Abstract
Microencapsulation of water-soluble drugs using coacervation-phase separation method is very challenging, as these drugs partitioned into the aqueous polymeric solution, resulting in poor drug entrapment. For evaluating the effect of ovalbumin on the microencapsulation of drugs with different solubility, pseudoephedrine HCl, verapamil HCl, propranolol HCl, paracetamol, and curcuminoid were used. In addition, drug mixtures comprising of paracetamol and pseudoephedrine HCl were also studied. The morphology, encapsulation efficiency, particle size, and in vitro release profile were investigated. The results showed that the solubility of the drug determined the ratio of ovalbumin to be used for successful microencapsulation. The optimum ratios of drug, ovalbumin, and gelatin for water-soluble (pseudoephedrine HCl, verapamil HCl, and propranolol HCl), sparingly water-soluble (paracetamol), and water-insoluble (curcuminoid) drugs were found to be 1:1:2, 2:3:5, and 1:3:4. As for the drug mixture, the optimum ratio of drug, ovalbumin, and gelatin was 2:3:5. Encapsulated particles prepared at the optimum ratios showed high yield, drug loading, entrapment efficiency, and sustained release profiles. The solubility of drug affected the particle size of the encapsulated particle. Highly soluble drugs resulted in smaller particle size. In conclusion, addition of ovalbumin circumvented the partitioning effect, leading to the successful microencapsulation of water-soluble drugs.
Key words: coacervation-phase separation method, gelatin, microencapsulation, ovalbumin, solubility
INTRODUCTION
Microencapsulation of water-soluble drugs using coacervation-phase separation method is very challenging. In the case of poorly or insoluble drugs, they can be encapsulated easily by aqueous polymeric coat. In contrast, when water-soluble drugs are involved, there is a high tendency of partitioning of the water-soluble drugs from the polymeric phase into the aqueous phase, resulting in poor entrapment.
Studies have been carried out to resolve drug-partitioning effect. Dispersion of drugs (with or without polymer) into aqueous medium, oil, or non-aqueous solvents to prevent drug loss and emulsification as water-in-oil (w/o) emulsion were reported (1–5). However, the non-aqueous medium was often expensive and difficult to eliminate (1). In addition, emulsification of the drug into an emulsion (o/w) produced irregular and rough surfaces (2). This might be attributed to drug partitioning into the external phase (aqueous) and drug crystals deposited on the outer layer of the microsphere. In another study, drug was encapsulated by wax using spray congealing (5). As a result of the wetting difficulty between the highly hydrophilic drug crystals and the highly lipophilic molten wax, a limited loading was obtained even after the addition of surfactants. Other groups of researcher encapsulated the water-soluble drugs using w/o/w double emulsion or double emulsion with biodegradable polymer (3,4,6–10). Yeo et al. used the ink-jet-interfacial-phase separation method to cover a single hydrophilic core with a thin biodegradable polymeric membrane (11). Lecomte et al. used polymer blends to coat propranolol hydrochloride loaded pellets (12). Microencapsulation by forming liposome was also reported to contain water-soluble drugs (13). Although these studies could prevent the partitioning effect of water-soluble drugs, a large amount of excipient was required and the drug loading was low. In order to increase the capacity of drug loading in particles, alternative methods such as supercritical anti-solvent (14), electrospinning process using biodegradable polymers (15), culturing of drug into yeast cells (16), gelatin coacervation-phase separation method (17), and using hydrogen peroxide to make porous microspheres (18) have been investigated. Nevertheless, most of these methods involved complicated procedures with limitations of their own.
Generally, coacervation-phase separation method is used to encapsulate water-insoluble materials and one of the commonly used microencapsulating agents is gelatin (21,22). The method involves removal of associated water molecules from the dispersed colloid by coacervating agents with a greater affinity for water. Dehydrated molecules of polymer tend to aggregate with surrounding molecules to form the coacervate. In previous studies (19,20), gelatin was unable to encapsulate water-soluble drugs, pseudoephedrine HCl (PE), diclofenac sodium, and paracetamol (PC), in coacervation-phase separation method. During phase separation process, the drugs were separated from gelatin phase and partitioned into the aqueous phase when coacervated with ethanol.
Chicken egg white is the major source of ovalbumin (23–25). Chicken egg white has often been used as the ingredient in food processing for their unique functional properties, such as gelling, foaming, heat setting, and binding adhesion (26). Ovalbumin constitutes over half of the egg white albumen. It is a monomeric phosphoglycoprotein and the only protein that contains free sulfhydril groups, which is buried in the protein core. Heat-induced denaturation of ovalbumin results in the external exposure of these sulfhydril groups, accompanied by a decrease in the total sulfhydril content, due to the oxidation of SH groups to disulfide bonds (27). Egg albumin has been used for the microencapsulation of drugs. These included the capillary extrusion method (denaturation by dispersion of drug-egg albumin solution into hot oil), w/o emulsion method (physical denaturation of the emulsified oil-drug-egg albumin with different temperature), and chemical method using glutaraldehyde (28–32). In another study, a w1/o/w2 emulsion solvent evaporation method was employed to encapsulate hydrophilic peptide into microspheres. Ovalbumin was used to stabilize the inner emulsion to prevent the destruction of the internal globules and leakage of the peptide to the outer aqueous phase (33). However, the peptide eluted from the surface of the microspheres through pores formed by ovalbumin aggregation that occurred during the washing process, and these pores constituted a pathway for the fast drug release (33).
In view of these aspects, the study aimed to improve the encapsulation of water-soluble drugs by incorporation of ovalbumin to circumvent drug partitioning from gelatin phase to aqueous phase, in coacervation-phase separation method.
EXPERIMENTS
Materials
Ovalbumin (grade 2) and gelatin (Type B, bloom 225) were purchased from Sigma, USA. Curcuminoid (CU) standard 98% (containing three compounds: curcumin 1 (CU1), curcumin 2 (CU2), and curcumin 3 (CU3)) was purchased from Acros Organic, USA. Acetone and glacial acetic acid were purchased from R & M Chemicals, UK. PC was purchased from Vangfou Pharma, China. Formaldehyde solution (37%) was purchased from Merck, Germany. Ethanol (95%) and methanol were purchased from Fisher Scientific, UK. Verapamil HCl (VP) was purchased from Nicholas Piramal India Ltd, India. Propranolol HCl (PP) was purchased from SM Pharmaceuticals, Malaysia. PE was purchased from Emmellen Pharmaceutical and Biotech, India.
Encapsulation of Drugs Using Gelatin
The drug compounds, namely, PE, VP, PP, PC, and CU were used, as well as two drugs, PC and PE, 1:1, w/w (PCPE). The coacervated layer determined was similar to that previously described (19,20). The ratio of drug to gelatin was set at 1:1. For each gram of gelatin, 20 mL of water was used and coacervated with 20 mL of ethanol. The drug compounds were either dissolved or dispersed in the gelatin solution at 50°C with a constant stirring using a magnetic stirrer (Heidolph, Germany). Ethanol was added to the drug dispersion using a syringe pump (Argus 600, Switzerland) at a feeding rate of 1 mL/min and a stirring speed of 500 rpm. The mixture was stirred continuously at 500 rpm for an additional hour to ensure a complete deposition of gelatin onto the drug compound. Formaldehyde solution (37%, v/v) was added to rigidize the gelatin coating. The volume of formaldehyde solution added was equivalent to the volume of coacervated layer obtained (1:1, v/v). The encapsulated particles collected were washed three times with ethanol, followed by cold water (5°C), re-dispersed in water, kept frozen at −70°C for 24 h, dried by lyophilization (Labconco, Missouri, USA), and finally sieved through a 100-mesh sieve (150 μm).
