Abstract
The aim of this study was to develop a taste-masked oral disintegrating film (ODF) containing donepezil, with fast disintegration time and suitable mechanical strength, for the treatment of Alzheimer’s disease. Hydroxypropyl methylcellulose, corn starch, polyethylene glycol, lactose monohydrate and crosspovidone served as the hydrophilic polymeric bases of the ODF. The uniformity, in vitro disintegration time, drug release and the folding endurance of the ODF were examined. The in vitro results showed that 80% of donepezil hydrochloride was released within 5 minutes with mean disintegration time of 44 seconds. The result of the film flexibility test showed that the number of folding time to crack the film was 40 times, an indication of sufficient mechanical property for patient use. A single-dose, fasting, four-period, eight-treatment, double-blind study involving 16 healthy adult volunteers was performed to evaluate the in situ disintegration time and palatability of ODF. Five parameters, namely taste, aftertaste, mouthfeel, ease of handling and acceptance were evaluated. The mean in situ disintegration time of ODF was 49 seconds. ODF containing 7 mg of sucralose were more superior than saccharin and aspartame in terms of taste, aftertaste, mouthfeel and acceptance. Furthermore, the ODF was stable for at least 6 months when stored at 40°C and 75% relative humidity.
KEY WORDS: Alzheimer disease, donepezil HCl, oral disintegrating film, palatability
INTRODUCTION
Alzheimer disease is the most common cause of dementia, accounting for more than 60% of cases of late-life cognitive dysfunction (1). In the treatment algorithm of Alzheimer disease, donepezil is prescribed to treat mild to moderate Alzheimer disease. Donepezil hydrochloride is a reversible inhibitor of the enzyme acetylcholinesterase, which is postulated to exert its therapeutic effect by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by acetylcholinesterase. The structure of donepezil hydrochloride is shown in Fig. 1. Though effective as a drug, donepezil is very bitter in taste (2).
Fig. 1.
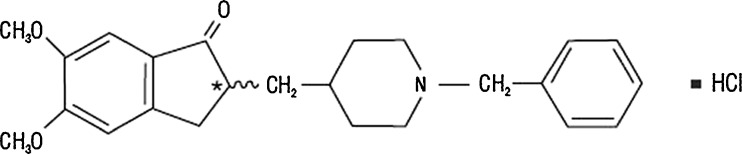
Molecular structure of donepezil hydrochloride
Many geriatric patients find it difficult to swallow solid dosage forms such as tablets or capsules (3). Therefore, ease of administration of dosage forms is of paramount importance, especially amongst certain Alzheimer’s disease patients who tend to be even less cooperative (4). In recent years, various novel drug delivery systems have been developed to improve patient compliance.
Orally disintegrating drug delivery systems were first developed in the late 1970 as an alternative to tablets and capsules for geriatric patients, who had difficulties in swallowing conventional solid dosage forms (5). Orally disintegrating tablet (ODT), an example of the oral disintegrating drug delivery systems, is a solid single unit that is placed in the mouth, and is allowed to disperse or dissolve in the saliva before swallowing (6). In most cases, the disintegrated materials are insoluble and remain in the buccal cavity until swallowed. On the other hand, oral disintegrating film (ODF) is a thin film prepared using hydrophilic polymers, which dissolve rapidly on the tongue or in the buccal cavity (7). The superiorities of ODF include larger surface area for rapid disintegrating, accuracy in the administered dose and consumer-friendly due to its ease of swallowing property (8). Nevertheless, developing a novel oral disintegrating drug delivery system is a challenging task. Among other factors, palatability (taste, smell, texture and aftertaste) of the formulation is one of the important factors ensuring patient compliance to the therapeutic regimen. On the other hand, disintegration time, mechanical strength and stability of the drug in the formulation are other important factors essential for the formulator to take into consideration (9). Hitherto, there are only a handful of published works on ODF (10–13), and taste-masked oral disintegrating system containing donepezil is only available in tablet form (14).
