hybrids of types II and III, suggesting a simultaneous coverage of the adsorbent with water as well as water–water and/or water–polymer interactions at the lower relative RHs. Doshion P544 DS was capable of incorporating larger amounts of water compared to the other brands. The following rank order was observed in terms of the amount of moisture adsorbed:
Fig. 1.
Weight change of Amberlite IRP 88 as% RH increase s at room temperature
Fig. 2.
Weight change of Doshion P 544 DS as% RH increase s at room temperature
Fig. 3.
Weight change of Indion 294 as% RH increase s at room temperature
Fig. 4.
Weight change of Tulsion 339 as% RH increase s at room temperature
Doshion P544 DS>Amberlite IRP 88>Indion 294∼Tulsion 339
Because Doshion P 544 DS absorbed more amount of moisture, we could predict that its performance as a disintegrant would be better compared to the other three brands, and the rank order for disintegrant performance would follow the rank order for the amount of moisture absorbed. It is also predictable, that storage and handling of Doshion P 544DS in terms of protection from moisture, before incorporation into the tablet formulations, would be more challenging than the other three brands.
Equilibrium Moisture Contents
Table III lists the average initial moisture contents and %LOD specified for Polacrilin Potassium NF according to the USP NF monograph. Figure 5 indicates the static vapor sorption analysis as determined after 17 days storage at different relative humidities. The equilibrium moisture gains were in accordance with the dynamic vapor sorption profiles as shown in Figs. 1, 2, 3, and 4, and hence are expected to have the similar implications on the disintegrant performance, storage, and handling.
Table III.
Intial %LOD and the Moisture Content Specifications for Polacrilin Potassium NF
| Disintegrant | Average% LOD (Mean ± SD, n = 3) | % LOD limit prescribed by USP NF |
|---|---|---|
| Amberlite IRP 88 | 9.819 (1.2) | <10 |
| Doshion P 544 DS | 9.826 (0.8) | <10 |
| Indion 294 | 8.747 (0.5) | <10 |
| Tulsion 339 | 6.872 (0.9) | <10 |
Fig. 5.
Equilibrium moisture gain of Polacrilin Potassium NF brands after 17 days of storage at room temperature
Particle Size Measurements
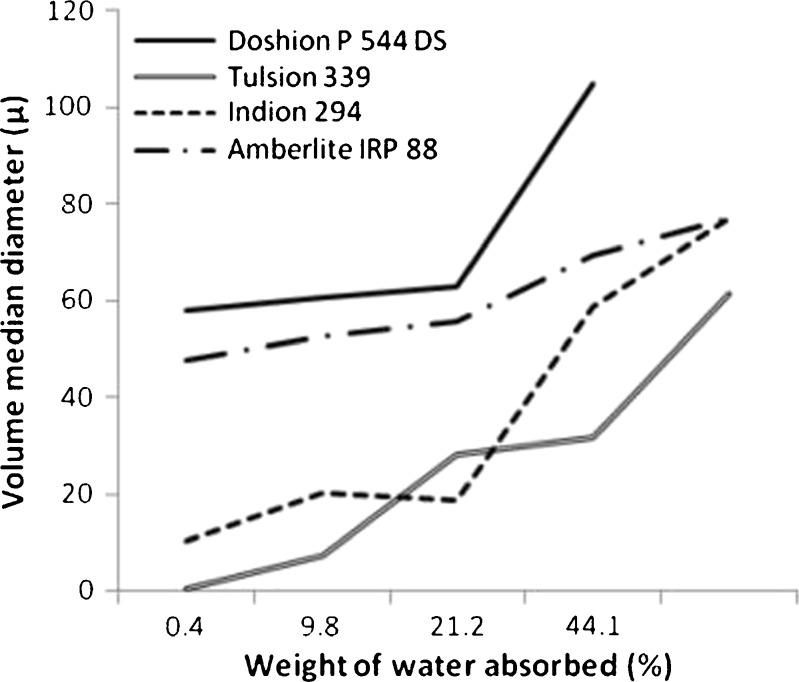
Doshion P544 DS samples stored at 94% RH were aggregated and could not be introduced into Mastersizer for measurement. It was possible to introduce Amberlite IRP88, Indion 294, Tulsion 339 powders stored at 94% RH, but the mean particle sizes were excessively large. Even powders stored at 75% RH had excessively large particle sizes, compared to the particle sizes of the powders sampled as such from their containers. This was probably due to aggregation, and did not seem to reflect the particle sizes of the individual particles (see Fig. 6). The particle agglomeration was later confirmed by microscopic observation.