Preparation of Drug–Ovalbumin Particles
The drugs consisted of PE, VP, PP, PC, and CU. The ovalbumin solution (20%, w/v) was prepared in distilled water at room temperature (28°C) using a magnetic stirrer (Heidolph, Germany). A known amount of the drug materials was either dissolved or dispersed in 20 to 40 mL of distilled water. The solutions or dispersions were dispersed in the ovalbumin solution, with drug to ovalbumin ratios at 1:3, 1:2, 2:3, 1:1, and 3:2 (w/w) as shown in Table I. The mixtures were homogenized at 5,000 rpm for 1 min (Ultra-Turrax T18 Homogenizer, USA) before transferred to a Teflon plate and dried in an oven (Carbolite, UK) at 50°C for 12 h. The resulting drug–ovalbumin particles were gently triturated using the mortar and pestle and sieved through a sieve (150 μm).
Table I.
Preparation of Drug–Ovalbumin–Gelatin Microcapsules
| Drug compound or powder | Drug solution or dispersion | 20% ovalbumin solution | Ratio of D/O (w/w) | Code | 5% gelatin solution | Ratio of D/O/G (w/w/w) | Code | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name (solubility) | Codea | Drug (mg) | Water (mL) | Ovalbumin (mg) | Water (mL) | Gelatin (mg) | Water (mL) | ||||
| Pseudoephedrine HCl (very water-soluble, 1 g/0.5 mL) | PE | 250 | 0.13 | 750 | 3.75 | 1:3 | PEO13 | 1,000 | 20 | 1:3:4 | PEOG134 |
| 333 | 0.17 | 666 | 3.33 | 1:2 | PEO12 | 1,000 | 20 | 1:2:3 | PEOG123 | ||
| 400 | 0.20 | 600 | 3.00 | 2:3 | PEO23 | 1,000 | 20 | 2:3:5 | PEOG235 | ||
| 500 | 0.25 | 500 | 2.50 | 1:1 | PEO11 | 1,000 | 20 | 1:1:2 | PEOG112 | ||
| 600 | 0.30 | 400 | 2.00 | 3:2 | PEO32 | 1,000 | 20 | 3:2:5 | PEOG325 | ||
| Verapamil HCl (water-soluble, 1 g/15 mL) | VP | 250 | 3.75 | 750 | 3.75 | 1:3 | VPO13 | 1,000 | 20 | 1:3:4 | VPOG134 |
| 333 | 5.00 | 666 | 3.33 | 1:2 | VPO12 | 1,000 | 20 | 1:2:3 | VPOG123 | ||
| 400 | 6.00 | 600 | 3.00 | 2:3 | VPO23 | 1,000 | 20 | 2:3:5 | VPOG235 | ||
| 500 | 7.50 | 500 | 2.50 | 1:1 | VPO11 | 1,000 | 20 | 1:1:2 | VPOG112 | ||
| 600 | 9.00 | 400 | 2.00 | 3:2 | VPO32 | 1,000 | 20 | 3:2:5 | VPOG325 | ||
| Propranolol HCl (water-soluble, 1 g/20 mL) | PP | 250 | 5.00 | 750 | 3.75 | 1:3 | PPO13 | 1,000 | 20 | 1:3:4 | PPOG134 |
| 333 | 6.66 | 666 | 3.33 | 1:2 | PPO12 | 1,000 | 20 | 1:2:3 | PPOG123 | ||
| 400 | 8.00 | 600 | 3.00 | 2:3 | PPO23 | 1,000 | 20 | 2:3:5 | PPOG235 | ||
| 500 | 10.00 | 500 | 2.50 | 1:1 | PPO11 | 1,000 | 20 | 1:1:2 | PPOG112 | ||
| 600 | 12.00 | 400 | 2.00 | 3:2 | PPO32 | 1,000 | 20 | 3:2:5 | PPOG325 | ||
| Paracetamol (sparingly water-soluble, 1 g/70 mL) | PC | 250 | 17.50 | 750 | 3.75 | 1:3 | PCO13 | 1,000 | 20 | 1:3:4 | PCOG134 |
| 333 | 23.31 | 666 | 3.33 | 1:2 | PCO12 | 1,000 | 20 | 1:2:3 | PCOG123 | ||
| 400 | 28.00 | 600 | 3.00 | 2:3 | PCO23 | 1,000 | 20 | 2:3:5 | PCOG235 | ||
| 500 | 35.00 | 500 | 2.50 | 1:1 | PCO11 | 1,000 | 20 | 1:1:2 | PCOG112 | ||
| 600 | 42.00 | 400 | 2.00 | 3:2 | PCO32 | 1,000 | 20 | 3:2:5 | PCOG325 | ||
| Curcuminoid (water insoluble)a | CU | 250 | 50 | 750 | 3.75 | 1:3 | CUO13 | 1,000 | 20 | 1:3:4 | CUOG134 |
| 333 | 50 | 666 | 3.33 | 1:2 | CUO12 | 1,000 | 20 | 1:2:3 | CUOG123 | ||
| 400 | 50 | 600 | 3.00 | 2:3 | CUO23 | 1,000 | 20 | 2:3:5 | CUOG235 | ||
| 500 | 50 | 500 | 2.50 | 1:1 | CUO11 | 1,000 | 20 | 1:1:2 | CUOG112 | ||
| 600 | 50 | 400 | 2.00 | 3:2 | CUO32 | 1,000 | 20 | 3:2:5 | CUOG325 | ||
| Paracetamol + pseudoephedrine HCl | PCPE | 200 + 200 | 28 | 600 | 3.00 | 2:3 | PCPEO23 | 1,000 | 20 | 2:3:5 | PCPEOG235 |
PE pseudoephedrine HCl, VP verapamil HCl, PP propranolol HCl, PC paracetamol, CU curcuminoid, PCPE paracetamol and pseudoephedrine HCl at a ratio of 1:1, D/O drug/ovalbumin, D/O/G drug/ovalbumin/gelatin, PEO13 pseudoephedrine HCl and ovalbumin at a ratio of 1:3, PEO12 pseudoephedrine HCl and ovalbumin at a ratio of 1:2, PEO23 pseudoephedrine HCl and ovalbumin at a ratio of 2:3, PEO11 pseudoephedrine HCl and ovalbumin at a ratio of 1:1, PEO32 pseudoephedrine HCl and ovalbumin at a ratio of 3:2, VPO13 verapamil HCl and ovalbumin at a ratio of 1:3, VPO12 verapamil HCl and ovalbumin at a ratio of 1:2, VPO23 verapamil HCl and ovalbumin at a ratio of 2:3, VPO11 verapamil HCl and ovalbumin at a ratio of 1:1, VPO32 verapamil HCl and ovalbumin at a ratio of 3:2, PPO13 propranolol HCl and ovalbumin at a ratio of 1:3, PPO12 propranolol HCl and ovalbumin at a ratio of 1:2, PPO23 propranolol HCl and ovalbumin at a ratio of 2:3, PPO11 propranolol HCl and ovalbumin at a ratio of 1:1, PPO32 propranolol HCl and ovalbumin at a ratio of 3:2, PCO13 paracetamol and ovalbumin at a ratio of 1:3, PCO12 paracetamol and ovalbumin at a ratio of 1:2, PCO23 paracetamol and ovalbumin at a ratio of 2:3, PCO11 paracetamol and ovalbumin at a ratio of 1:1, PCO32 paracetamol and ovalbumin at a ratio of 3:2, CUO13 curcuminoid and ovalbumin at a ratio of 1:3, CUO12 curcuminoid and ovalbumin at a ratio of 1:2, CUO23 curcuminoid and ovalbumin at a ratio of 2:3, CUO11 curcuminoid and ovalbumin at a ratio of 1:1, CUO32 curcuminoid and ovalbumin at a ratio of 3:2, PCPEO23 paracetamol–pseudoephedrine HCl and ovalbumin at a ratio of 2:3, PEOG134 pseudoephedrine HCl, ovalbumin, and gelatin at a ratio of 1:3:4, PEOG123 pseudoephedrine HCl, ovalbumin, and gelatin at a ratio of 1:2:3, PEOG235 pseudoephedrine HCl, ovalbumin, and gelatin at a ratio of 2:3:5, PEOG112 pseudoephedrine HCl, ovalbumin, and gelatin at a ratio of 1:1:2, PEOG325 pseudoephedrine HCl, ovalbumin, and gelatin at a ratio of 3:2:5, VPOG134 verapamil HCl, ovalbumin, and gelatin at a ratio of 1:3:4, VPOG123 verapamil HCl, ovalbumin, and gelatin at a ratio of 1:2:3, VPOG235 verapamil HCl, ovalbumin, and gelatin at a ratio of 2:3:5, VPOG112 verapamil HCl, ovalbumin, and gelatin at a ratio of 1:1:2, VPOG325 verapamil HCl, ovalbumin, and gelatin at a ratio of 3:2:5, PPOG134 propranolol HCl, ovalbumin, and gelatin at a ratio of 1:3:4, PPOG123 propranolol HCl, ovalbumin, and gelatin at a ratio of 1:2:3, PPOG235 propranolol HCl, ovalbumin, and gelatin at a ratio of 2:3:5, PPOG112 propranolol HCl, ovalbumin, and gelatin at a ratio of 1:1:2, PPOG325 propranolol HCl, ovalbumin, and gelatin at a ratio of 3:2:5, PCOG134 paracetamol, ovalbumin, and gelatin at a ratio of 1:3:4, PCOG123 paracetamol, ovalbumin, and gelatin at a ratio of 1:2:3, PCOG235 paracetamol, ovalbumin, and gelatin at a ratio of 2:3:5, PCOG112 paracetamol, ovalbumin, and gelatin at a ratio of 1:1:2, PCOG325 paracetamol, ovalbumin, and gelatin at a ratio of 3:2:5, CUOG134 curcuminoid, ovalbumin, and gelatin at a ratio of 1:3:4, CUOG123 curcuminoid, ovalbumin, and gelatin at a ratio of 1:2:3, CUOG235 curcuminoid, ovalbumin, and gelatin at a ratio of 2:3:5, CUOG112 curcuminoid, ovalbumin, and gelatin at a ratio of 1:1:2, CUOG325 curcuminoid, ovalbumin, and gelatin at a ratio of 3:2:5, PCPEOG23 paracetamol–pseudoephedrine HCl, ovalbumin, and gelatin at a ratio of 2:3:5