From the patient point of view, some marketed ODT products are too fragile and friable. Compared to ODT, ODF is more robust and offers ease of administration and improved compliance (7). Many geriatric patients find it difficult to swallow solid dosage forms such as tablets or capsules. One of the major problems that health care professionals face in treating geriatric patients is compliance. Difficulty in swallowing tablets or capsules has been identified as one of the contributing factors to geriatric patient compliance. Although ODT was designed for fast disintegration in the mouth, the fear of taking solid tablets and the risk of choking for certain patient populations still exist (15). The use of ODF can resolve the problem of swallowing and hence the non-compliance. Geriatric patients usually take more than one medication per day and most of the medications are solid dosage forms. Therefore, ease of administration of dosage forms is of paramount importance, especially among patients suffering from schizophrenia, bipolar disorder, Parkinson’s disease and Alzheimer disease. With this innovative dosage form, it can help to increase patient compliance. Clinical research findings suggested that patients preferred orally dissolving dosage form than conventional oral solid dosage forms (16–19). From the clinical point of view, this novel dosage form has great potential to solve non-compliance issue. ODF is a patient-friendly dosage form with a few added advantages over other dosage forms: (1) The dosage form can be taken without water. (2) Patient is not required to swallow the dosage form because it dissolves in the oral cavity. (3) It is more stable compared to liquid dosage form. (4) The absorption of the drug is more significant as the dosage form dissolves in buccal cavity and the absorption takes place starting from buccal cavity to intestine.
The aim of this study was to develop a taste-masked ODF containing donepezil with fast disintegration time and suitable mechanical strength for the treatment of Alzheimer disease.
MATERIALS AND METHODS
Materials
Donepezil hydrochloride was a gift from Ind-Swift Laboratory Limited (India). Crospovidone was purchased from ISP Technologies INC, (USA). Microcrystalline cellulose and corn starch were obtained from Intermed Sdn. Bhd. (Malaysia). Hydroxypropyl methylcellulose and polyethylene glycol were purchased from Sigma Chemical Co. (USA). Lactose monohydrate (Starlac®) was ordered from Meggle Group (Germany). Sweetening agents (saccharin sodium, aspartame, sucralose) and pineapple flavour were purchased from Nutrisweet & Food Specialities Sdn. Bhd. (Malaysia).
Preparation of Oral Disintegrating Film
Hydroxypropyl methylcellulose, corn starch, polyethylene glycol, microcrystalline cellulose, lactose monohydrate and Crospovidone, were sieved through a no. 40 mesh screen (diameter 0.5 mm) and mixed uniformly using geometrical dilution method. The powder mixture was dispersed in 30 g of distilled water heated at 60°C and homogenised at 2,000 rpm for 30 min (IKA Works, INC. USA). The sweetener, flavouring agent and donepezil hydrochloride, were separately dissolved in 10 g of distilled water and added to the mixture prepared earlier. The weight was adjusted to 50 g with distilled water and homogenization was continued for another 30 min. The final mixture of 1 g was weighed and transferred into 20 × 20 × 8 mm flat bottom polypropylene weighing boat each. The weighing boats were dried in an oven at 60°C for 2 h. The film was removed from the weighing boat and stored in a desiccator. The formulations containing different amount of sweetening agents are presented in Table I.
Table I.
Various Formulations Containing Different Amount of Sweetening Agents
| Ingredients | Formulation code (mg/film) | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| Donepezil | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Aspartame | – | 5 | 7 | – | – | – | – | – |
| Sucralose | – | – | – | 5 | 7 | – | – | – |
| Saccharine sodium | – | – | – | – | – | 5 | 7 | – |
| Pineapple flavour | – | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Film base | 140 | 133 | 131 | 133 | 131 | 133 | 131 | 138 |
Uniformity of Thickness
The thickness of each ODF formulation (20 × 20 mm) was measured using a micrometre (Mitutoyo, Japan) at the four corners and centre. Six samples of each ODF formulation were measured.
Tensile Strength Measurement
The tensile strength of the ODF was measured using a texture analyser (TX-XT2 texture analyser, North America). The samples of ODF at dimension of 20 × 20 mm, were held vertically between two clamps of 1 cm apart. The ODF was pulled by the clamp at a rate of 100 mm/min and contact force of 0.05 N. The tensile strength was defined as the maximum load force to break the ODF and calculated by dividing the applied load at rupture with the cross-sectional area of the film (21). For each formulation, six samples were measured.
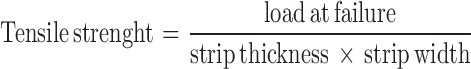 |
Film Flexibility Determination
The ODF (20 × 20 mm) was repeatedly folded at the same place. The total number of foldings made before the film cracked was denoted as film flexibility value. The ODF was examined for cracks over the area of the bend under a strong light. For each formulation, six samples were examined.