Fig. 6.
Volume mean diameters of Polacrilin Potassium NF brands containing varying amounts of moisture
At the lower RH (11%), where the moisture content was 0.46%, Tulsion 339 particles appeared to have shrunk in size, and as a consequence, the increase in volume median diameter with subsequent increase in moisture content appeared to be larger compared to the other three brands.
Doshion P544 DS and Amberlite IRP 88 showed a slow and steady increase in particle size with increase in moisture content until the particles were agglomerated. This could be attributed to their sponge-like porous structure that expands in volume with absorption of water. Indion 294 showed negligible increase in particle size until the humidity reached 75%. Possibly, Indion 294 had more rigid structure than the other brands, and which did not swell until enough water was absorbed in its void spaces to fill them up. The determination of yield pressure during compaction experiments may shed some light on this, and has been discussed in the next section. The particle size of disintegrant may affect its performance in formulation (27). Further testing was performed in order to study the effect of changes in particle size on disintegrant performance and has been discussed in the next sections.
Heckel Analyses
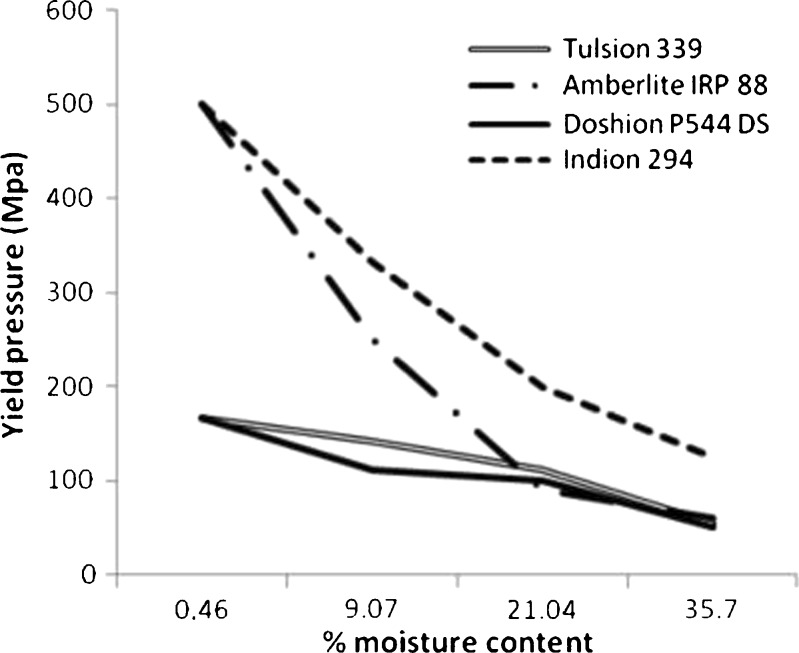
The plasticizing effect of moisture on the different brands of Polacrilin Potassium NF was demonstrated by the lowering of the yield pressure as the moisture content increases (see Fig. 7). The plasticizing effect of moisture was less on Tulsion 339 and Doshion P544 DS compared to the other two brands. Amberlite IRP 88 and Indion 294 showed the same initial yield pressures. However, the plasticizing effect of moisture was less for Amberlite IRP 88 than for Indion 294. The following rank order was observed for the decrease in yield pressure upon exposure to moisture:
Fig. 7.
Change in yield pressures of Polacrilin Potassium NF brands as a function of moisture content
Indion 294>Amberlite IRP88>Tulsion 339>Doshion P 544 DS
Indion 294 was the most rigid of all the brands and had the highest yield pressure before exposure to high RH. Though the decrease in its yield pressure upon exposure to high RH was maximum, still its compressibility upon moisture exposure was better than the other three brands. This explained the negligible increase in its particle size before it increased significantly upon exposure to 75% RH, as discussed in the previous section.
The effect of high RH on compressibility of different brands of polacrilin potassium was variable. As disintegrant is added in small proportions of the total tablet weight, the change in its compressibility may not significantly affect the compressibility of the powder blend that makes up the tablet. However, this needs to be considered while formulating tablets comprising higher proportions of disintegrant, e.g., fast disintegrating tablets and dispersible tablets.