aContains three compounds: curcumin 1, curcumin 2, and curcumin 3
Gelatin Coating of Drug–Ovalbumin Particles
The drug–ovalbumin particles comprising of pseudoephedrine–ovalbumin (PEO), verapamil–ovalbumin (VPO), propranolol–ovalbumin (PPO), paracetamol–ovalbumin (PCO), and curcuminoid–ovalbumin (CUO) at various compositions are shown in Table I. The drug–ovalbumin particles were dispersed in hot gelatin solution at 50°C with a constant stirring using a magnetic stirrer (Heidolph, Germany). Ethanol was added to the drug–ovalbumin–gelatin dispersion using a syringe pump (Argus 600, Switzerland) at a feeding rate of 1 mL/min and a stirring speed of 500 rpm. The mixture was stirred continuously at 500 rpm for an additional hour to ensure a complete deposition of gelatin onto the drug–ovalbumin. Formaldehyde solution (37%, v/v) was added to rigidize the gelatin coating. The drug–ovalbumin–gelatin particles collected were washed three times with ethanol, followed by cold water (5°C), re-dispersed in water, kept frozen at −70°C for 24 h, dried by lyophilization (Labconco, Missouri, USA), and finally sieved through a 100-mesh sieve (150 μm).
Microencapsulation of Drug Mixtures
Two drugs, PC (sparingly soluble, 1 g/70 mL water) and PE (very soluble, 1 g/0.5 mL water) were mixed together at a ratio of 1:1 (w/w) (Table I). The drug mixtures were dissolved completely in water and encapsulated according to the procedures described in “Microencapsulation Using Ovalbumin and Gelatin,” “Percent of Yield, Drug Loading, and Entrapment Efficiency,” and “In vitro Drug Release Study.”
Morphology Evaluation
The morphology was investigated using a light microscope (Leica DMLB, Cambridge, UK) connected to a camera (Leica DC 300, Cambridge, UK) and a compact workstation (Leica Compact, UK). The encapsulated particles were dispersed in a drop of liquid paraffin prior to observation under a light microscope. The surface morphology of selected particles was also examined using a scanning electron microscope (Leica Cambridge S360, UK) at operating voltage of 5.00 kV. The samples were first sputter coated with gold under an argon atmosphere (Emitech K750, Kent, UK).
Particle Size Determination
The particle size was determined with a Mastersizer S (Malvern Instruments, MAM5005, UK) fitted with a small sample dispersion unit (MS1) connected to a dispersion unit controller. A beam length of 2.4 mm and 300 RF lens (range, 0.05–900 μm) was used. The drug materials and the encapsulated formulations were sonicated in hexane or water for 1 min before loading into the small sample dispersion unit and stirred at a speed of 1,000 rpm until an obscuration value between 12% and 17% was obtained. Before running each sample, the system was aligned and a background measurement was taken using filtered hexane or water (0.45 μm nylon membrane filters, Whatman, Maidstone, UK) as dispersing solvent. The sample was measured thrice with 12,000 sweeps over 10 runs.
Drug Analysis
The concentration of PE, VP, PP, and PC were analyzed using UV-spectrophotometer (Hitachi, model U-2000, Tokyo, Japan) at detection wavelengths of 206, 278, 290, and 242 nm, respectively. The CU content (CU1, CU2, and CU3) was analyzed using HPLC method (19,20). Briefly, the HPLC system was comprised of a pump (Model 307, Gilson), 6-valve injection port (Rheodyne, Cotati, CA, USA), a UV detector (Model 115, Gilson, France) and an integrator (D-2500 Chromato-Integrator, Hitachi, Japan). The detector was operated at a detection wavelength of 375 nm. A reversed-phase column (Luna C18 (2), 5 μm, 150 × 4.5 mm ID, Phenomenex) fitted with a refillable guard column (Upchurch Scientific, Oak Harbor, WA, USA) was used for the chromatographic separation. The mobile phase was comprised of acetonitrile, methanol, and water (35:10:55, v/v) adjusted to pH 3.0 with glacial acetic acid. The analysis was run at a flow rate of 1.3 mL/min. The drug content of encapsulated particles (ground, centrifuged, filtered, and extracted using water for water-soluble drugs and ethanol for CU) was determined by injecting the sample into the column.
Determination of Percent Yield, Drug Loading, and Entrapment Efficiency
The percentage of yield, drug loading, and entrapment efficiency (EE) of the drug-loaded particles were calculated using the following equations (19,34).
 |
In addition, the percentage of compositions of CU1, CU2, and CU3 in CU powder and encapsulated CU particles were compared. The percentages of PC and PE entrapped in drug–gelatin and drug–ovalbumin–gelatin particles were determined and compared.
In vitro Drug Release Study
The release of pure drug powder or encapsulated formulations was investigated using modified Franz diffusion cells. Twenty-five milligrams of pure drug powder or encapsulated formulation containing an equivalent amount of drug was carefully transferred onto the cellulose nitrate membrane. Water and ethanol were used as the dissolution medium for the water-soluble drugs and CU, respectively. At pre-set time intervals of 0, 5, 10, and 20 min, 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 h after the commencement of the study, 0.2-mL samples were removed and replaced with the same volume of fresh dissolution medium. In addition, the percentage of PC and PE released from drug–gelatin and drug–ovalbumin–gelatin particles were determined and compared.
Statistical Analysis
The results were treated statistically using SPSS software (version 15, USA). One-way analysis of variance was employed for the analysis of results. When there was a statistically significant difference, post hoc Tukey’s honestly significant difference (HSD) test was applied. Independent samples t test was used to compare the means of variables for two groups. A statistically significant difference was defined as P < 0.05.
RESULTS AND DISCUSSION
Microencapsulation Using Gelatin
When examined under microscope, only curcuminoid–gelatin particles appeared as discrete particles and were spherical in shape (19). In contrast, the water-soluble drugs, PE, VP, PP, and PC, existed in agglomeration and were not encapsulated. Previous studies demonstrated the inability of coacervation-phase separation method to encapsulate water-soluble drugs when gelatin solution was coacervated with ethanol (19,20).
When the hydrophilic drugs, PE, VP, PP, and PC, were added to the aqueous gelatin solution at 50°C, clear solutions were obtained but upon cooling turbid layer was observed. When examined under microscope after freeze-dried, the particles appeared in agglomeration and the drug particles could be seen deposited on the gelatin coat.
The successful encapsulation of CU involved the removal of only water from the gelatin–curcuminoid dispersion upon the addition of ethanol. The resultant dehydrated molecules of gelatin aggregated and surrounded CU molecule to form the precipitated coacervate-phase containing curcuminoid–gelatin particles and the aqueous phase. However, in the case of water-soluble drugs, upon addition of ethanol, due to the high mutual affinity, water and water-soluble drugs were separated from gelatin phase and partitioned into the aqueous phase.
Microencapsulation Using Ovalbumin and Gelatin
Preparation of Drug–Ovalbumin Particles
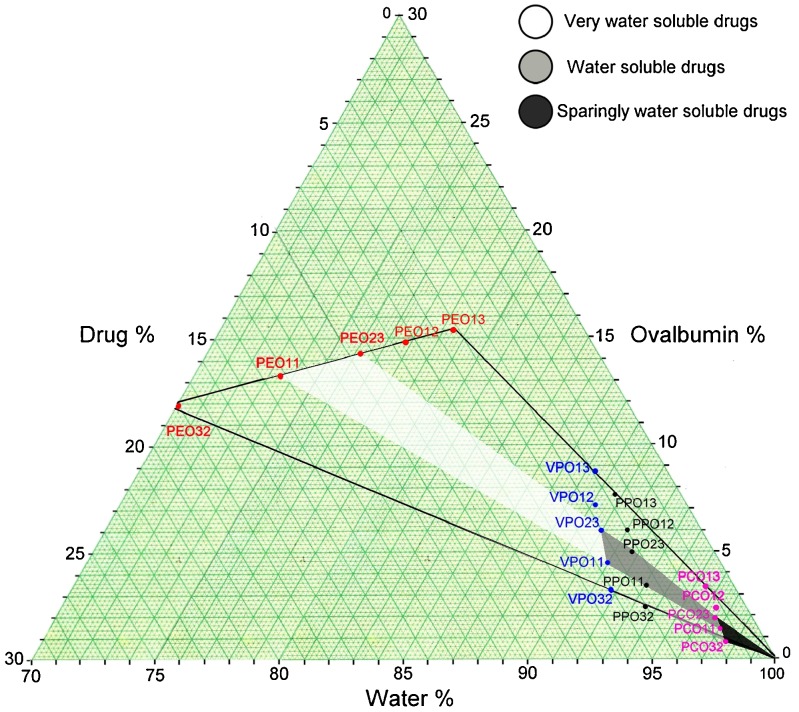
The phase diagram in Fig. 1 shows the suitable area for preparing drug–ovalbumin dispersion. pseudoephedrine–ovalbumin particles (PEO11), verapamil–ovalbumin particles (VPO11), and propranolol–ovalbumin particles (PPO11) were obtained at a ratio of 1:1. However, the paracetamol–ovalbumin particles (PCO23) were produced at a ratio of 2:3.
Fig. 1.
Phase diagram showing the optimum area obtained for producing the drug–ovalbumin particles
When drug solution blended with ovalbumin solution (20%), the amount of water used for preparing drug solutions were varied according to the drug’s solubility. The phase diagram demonstrated that the drug’s solubility is directly proportional to the amount of ovalbumin used but inversely proportional to the amount of water used. Thus, drug with lower solubility requires lesser amount of ovalbumin but more amount of water.
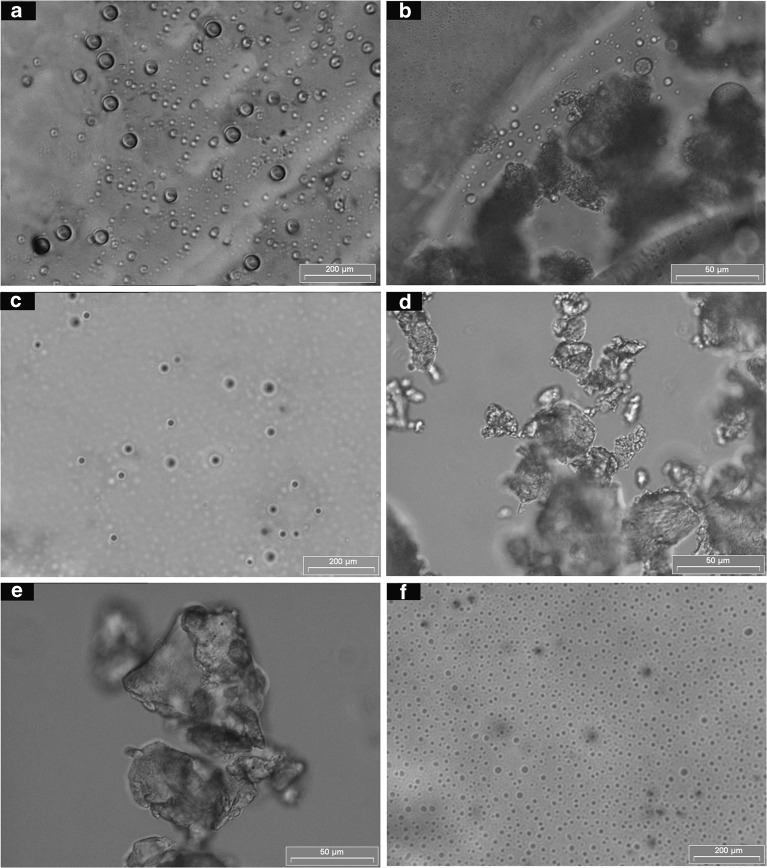
Figure 2 shows some typical examples of the morphology of these particles. When PE (Fig. 2a) and ovalbumin were prepared at a ratio of 1:1, the pseudoephedrine–ovalbumin particles were freely produced (Fig. 2b). While, at a ratio of 2:3, agglomerated particles were obtained (Fig. 2c). In contrast, agglomerated particles were determined when PC (Fig. 2d) was prepared with ovalbumin at a ratio of 1:1 (Fig. 2e). However, at a ratio of 2:3, paracetamol–ovalbumin particles were freely produced (Fig. 2f). Formulations PEO13, PEO12, VPO13, VPO12, PPO13, PPO12, PCO32, CUO12, CUO23, CUO11, CUO32, PEO32, VPO32, PPO32, PCO13, and PCO12 (Fig. 3), failed to form discrete particles and agglomeration was observed (Table II).
Fig. 2.
Photographs of a PE, b pseudoephedrine–ovalbumin (1:1), c pseudoephedrine–ovalbumin (2:3), d paracetamol, e paracetamol–ovalbumin (1:1), and f paracetamol–ovalbumin (2:3). Magnification, ×200
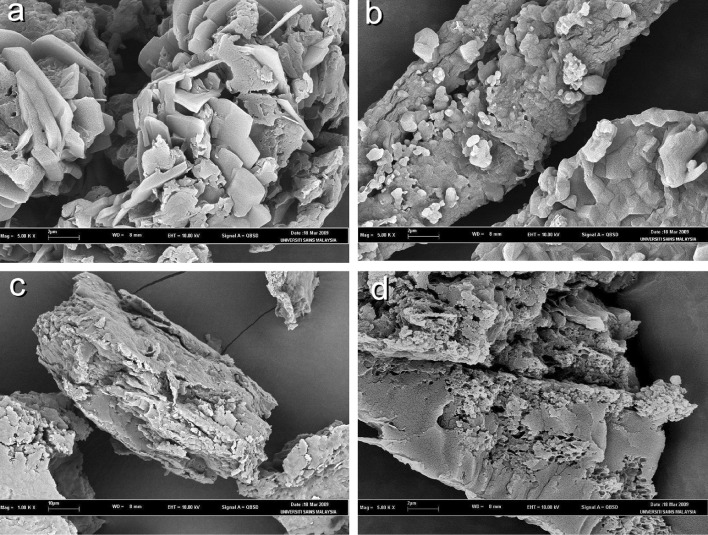
Fig. 3.