Tensile Strength
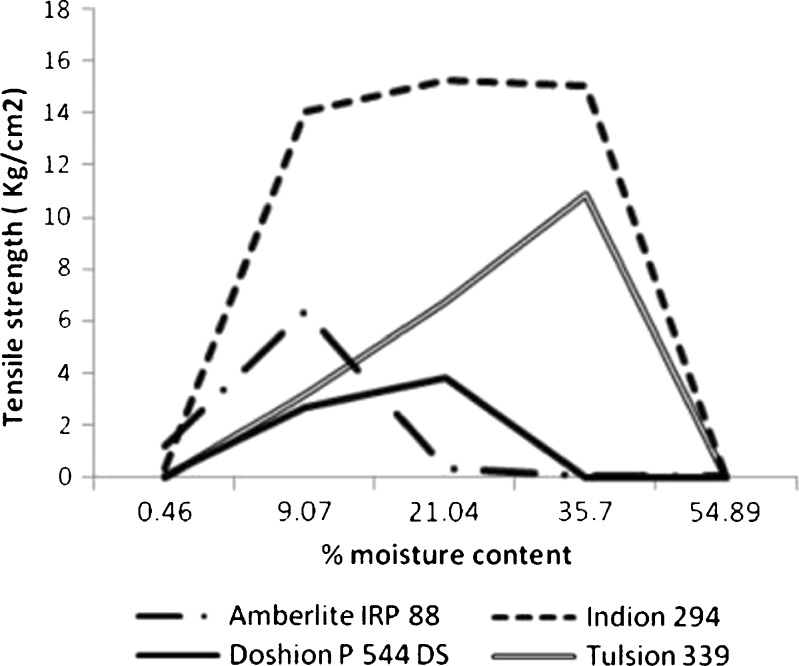
For each brand of polacrilin potassium, there appeared to be an optimum moisture level that yielded the greatest tensile strengths of the tablets (see Fig. 8). The effect of moisture on the tensile strength of Indion 294 compacts was marked. The following rank order was observed for maximum tensile strengths:
Fig. 8.
Tensile strengths of compacts of different brands of Polacrilin Potassium NF as a function of moisture content
Indion 294>Tulsion 339>Amberlite IRP 88>Doshion P 544 DS
Indion 294 had better compressibility and compactability than the other three brands. Doshion P544 DS, on the other hand, was the most vulnerable to moisture exposure in terms of compressibility and compactability. The effect of high RH on the compactability and compressibility of the polacrilin potassium brands may be worth consideration while formulating tablets containing higher proportions of disintegrants viz. fast disintegrating or dispersible tablets.
X-ray Powder Diffraction
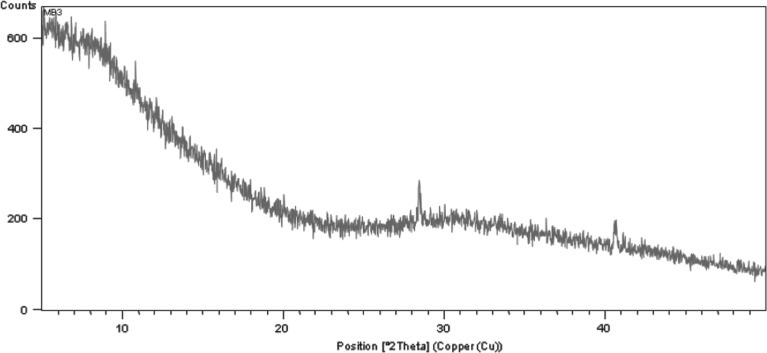
All the four brands of Polacrilin Potassium NF showed halo-like patterns indicative of amorphous solids. The XRD patterns for the powders, stored at low (11%) and high (75.3%) RH, were similar which indicated that the moisture did not affect their amorphous state. However, samples of Doshion P 544 DS that were not exposed to moisture showed peaks at two distinct positions (2θ = 28 and 2θ = 40, Fig. 9). These peaks were not present in the Doshion P544DS sample stored at 75.3% RH. It could be assumed that these peaks were of some known water-soluble impurities in Doshion P544DS which were solvated upon exposure to moisture. To determine if such structural change due to humidity was reversible, Doshion P 544 DS sample that had been stored in 75.3% RH for 2 weeks was re-equilibrated in the 11.3% RH chamber for 19 days. However, the sharp peaks did not re-appear, indicating the structural changes were irreversible. It was unlikely that any impurities would have disappeared. Probably, upon hydration, the impurities were no longer in crystalline form, but got solvated and redistributed in the moisture. Perhaps, when the rehydration occurred, the particles of the impurities were too small to get recrystallized in their original form. Thus, it was clear that the molecular structural alterations that occurred upon incorporation of water were irreversible for Doshion P 544 DS. This should be given due consideration while exposure of Doshion P 544 DS to higher RH during storage and handling in general, and while using wet granulation tabletting in particular.