Scanning electron micrographs of a verapamil–ovalbumin (1:1; VPO11—magnification, ×5,000), b propranolol–ovalbumin (1:1; PPO11—magnification, ×5,000), c pseudoephedrine–ovalbumin (1:1; PEO11—magnification, ×1,000), and d paracetamol–ovalbumin (2:3; PCO23—magnification, ×5,000)
Table II.
Preparation of Drug–Ovalbumin Complex
| Drug–ovalbumin complex | Phase diagram composition (%) | Observation | Particle size D[4,3] μm | ||
|---|---|---|---|---|---|
| Drug (%) | Ovalbumin (%) | Water (%) | |||
| PEO13 | 5.1 | 15.4 | 79.5 | Agglomeration | 45.82 ± 3.31 |
| PEO12 | 7.4 | 14.8 | 77.8 | Agglomeration | 43.29 ± 2.80 |
| PEO23 | 9.5 | 14.3 | 76.2 | Microparticles | 17.28 ± 0.32* |
| PEO11 | 13.3 | 13.3 | 73.3 | Microparticles | 15.33 ± 1.19* |
| PEO32 | 18.2 | 12.1 | 69.7 | Agglomeration | 41.03 ± 2.63 |
| VPO13 | 2.9 | 8.8 | 88.2 | Agglomeration | 47.58 ± 3.15 |
| VPO12 | 3.6 | 7.1 | 89.3 | Agglomeration | 46.10 ± 3.79 |
| VPO23 | 4.0 | 6.0 | 90.0 | Microparticles | 18.53 ± 1.15* |
| VPO11 | 4.5 | 4.5 | 90.9 | Microparticles | 16.61 ± 1.11* |
| VPO32 | 5.0 | 3.3 | 91.7 | Agglomeration | 42.00 ± 2.18 |
| PPO13 | 2.6 | 7.7 | 89.7 | Agglomeration | 49.69 ± 2.47 |
| PPO12 | 3.0 | 6.1 | 90.9 | Agglomeration | 48.51 ± 2.80 |
| PPO23 | 3.3 | 5.0 | 91.7 | Microparticles | 20.08 ± 1.70* |
| PPO11 | 3.7 | 3.7 | 92.6 | Microparticles | 17.25 ± 1.05* |
| PPO32 | 4.0 | 2.7 | 93.3 | Agglomeration | 43.08 ± 2.94 |
| PCO13 | 1.1 | 3.4 | 95.5 | Agglomeration | 51.04 ± 2.53 |
| PCO12 | 1.2 | 2.4 | 96.4 | Agglomeration | 49.86 ± 2.18 |
| PCO23 | 1.3 | 1.9 | 96.9 | Microparticles | 25.05 ± 1.30* |
| PCO11 | 1.3 | 1.3 | 97.4 | Microparticles | 28.06 ± 1.18* |
| PCO32 | 1.3 | 0.9 | 97.8 | Agglomeration | 45.21 ± 3.23 |
| CUO13 | 0.5 | 1.4 | 98.2 | Microparticles | 71.81 ± 2.89* |
| CUO12 | 0.6 | 1.2 | 98.2 | Agglomeration | 68.13 ± 3.85* |
| CUO23 | 0.7 | 1.1 | 98.1 | Agglomeration | 90.62 ± 3.40 |
| CUO11 | 0.9 | 0.9 | 98.1 | Agglomeration | 96.03 ± 2.37 |
| CUO32 | 1.1 | 0.8 | 98.1 | Agglomeration | 98.73 ± 2.32 |
*p < 0.05
The scanning electron micrographs of drug–ovalbumin particles are shown in Fig. 4. It can be observed that ovalbumin and drug adsorbed onto each other forming solid aggregates. The aggregates were irregular in shape with rough surfaces. The formation of aggregates could have affected the physical properties of drug.
Fig. 4.
Photographs of encapsulated particles of a verapamil–ovalbumin–gelatin (1:1:2), b verapamil–ovalbumin–gelatin (2:3:5), c propranolol–ovalbumin–gelatin (1:1:2), d propranolol–ovalbumin–gelatin (2:3:5), e paracetamol–ovalbumin–gelatin (1:1:2), and f paracetamol–ovalbumin–gelatin (2:3:5). Magnification, ×200
The particle sizes at drug to ovalbumin ratio of 1:1 (PEO11, VPO11, PPO11, and PCO11) and 2:3 (PEO23, VPO23, PPO23, and PCO23) were significantly smaller than the particles produced at drug to ovalbumin ratio of 1:3 (PEO13, VPO13, PPO13 and PCO13), 1:2 (PEO12, VPO12, PPO12, and PCO12) and 3:2 (PEO32, VPO32, PPO32, and PCO32). However, the curcuminoid–ovalbumin particles (CUO12 and CUO13) produced at ratios of 1:2 and 1:3, were significantly smaller than other ratios (CUO23, CUO11, and CUO32) (Table II).
Gelatin Coating of Drug–Ovalbumin Particles
Referring to photographs in Fig. 4, PEOG112, VPOG112, PPOG112, PCOG235, and CUOG134 appeared as discrete particles. However, formulations PEOG235, VPOG235, PPOG235, PCOG112, and CUOG123 existed in agglomeration.
The results of particle size of drug and drug–ovalbumin–gelatin particles at various ratios are presented in Table III. The particle size results of water-soluble drugs, at drug to ovalbumin to gelatin of 1:1:2 (PEOG112, VPOG112, and PPOG112) and 2:3:5 (PEOG235, VPOG235, and PPOG235), were significantly smaller than that of the corresponding drug powders. Increasing the amount of ovalbumin from ratio of 1:1:2 (PEOG112, VPOG112, and PPOG112) to 2:3:5 (PEOG235, VPOG235, and PPOG235), resulted in a larger particle size. However, the particle size results of particles prepared from PC (PCOG112 and PCOG235), and CU (CUOG123 and CUOG134) were significantly larger when compared with their corresponding drug powders. An increase in the amount of ovalbumin increased the particle size of CU particles from ratio of 1:2:3 to 1:3:4 (CUOG123 < CUOG134). In the case of PC, an increase in ovalbumin decreased the particle size from ratio of 1:1:2 to 2:3:5 (PCOG112 > PCOG235).
Table III.
Particle Size of the Formulations
| Formulations | Particle size D[4,3] μm | ||||
|---|---|---|---|---|---|
| PE | VP | PP | PC | CU | |
| Drug powder (A) | 37.52 ± 1.91 | 40.65 ± 1.00 | 47.81 ± 1.76 | 38.07 ± 1.67 | 31.39 ± 0.98 |
| Drug/ovalbumin/gelatin at 1:1:2 (B) | 25.43 ± 2.57 | 29.89 ± 0.85 | 31.31 ± 1.35 | 75.93 ± 1.14 | – |
| Drug/ovalbumin/gelatin at 2:3:5 (C) | 32.34 ± 1.07 | 35.78 ± 1.55 | 36.70 ± 0.93 | 64.75 ± 4.45 | – |
| Drug/ovalbumin/gelatin at 1:2:3 (D) | – | – | – | – | 121.36 ± 1.96 |
| Drug/ovalbumin/gelatin at 1:3:4 (E) | – | – | – | – | 184.68 ± 3.19 |
| Statistical analysis | |||||
| F | 29.048 | 63.780 | 110.013 | 142.872 | 3,565.634 |
| Significance | P < 0.01 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
| Tukey’s HSD | |||||
| A and B | P < 0.01 | P < 0.001 | P < 0.001 | P < 0.001 | – |
| A and C | P < 0.05 | P < 0.01 | P < 0.001 | P < 0.001 | – |
| B and C | P < 0.05 | P < 0.01 | P < 0.01 | P < 0.01 | – |
| A and D | – | – | – | – | P < 0.001 |
| A and E | – | – | – | – | P < 0.001 |
| D and E | – | – | – | – | P < 0.001 |
Mean ± SD (N = 3)
In short, the solubility of drug in ovalbumin solution affects the particle size of its formulation. Drugs that dissolved in ovalbumin solution showed smaller particle size than drugs that were dispersed (PEOG112 < VPOG112 < PPOG112 < PCOG235 < CUOG235).
Percent of Yield, Drug Loading, and Entrapment Efficiency
Single Drug
The percent of yield, drug loading, and entrapment efficiency values of drug–ovalbumin–gelatin at various compositions are shown in Table IV. The percent of yield, drug loading, and entrapment efficiency values of PEOG112, VPOG112, PPOG112, PCOG235, and CUOG134 were higher than PEOG235, VPOG235, PPOG235, PCOG112, and CUOG123. It can be observed that the percent of yield, drug loading, and entrapment efficiency for soluble drugs (PE, VP, and PP) decreased but for sparingly soluble (PC) and non-soluble (CUO) drugs increased, when the amount of ovalbumin was increased. The difference in values of percent yield, drug loading, and entrapment efficiency was found to be significant statistically except for CUOG.
Table IV.
Yield, Drug Loading, and Entrapment Efficiency of Formulations
| Formulations | Yield (%) | Drug loading (%) | Entrapment efficiency (%) |
|---|---|---|---|
| PEOG235 (A) | 84.36 ± 1.08 | 11.91 ± 0.77 | 50.22 ± 2.82 |
| VPOG235 (B) | 82.11 ± 1.29 | 10.15 ± 0.97 | 41.72 ± 4.60 |
| PPOG235 (C) | 83.22 ± 1.25 | 12.54 ± 0.81 | 52.22 ± 4.12 |
| PCOG235 (D) | 90.10 ± 1.70 | 19.83 ± 0.87 | 89.32 ± 3.67 |
| CUOG134 (F) | 89.65 ± 1.17 | 13.77 ± 0.31 | 98.73 ± 1.06 |
| PEOG112 (a) | 94.42 ± 1.20 | 23.96 ± 1.63 | 90.56 ± 7.23 |
| VPOG112 (b) | 89.85 ± 1.09 | 23.84 ± 0.84 | 85.71 ± 4.02 |
| PPOG112 (c) | 89.77 ± 1.13 | 23.00 ± 1.53 | 82.65 ± 6.48 |
| PCOG112 (d) | 81.86 ± 1.65 | 16.29 ± 0.74 | 53.38 ± 3.45 |
| CUOG123 (e) | 86.30 ± 2.58 | 13.63 ± 0.58 | 70.66 ± 3.31 |
| Independent samples t test | |||
| A and a | P < 0.05 | P < 0.01 | P < 0.01 |
| B and b | P < 0.05 | P < 0.01 | P < 0.01 |
| C and c | P < 0.001 | P < 0.01 | P < 0.01 |
| D and d | P < 0.05 | P < 0.05 | P < 0.01 |
| E and e | P > 0.05 | P > 0.05 | P < 0.01 |
Mean ± SD (N = 3)
It can be seen from Table IV that the encapsulation efficiency of water-soluble drugs (PE, VP, and PP) at drug–ovalbumin–gelatin ratio of 1:1:2 were more than 80%. The formation of drug–ovalbumin aggregates circumvented the partitioning effect of water-soluble drugs, allowing encapsulation by gelatin leading to the successful formation of microparticles. An increase in the amount of ovalbumin is expected to increase the size of drug–ovalbumin aggregates, which compromises the entrapment efficiency.
Drug Mixtures
The encapsulation of equal amount of PC and PE mixtures showed that the PC and PE composition in paracetamol–pseudoephedrine–gelatin (PCPEG) and paracetamol–pseudoephedrine–ovalbumin–gelatin (PCPEOG235) particles were significantly different. The composition of PC and PE remained almost unchanged in PCPEOG235 particles, but not in PCPEG particles, which was attributed to the partitioning effect of PE and PC at different extent from the gelatin phase into the aqueous phase (Table V). Hence, it is demonstrated that the formation of drug–ovalbumin aggregates could circumvent the partitioning effect of drug mixtures of different solubility.
Table V.
Effect of Microencapsulation on the Composition of Paracetamol and Pseudoephedrine HCl
| Formulations | Composition (%) | ||
|---|---|---|---|
| PC | PE | Total | |
| PCPE (A) | 50.10 ± 0.06 | 50.17 ± 0.08 | 100.27 ± 0.02 |
| PCPEG (B) | 19.46 ± 3.70 | 3.20 ± 2.03 | 22.66 ± 2.04 |
| PCPEOG235 (C) | 45.37 ± 1.68 | 42.86 ± 1.59 | 88.23 ± 3.01 |
| Statistical analysis | |||
| F | 148.709 | 864.225 | 1,189.097 |
| Significance | P < 0.001 | P < 0.001 | P < 0.001 |
| Tukey’s HSD | |||
| A and B | P < 0.001 | P < 0.001 | P < 0.001 |
| A and C | P > 0.05 | P < 0.01 | P < 0.01 |
| B and C | P < 0.001 | P < 0.001 | P < 0.001 |
Mean ± SD (N = 3)
The scanning electron micrographs of PCPEG and PCPEOG235 particles are shown in Fig. 5. PCPEG (Fig. 5a) appeared to be discrete spherical particles with drug crystals adsorbed on the surface. On the other hand, PCPEOG235 (Fig. 5b) particles were spherical in shape with smooth surface. The presence of drug crystals adsorbed on the surface of PCPEG particles could be attributed to the partitioning effect of drugs.
Fig. 5.
Scanning electron micrographs of a PCPEG (magnification, 100×) and b PCPEOG235 (magnification, 250×)
In vitro Drug Release Study
In vitro Release Profiles of CU
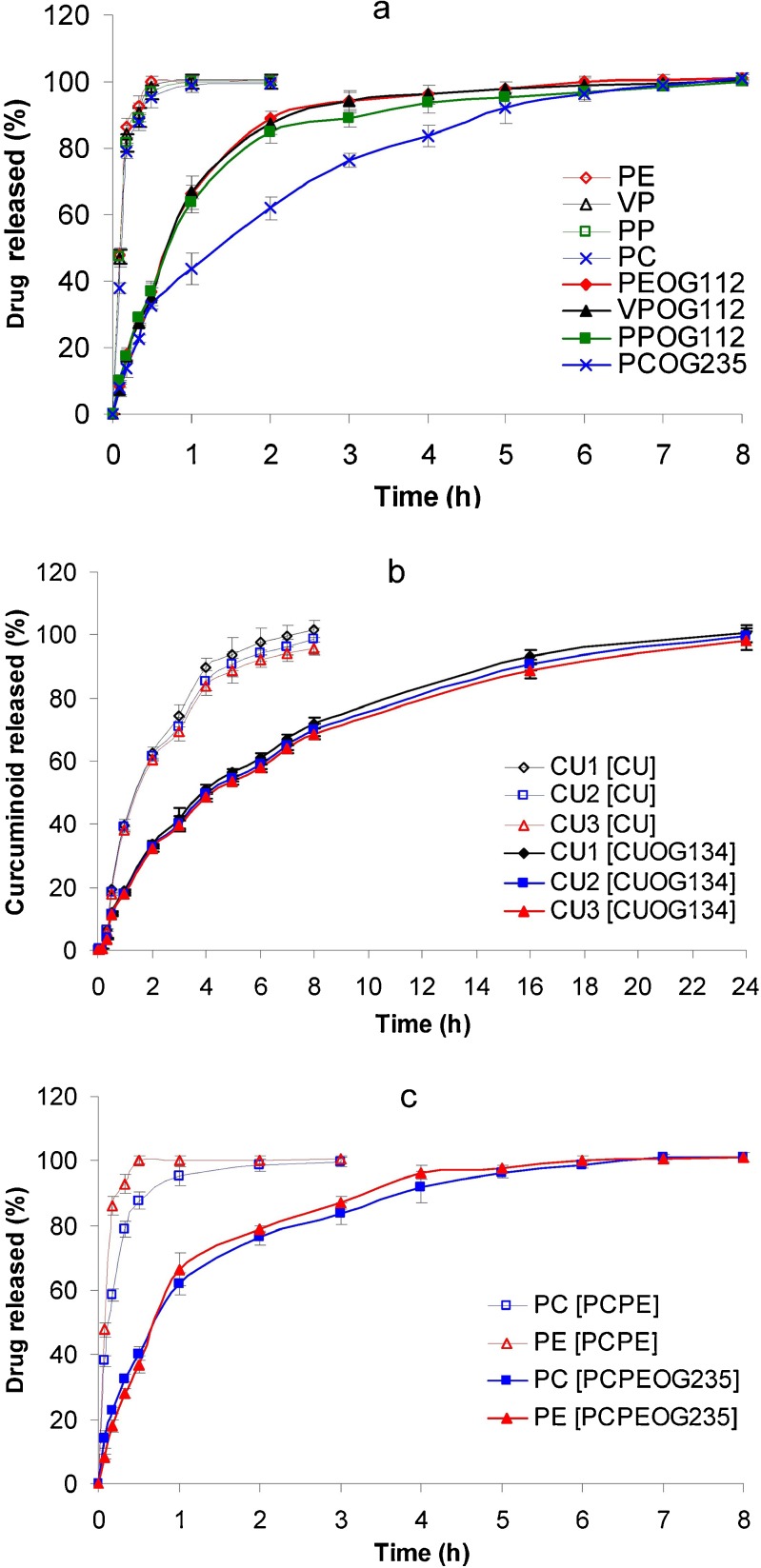
As shown in Fig. 6, the release of curcuminoid–ovalbumin–gelatin particles (CU1OG134, CU2OG134, and CU3OG134), exhibited sustained release profiles different from their corresponding CU powders (CU1, CU2, and CU3). CU1, CU2, and CU3 were completely released at 8 h while the release of curcuminoid–ovalbumin–gelatin particles was complete at 24 h.