Fig. 9.
XRPD of Doshion P 544 DS before exposure to moisture
Microscopic Studies
Table IV shows the effects of exposure to “high” and “low” moisture content on particles of polacrilin potassium brands and estimates of their Martin’s diameters. Indion 294 and Tulsion 339 particles exposed to high relative humidity showed a significant increase in their sizes. However, increase in particle sizes of Amberlite IRP 88 and Doshion P 544 DS was significantly more than the Indion 294 and Tulsion 339 particles. More precisely, despite the sieving prior to viewing in the microscope, the particles seemed to have aggregated, making them appear bigger. A closer examination, which is possible using the zoom function in the analyzer, revealed that the individual particles could be distinguished. Doshion P 544DS and Amberlite IRP 88 hydrate more, and are more hydrophilic, and hence probably are better disintegrants than Indion 294 and Tulsion 339.
Table IV.
Photomicrographs of Polacrilin Potassium NF Brands with or without Pre-storage in 75.3% RH (Average Martin’s Diameter ± S.D., N = 20)

Water Uptake Rate S of Disintegrant Powders
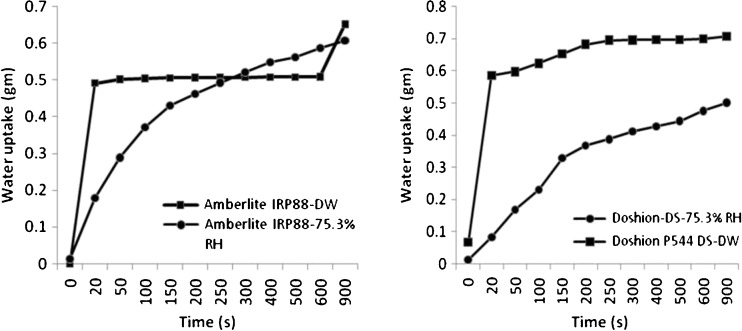
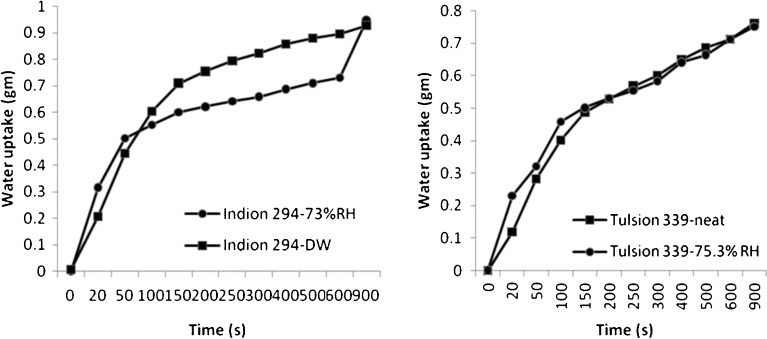
Figures 10 and 11 show the water uptakes of the four polacrilin potassium powders stored in original containers and those exposed to 75.3% RH. Moisture exposure substantially decreased both the rate and extent of water uptake of Doshion P544 DS and Amberlite IRP 88 (p < 0.005) (Fig. 10). The water uptake rate was increased on exposure to high humidity for both Indion 294 and Tulsion 339, however, the extent remained more or less the same (Fig. 11). This could be explained on the basis of the fact that the resin particles swelled in presence of moisture and the increase in particle size increased the void volume and porosity. The water not only occupied the voids but probably was also taken up into the polymer itself. This water may have plasticized the polymer and allowed it to expand, thus allowing yet more water to be taken up. It could be seen from the figures that Amberlite IRP 88 and Doshion P544 DS powders that were not exposed to high RH, had better rates of water uptake than the other two polacrilin potassium brands. However, Amberlite IRP 88and Doshion P544 DS particles aggregated in presence of 75.3% RH and lost their porosities, or probably the intra-particulate capillaries got saturated with water, which negatively affected their water uptake rate. The negative effect of moisture on the water uptake rate of disintegrants could be rated in the following rank order:
Fig. 10.