Fig. 6.
The release profiles of powder and encapsulated particles of a PE, VP, PP, and PC and PEOG112, VPOG112, PPOG112, and PCOG235; b CU and CUOG134; and c PCPE and PCPEOG235. Mean ± SD (N = 3)
Table VI shows the mean T50% values of CU powders (CU1, CU2, and CU3) and curcuminoid–ovalbumin–gelatin particles (CU1OG134, CU2OG134, and CU3OG134). The mean T50% values of CU1, CU2, and CU3 were 86.90 ± 4.19, 89.18 ± 1.51, and 92.04 ± 1.47 min, respectively.
Table VI.
The Mean T 50% Values of Drugs Compared with Drug–Ovalbumin–Gelatin Microcapsules
| Drug | T 50% (min) | Independent samples t test | |
|---|---|---|---|
| Powder | Microcapsules | ||
| PE (A) | 5.29 ± 0.27 | 43.40 ± 1.15 | P < 0.001 |
| VP (B) | 5.39 ± 0.36 | 44.01 ± 2.32 | P < 0.01 |
| PP (C) | 5.36 ± 0.31 | 44.76 ± 2.01 | P < 0.01 |
| PC (D) | 6.46 ± 0.22 | 79.32 ± 14.82 | P < 0.05 |
| Statistical analysis | |||
| F | 10.838 | 16.204 | |
| Significance | P < 0.01 | P < 0.01 | |
| Tukey’s HSD | |||
| D > A, B, and C | P < 0.01 | P < 0.01 | |
| CU1 (A) | 86.90 ± 4.19 | 231.56 ± 12.45 | P < 0.01 |
| CU2 (B) | 89.18 ± 1.51 | 246.45 ± 13.22 | P < 0.01 |
| CU3 (C) | 92.04 ± 1.47 | 257.03 ± 15.29 | P < 0.01 |
| Statistical analysis | |||
| F | 2.715 | 2.615 | |
| Significance | P > 0.05 | P > 0.05 | |
| PC | 8.62 ± 0.72 | 43.64 ± 3.73 | P < 0.01 |
| PE | 5.11 ± 0.29 | 42.40 ± 2.72 | P < 0.01 |
| Independent samples t test | P < 0.01 | P > 0.05 | |
Mean ± SD (N = 3)
CU1 curcumin 1, CU2 curcumin 2, CU3 curcumin 3
When analyzed statistically using independent samples t test, the T50% values of CU powder were significantly faster than the curcuminoid–ovalbumin–gelatin particles. On the other hand, the mean T50% values among CU1, CU2, and CU3 powders as well as encapsulated particles were not significantly different.
In vitro Release Profiles of Drug Mixtures
The release profiles of PC and PE, from paracetamol–pseudoephedrine mixture (PCPE) and paracetamol–pseudoephedrine–ovalbumin–gelatin particles (PCPEOG235) were determined to evaluate the consistency of release patterns. The results showed that PCPEOG235 exhibited sustained release profiles, where PC and PE were completely released from the encapsulated particles at 8 h. In contrast, PE was completely released at 0.5 h and PC at 3 h (Fig. 5c).
The independent samples t test showed that the release of PC and PE were significantly faster from powder than from the drug–ovalbumin–gelatin particles PCPEOG235 (P < 0.05). Similarly, there was a statistically significant difference in the T50% values between PC and PE powder. In contrast, no statistically significant difference in the T50% values between PC and PE from PCPEOG235 (P > 0.05) (Table VI).
CONCLUSIONS
Ovalbumin formed aggregates with water-soluble drugs, circumventing the partitioning of drug from the gelatin phase to aqueous phase in coacervation-phase separation method, increasing the entrapment efficiency of water-soluble drugs. Moreover, gelatin coating of drug–ovalbumin aggregates could sustain the drug release. Ovalbumin demonstrated an important role in gelatin coacervation-phase separation method for encapsulation of water-soluble drugs.
Acknowledgments
Fellowship from Institute of Postgraduate Studies (IPS) and funding RU-grant (1001/815001) from Universiti Sains Malaysia (USM) are gratefully acknowledged.
Contributor Information
Hesham Abdul Aziz, Email: heshamaziz424@yahoo.co.uk.
Yvonne Tze Fung Tan, Email: yvonne@usm.my.
Kok Khiang Peh, Phone: +60-4-6533888, FAX: +60-4-6570017, Email: kkpeh@usm.my, Email: kkpehken@gmail.com.