Water uptake rate of Amberlite IRP 88 and Doshion P 544 DS stored at different RH
Fig. 11.
Water uptake rate of Indion 294 and Tulsion 339 stored at different RH
Doshion P 544 DS>Amberlite IRP 88>Indion 294>Tulsion 339
Disintegrant Ability
In a preliminary study using a soluble matrix comprising anhydrous lactose and an insoluble matrix comprising DCP, disintegrants containing high and low levels of moisture were evaluated for their disintegrant performance. However, the results were not very clear because the tablets disintegrated too quickly. The DCP tablets disintegrated instantaneously making it impossible to distinguish between polacrilin potassium samples. Even the more slowly disintegrating lactose tablets gave disintegration times (DTs) of less than 5 min. Therefore, it was necessary to use a more slowly disintegrating matrix, to be able to discriminate between different samples of polacrilin potassium.
PEG 4,000 powder was chosen as an alternative to spray-dried lactose because PEG tablets containing 3% disintegrant and 1% lubricant gave tablets having disintegration time of about 13 min which was believed to be sufficiently long to allow the differences between the four materials to be seen. In addition, it has a low hygroscopicity when exposed to relative humidity, of less than 80%, at room temperature. DCP was used in combination with PEG 4,000.
The DTs of the slow disintegrating tablets containing polacrilin potassium samples which had either been stored in their original containers or were exposed to 75.3% RH are shown in Table V. The DTs of tablets containing Amberlite IRP 88 and Doshion P544 DS exposed to 75.3% RH were found to be significantly greater than for the tablets prepared using the materials stored in their original containers (p < 0.005). It was observed that there was a considerable increase in the size of Amberlite IRP 88 and Doshion P 544 DS particles exposed to 75% RH. Microscopic studies also showed that the particles of these disintegrants were agglomerated upon storage at 75% RH. Also, there was a significant (p < 0.005) decrease in water uptake rate upon exposure to 75% RH.
Table V.
Disintegration Time of Slow-Disintegrating Tablets Containing Disintegrants Exposed to Different Relative Humidities (Mean ± Sd, N = 6)
| Disintegrant | DT of tablet containing 3% disintegrant exposed to 11% RH (s) | DT of tablet containing 3% disintegrant exposed to 73.3% RH (s) |
|---|---|---|
| Amberlite IRP 88 | 32.6 (2.0) | 73 (8.1) |
| Doshion P 544 DS | 46.23 (8.9) | 107.66 (14.3) |
| Indion 294 | 103 (12.0) | 51.12 (3.8) |
| Tulsion 339 | 163 (10.5) | 151.6 (8.3) |
However, the disintegration times of tablets containing Indion 294 and Tulsion 339 exposed to 75.3% RH were significantly less (p < 0.005 and p < 0.05, respectively) than of those containing the same disintegrants stored in their original containers. This is in line with the fact that they showed a moderate increase in particle size due to swelling that was not accompanied by agglomeration, and they also showed improved water uptake rates.
We can conclude that exposure of Indion 294 and Tulsion 339 to moisture increased the water uptake rate and in turn decreased disintegration time. This was probably, because, these two brands were less hydrophilic and hence were slower to hydrate. Hence, the storage at high RH was not detrimental to their disintegrant performance. Rather, it plasticized the polymers and made them draw more amount of water and increased their intra-particulate porosities. Amberlite IRP 88 and Doshion P 544 DS, on the other hand, were found to be more hydrophilic and hence are better disintegrants than Indion 294 and Tulsion 339. However, they lost their intra-particulate porosities and agglomerated upon exposure to high RH. This decreased the water uptake rate and increased the disintegration time. Probably, Indion 294 and Tulsion 339 particles would also have agglomerated, if they were stored at the same level of moisture for longer times. Thus, Indion 294 and Tulsion 339 are inferior disintegrants, and the fact that they perform better after exposure to high humidity suggests that they do not hydrate as quickly which reduces their effectiveness. However, once they are hydrated, they perform much better.
CONCLUSIONS
In an earlier study in our laboratory, the physical characterization and functionality evaluation of four brands of Polacrilin Potassium NF was studied. In this study, it was observed that Doshion P 544 DS and Amberlite IRP 88 are better disintegrants than the other two brands. It was also concluded that the disintegrants function by wicking, and hence, water uptake measurement is a better test for their functionality evaluation. Since the resins swell little, measurement of settling volumes is not a useful test for their functionality evaluation (23,28).
The objective of this study was to understand the effects of moisture on the physical characteristics and functionality of four brands of Polacrilin Potassium NF. All four brands were hygroscopic. However, Doshion P544 DS was the most water absorbing brand. Moisture plasticized the disintegrants and reduced their yield pressures. Indion 294 and Amberlite IRP 88 were more compressible than the other two brands at low RHs. However, their compressibility was reduced to a greater extent upon exposure to moisture. Indion 294 showed maximum compactability (in terms of tensile strength) which was maintained over a wider range of RH. This was followed by Tulsion 339, Amberlite IRP 88, and Doshion P 544 DS. In earlier studies in our lab (28), Doshion P 544 DS and Amberlite IRP 88 were found to have significantly larger particle sizes than the other two brands. They also demonstrated better performance as disintegrants owing to their greater extent of water uptake. In this study, upon exposure to higher RH, particles of these two better performing disintegrants aggregated. Exposure to high humidity reduced their water uptake rate and in turn, their disintegrant performance. The tablets containing these two disintegrants previously exposed to higher RH showed increased disintegration times. It could be concluded that owing to their higher hygroscopicity, these two brands were better disintegrants than the other two brands, but were more sensitive to moisture and this should be taken into account while using them. The other two brands, Indion 294 and Tulsion 339, though not as effective in their disintegrant performance, showed better moisture stability. In fact, if protected from aggregation, moisture exposure improved their disintegrant performance. The effect of moisture on physical characteristics and functionality of the four brands of Polacrilin Potassium NF were different, and this should be taken into consideration before selecting a brand for incorporation in tablets.
ACKNOWLEDGMENTS
The authors would like to thank the Sophisticated Analytical Instrumentation Facility (SAIF) at Punjab University, Chandigarh for the PXRD studies, TA Instruments, Bangalore for the DVS analysis, and Dr. Ganesh jadhav, Dr. reddy’s Labs, Hyderabad for the particle size analysis on Malvern mastersizer using dry powder dispenser.
ABBREVIATIONS
- DCP
Dicalcium phosphate
- DT
Disintegration time
- LOD
Loss on drying
- PEG
Polyethylene glycol
- RH
Relative humidity
REFERENCES
- 1.Banker G, Perck G, Baley G. Compressed tablets. In: Lieberman H, Lachman L, editors. Pharmaceutical dosage forms: tablets, Volume-I. New York: Marcel Dekker; 1980. p. 86. [Google Scholar]
- 2.Marshall K, Rudinic EM. Tablet Dosage Forms. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. New York: Marcel Dekker, Inc; 1990. pp. 355–426. [Google Scholar]
- 3.Gordon, R. (1981). The thermodynamic characterization of the solid – water surface interaction of three tablet disintegrating agents. Doctoral dissertation, Purdue University –West Lafayette,1981.
- 4.Augsburger Larry L., Hahm Huijeong A., Brzeczko Albert W. and Shah Umang, “Super Disintegrants: Characterization and Function”, Encyclopedia of Pharmaceutical Technology, 2623–2637.