References
- 1.Benoit JP, Marchais H, Rolland H, Velde VV. Biodegradable microspheres: advances in production technology. In: Benita S, editor. Microencapsulation methods and industrial applications. New York: Marcel Dekker; 1996. pp. 36–62. [Google Scholar]
- 2.Cruaud O, Benita S, Benoit JP. The characterization and release kinetics evaluation of baclofen microspheres designed for intrathecal injection. Int J Pharm. 1999;177(2):247–57. doi: 10.1016/S0378-5173(98)00350-0. [DOI] [PubMed] [Google Scholar]
- 3.Freytag T, Dashevsky A, Tillman L, Hardee GE, Bodmeier R. Improvement of the encapsulation efficiency of oligonucleotide-containing biodegradable microspheres. J Control Release. 2000;69(1):197–207. doi: 10.1016/S0168-3659(00)00299-6. [DOI] [PubMed] [Google Scholar]
- 4.Hombreiro Pérez M, Zinutti C, Lamprecht A, Ubrich N, Astier A, Hoffman M, et al. The preparation and evaluation of poly([epsilon]-caprolactone) microparticles containing both a lipophilic and a hydrophilic drug. J Control Release. 2000;65(3):429–38. doi: 10.1016/S0168-3659(99)00253-9. [DOI] [PubMed] [Google Scholar]
- 5.Passerini N, Perissutti B, Albertini B, Voinovich D, Moneghini M, Rodriguez L. Controlled release of verapamil hydrochloride from waxy microparticles prepared by spray congealing. J Control Release. 2003;88(2):263–75. doi: 10.1016/S0168-3659(03)00009-9. [DOI] [PubMed] [Google Scholar]
- 6.Ubrich N, Bouillot P, Pellerin C, Hoffman M, Maincent P. Preparation and characterization of propranolol hydrochloride nanoparticles: a comparative study. J Control Release. 2004;97(2):291–300. doi: 10.1016/j.jconrel.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Meng FT, Ma GH, Qiu W, Su ZG. W/O/W double emulsion technique using ethyl acetate as organic solvent: effects of its diffusion rate on the characteristics of microparticles. J Control Release. 2003;91(3):407–16. doi: 10.1016/S0168-3659(03)00273-6. [DOI] [PubMed] [Google Scholar]
- 8.Hasan AS, Socha M, Lamprecht A, Ghazouani FE, Sapin A, Hoffman M, et al. Effect of the microencapsulation of nanoparticles on the reduction of burst release. Int J Pharm. 2007;344(1–2):53–61. doi: 10.1016/j.ijpharm.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 9.Cohen-Sela E, Chorny M, Koroukhov N, Danenberg HD, Golomb G. A new double emulsion solvent diffusion technique for encapsulating hydrophilic molecules in PLGA nanoparticles. J Control Release. 2009;133(2):90–5. doi: 10.1016/j.jconrel.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 10.Puri S, Kallinteri P, Higgins S, Hutcheon GA, Garnett MC. Drug incorporation and release of water soluble drugs from novel functionalised poly(glycerol adipate) nanoparticles. J Control Release. 2008;125(1):59–67. doi: 10.1016/j.jconrel.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Yeo Y, Basaran OA, Park K. A new process for making reservoir-type microcapsules using ink-jet technology and interfacial phase separation. J Control Release. 2003;93(2):161–73. doi: 10.1016/j.jconrel.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Lecomte F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. Polymer blends used for the aqueous coating of solid dosage forms: importance of the type of plasticizer. J Control Release. 2004;99(1):1–13. doi: 10.1016/j.jconrel.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Nii T, Ishii F. Encapsulation efficiency of water-soluble and insoluble drugs in liposomes prepared by the microencapsulation vesicle method. Int J Pharm. 2005;298(1):198–205. doi: 10.1016/j.ijpharm.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Kim MS, Kim JS, Park HJ, Woo JS, Lee BC, et al. Controlled delivery of a hydrophilic drug from a biodegradable microsphere system by supercritical anti-solvent precipitation technique. J Microencapsul. 2006;23:741–9. doi: 10.1080/09687860600945552. [DOI] [PubMed] [Google Scholar]
- 15.Qi M, Li X, Yang Y, Zhou S. Electrospun fibers of acid-labile biodegradable polymers containing ortho ester groups for controlled release of paracetamol. Eur J Pharm Biopharm. 2008;70(2):445–52. doi: 10.1016/j.ejpb.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Shi G, Rao L, Yu H, Xiang H, Pen G, Long S, et al. Yeast-cell-based microencapsulation of chlorogenic acid as a water-soluble antioxidant. J Food Eng. 2007;80(4):1060–7. doi: 10.1016/j.jfoodeng.2006.06.038. [DOI] [Google Scholar]
- 17.Jin K-M, Kim Y-H. Injectable, thermo-reversible and complex coacervate combination gels for protein drug delivery. J Control Release. 2008;127(3):249–56. doi: 10.1016/j.jconrel.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Bae SE, Son JS, Park K, Han DK. Fabrication of covered porous PLGA microspheres using hydrogen peroxide for controlled drug delivery and regenerative medicine. J Control Release. 2009;133(1):37–43. doi: 10.1016/j.jconrel.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Aziz HA, Peh KK, Tan YTF. Solubility of core materials in aqueous polymeric solution effect on microencapsulation of curcumin. Drug Dev Ind Pharm. 2007;33(11):1263–72. doi: 10.1080/03639040701483967. [DOI] [PubMed] [Google Scholar]
- 20.Aziz HHHA. Microencapsulation of curcumin and turmeric oil extracted from turmeric rhizome and application in cream preparation. Pulau Pinang: Universiti Sains Malaysia; 2006. [Google Scholar]
- 21.Chang R-K, Robinson JR. Sustained drug release from tablets and particles throuph coating. In: Lieberman HA, Lachman L, Schwartz JB, editors. Pharmaceutical dosage forms-tablets. 2. Iowa: Informa Health Care; 1990. pp. 199–287. [Google Scholar]
- 22.Deasy PB. Coacervation-phase separation procedures using aqueous vehicles. In: Swarbrick J, editor. Drugs and pharmaceutical sciences: microencapsulation and related drug processes. New York: Marcel Dekker; 1984. pp. 61–95. [Google Scholar]
- 23.Awadé AC, Efstathiou T. Comparison of three liquid chromatographic methods for egg-white protein analysis. J Chromatogr B: Biomed Sci Appl. 1999;723(1–2):69–74. doi: 10.1016/S0378-4347(98)00538-6. [DOI] [PubMed] [Google Scholar]
- 24.Chang Y-K, Chang I-P. Method development for direct recovery of lysozyme from highly crude chicken egg white by stirred fluidized bed technique. Biochem Eng J. 2006;30(1):63–75. doi: 10.1016/j.bej.2006.02.006. [DOI] [Google Scholar]
- 25.Datta D, Bhattacharjee S, Nath A, Das R, Bhattacharjee C, Datta S. Separation of ovalbumin from chicken egg white using two-stage ultrafiltration technique. Sep Purif Technol. 2009;66(2):353–61. doi: 10.1016/j.seppur.2008.12.016. [DOI] [Google Scholar]
- 26.Jerez A, Partal P, Martínez I, Gallegos C, Guerrero A. Egg white-based bioplastics developed by thermomechanical processing. J Food Eng. 2007;82(4):608–17. doi: 10.1016/j.jfoodeng.2007.03.020. [DOI] [Google Scholar]
- 27.Van der Plancken I, Van Loey A, Hendrickx ME. Combined effect of high pressure and temperature on selected properties of egg white proteins. Innovat Food Sci Emerg Tech. 2005;6(1):11–20. doi: 10.1016/j.ifset.2004.10.002. [DOI] [Google Scholar]
- 28.Brophy MR, Deasy PB. Egg albumin microspheres containing sulphamethizole. J Microencapsul. 1984;1(2):157–68. doi: 10.3109/02652048409038519. [DOI] [PubMed] [Google Scholar]
- 29.Ishizaka T, Endo K, Koishi M. Preparation of egg albumin microcapsules and microspheres. J Pharm Sci. 1981;70(4):358–63. doi: 10.1002/jps.2600700404. [DOI] [PubMed] [Google Scholar]
- 30.Ishizaka T, Koishi M. In vitro drug release from egg albumin microcapsules. J Pharm Sci. 1983;72(9):1057–61. doi: 10.1002/jps.2600720922. [DOI] [PubMed] [Google Scholar]
- 31.Jun HW, Lai JW. Preparation and in vitro dissolution tests of egg albumin microcapsules of nitrofurantoin. Int J Pharm. 1983;16(1):65–77. doi: 10.1016/0378-5173(83)90129-1. [DOI] [Google Scholar]
- 32.Torrado JJ, Illum L, Cadorniga R, Davis SS. Egg albumin microspheres containing paracetamol for oral administration. I. In vitro characterization. J Microencapsul. 1990;7(4):463–70. doi: 10.3109/02652049009040468. [DOI] [PubMed] [Google Scholar]
- 33.Blanco-Príeto MJ, Fattal E, Gulik A, Dedieu JC, Roques BP, Couvreur P. Characterization and morphological analysis of a cholecystokinin derivative peptide-loaded poly(lactide-co-glycolide) microspheres prepared by a water-in-oil-in-water emulsion solvent evaporation method. J Control Release. 1997;43(1):81–7. doi: 10.1016/S0168-3659(96)01474-5. [DOI] [Google Scholar]
- 34.Hu Y, Jiang X, Ding Y, Zhang L, Yang C, Zhang J, et al. Preparation and drug release behaviors of nimodipine-loaded poly(caprolactone)-poly(ethylene oxide)-polylactide amphiphilic copolymer nanoparticles. Biomaterials. 2003;24(13):2395–404. doi: 10.1016/S0142-9612(03)00021-8. [DOI] [PubMed] [Google Scholar]