- 5.Smallenbroek AJ, Bolhuis GK, Lerk CF. The effect of particle size of disintegrants on the disintegration of tablets. Pharm Week Sci Ed. 1981;3:1048–1051. doi: 10.1007/BF02193321. [DOI] [Google Scholar]
- 6.Schwartz JB, Zelinskie JA. The binding and disintegrant properties of the corn starch fractions: amylase and amylopectin. Drug Dev Ind Pharm. 1978;4:463–483. doi: 10.3109/03639047809055654. [DOI] [Google Scholar]
- 7.Grai E, Ghanem AH, Mohmoud H. Studies on the direct compression of pharmaceuticals: part 15: effect of compression force on sulfadiazine–emcompresss tablets. Pharm Ind. 1984;46:279–284. [Google Scholar]
- 8.Khan KA, Rhodes CT. Disintegration properties of calcium phosphate dibasic dehydrate tablets. J Pharm Sci. 1975;64:166–167. doi: 10.1002/jps.2600640141. [DOI] [PubMed] [Google Scholar]
- 9.Shotton E, leonard GS. Effect of intragranular and extragranular disintegrating agents on particle size of disintegrated tablets. J Pharm Sci. 1976;65:1170–1174. doi: 10.1002/jps.2600650810. [DOI] [PubMed] [Google Scholar]
- 10.Khattab I, Menon A, Sakr A. Effect of mode of incorporation of disintegrants on the characteristics of fluid bed wet granulated tablets. J Pharm Pharmacol. 1992;45:687–691. doi: 10.1111/j.2042-7158.1993.tb07089.x. [DOI] [PubMed] [Google Scholar]
- 11.Mitrevej A, Hollenbeck R. Photomicrographic analysis of water vapour sorption and swelling of selected super-disintegrants. Pharm Tech. 1982;6:48–54. [Google Scholar]
- 12.Gould PL, Tan SB. The effect of recompression on the swelling kinetics of wet massed tablets containing superdisintegrants. Drug Dev Ind Pharm. 1985;11:1819–1836. doi: 10.3109/03639048509057701. [DOI] [Google Scholar]
- 13.Gould PL, Tan SB. The effect of recompression on disintegrant efficiency in tablets prepared by wet granulation. Drug Dev Ind Pharm. 1985;11:441–460. doi: 10.3109/03639048509056880. [DOI] [Google Scholar]
- 14.Hancock BC, Shamblin SL. Water vapour sorption by pharmaceutical sugars. Pharm Sci Tech Today. 1998;1(8):345–351. doi: 10.1016/S1461-5347(98)00088-1. [DOI] [Google Scholar]
- 15.Khan K, Rhodes CT. Water sorption properties of tablet disintegrants. J Pharm Sci. 1975;64(3):447–451. doi: 10.1002/jps.2600640321. [DOI] [PubMed] [Google Scholar]
- 16.Hahm HA. Effect of sorbed water on the efficiency of superdisintegrants: physical and mechanistic considerations. Ph.D.Dissertation, University of Maryland, Baltimore; 2002.
- 17.Faroongsarng D, Peck GE. The swelling and water uptake of tablets III: moisture sorption behavior of tablet disintegrants. Drug Dev Ind Pharm. 1994;20(5):779–798. doi: 10.3109/03639049409038331. [DOI] [Google Scholar]
- 18.Thibert R, Hancock BC. Direct visualisation of superdiisintegrant hydration by scanning electron microscopy. J Pharm Sci. 1995;85(11):1255–1258. doi: 10.1021/js960188d. [DOI] [PubMed] [Google Scholar]
- 19.Arwidsson HG, Larsson A, Graffner C. Water solid interactions II: effect of moisture sorption and glass transition temperature on compactability of microcrystalline cellulose alone or in binary mixtures with polyvinyl pyrrolidone. Int J Pharm. 1995;134(1–2):79–88. [Google Scholar]
- 20.Bravo-Osuna I, Ferrero C. Jimenez–Castellanos MR, Water sorption–desorption behaviour of methyl methacrylate-starch co polymers: effect of hydrophobic graft and drying method. Eur J Pharm Biopharm. 2005;59(30):537–548. doi: 10.1016/j.ejpb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Rowe RC, Sheskey PJ, Owen SC. Handbook of pharmaceutical excipients. London: Pharmaceutical Press; 2006. pp. 1410–1416. [Google Scholar]
- 22.Rohm and Haas Co. Amberlite IRP 88 (Product data sheet). Rohm and Haas Co.; February 2006.
- 23.Bele MH, Derle DV, On mechanism of action of Polacrilin Potassium NF, article communicated.
- 24.Fell JT, Newton JM. Determination of tablet strength by the diameteral-compression test. J Pharm Sc. 1970;59(5):688–691. doi: 10.1002/jps.2600590523. [DOI] [PubMed] [Google Scholar]
- 25.Young PM, Edge S, Staniforth J, Steele DF, Price R. Dynamic vapor sorption properties of sodium starch glycolate disintegrants. Pharm Dev Technol. 2005; 10. [DOI] [PubMed]
- 26.Brezeczko A. A critical evaluation of tablet diaintegration process, Ph.D. dissertation, University of Maryland, Baltimore, 1989.
- 27.Shah, U., Evaluation of the functional equivalence of different sources of superdisintegrants in pharmaceutical tablets, PhD dissertation, University of Maryland, Baltimore, (1996)
- 28.Bele MH, Derle DV. Brand to brand variation in disintegrant performance of polacrilin potassium NF. J Excip Food Chem. 2011;2(3):53–63. [Google Scholar]