Uniformity of Drug Content
The drug content was quantified using a validated high-performance liquid chromatographic (HPLC) method (Shimadzu VP series Kyoto, Japan) reported by Pappa et al. (22) with modification. The HPLC system was comprised of a pump (LC-10AT vp/FCV-10AL) equipped with an auto-injector (SIL-10AD) and a UV spectrophotometer connected to computer software (Class VP). A C18 (250 × 4.6 mm ID, 5 μm) analytical column (Agilent Eclipse Plus, USA) fitted with a guard column (Zorbax Eclipse Plus, Agilent, USA) packed with replaceable C-18 (12.5 × 4.6 mm ID, 5 μm) cartridge (Agilent, USA) was used for the chromatographic separation. The mobile phase consisted of a mixture of 0.01 M potassium dihydrogen phosphate buffer, methanol and acetonitrile (5:3:2, v/v) adjusted to pH 2.7 with 70% phosphoric acid. The analysis was run at a flow rate of 1.0 mL/min and the detection wavelength was 268 nm. The standard calibration curve of donepezil HCl was linear over a concentration range of 30–10,000 ng/mL. The mean accuracy values were 99.37 ± 0.37% and the mean precision values were 91.12 ± 0.23%. The limit of quantification was 30 ng/mL at a signal to noise ratio of 10:1. The limit of detection was 15 ng/mL at a signal to noise ratio of 3:1.
A piece of ODF film (20 × 20 mm) was dissolved in mobile phase by sonication. After appropriate dilution, 20 μL of the sample was injected into the HPLC and the amount of drug was determined. Six ODF (20 × 20 mm) of each formulation were examined and the acceptance value (AV) was calculated using the following equation:
 |
where M is the label claim (100%), X is the measured content of donepezil HCl, k is the acceptability constant (2.2) and s is the standard deviation. The drug content in the preparation should be within the range of 90% to 110% (USP31, 2010).
In Vitro Disintegration Time Study
The in vitro disintegration time of the ODF formulations (20 × 20 mm) was determined using a disintegration tester (Pharmatest, Germany) with distilled water at 37.0 ± 0.5°C. The disintegration time was defined as the time taken for ODF to completely disintegrate with no solid residue remaining on the screen. A total of six ODF samples were run for each formulation.
In Vitro Drug Dissolution Study
The in vitro drug dissolution study was carried out in 900 mL of 0.1 M HCl at 37.0 ± 0.5°C, using USP paddle method at a stirring speed of 50 rpm. At preset time intervals of 1, 3, 5, 10, 20 and 30 min, 3 mL of samples were withdrawn and immediately replaced with an equal volume of fresh dissolution medium. The samples were filtered through a 0.45-μm membrane filter and the amount of drug released was determined using HPLC method. Six ODF samples (20 × 20 mm) were analysed for each formulation. Six samples of Aricept® were analysed as reference product.
In Situ Disintegration Time and Palatability Studies
A total of 16 healthy adult volunteers (eight males and eight females) with a mean age of 22.5 years old (22–23 years old) participated in a single-dose, four-period, eight-treatment, double-blind study after providing written informed consent. Prior to the study, the volunteers were briefed on the nature, purpose, duration and risk of the study. The study protocol was approved by the Joint Ethics Committee of School of Pharmaceutical Sciences, USM and Hospital Lam Wah Ee on Clinical Studies.
The study was divided into 4 days, with two treatments and two phases on each day. There was a washout period of 2 h between the two phases on the same day. Prior to the study, the volunteers were required to gargle their mouth with 200 mL of distilled water. One ODF film (20 × 20 mm) was placed on the tongue of the volunteer. The volunteers were requested to record the disintegration time of the ODF and gave the score based on the parameters, namely taste, aftertaste, mouthfeel, ease of handling and acceptance as presented in Table II. The volunteers were told to spit out the test sample, followed by rinsing their mouths with 200 mL of distilled water. In each phase of the study, one ODF formulation was given to all the 16 volunteers. Another two ODF formulations were given to the volunteers the next day. The same procedure was repeated up to 4 days to complete the evaluation of all the eight formulations.
Table II.
Parameters and Score in Palatability Study
| Parameters | Score | ||||
|---|---|---|---|---|---|
| Taste | Aftertaste | Mouthfeel | Ease of handling | Acceptance | |
| Very bitter | Very bitter | Gritty and irritating | Very brittle | Very poor | 1 |
| Bitter | Bitter | Gritty | Brittle | Poor | 2 |
| Slightly bitter | Slightly bitter | Slightly gritty | Does not break | Acceptable | 3 |
| Slightly sweet | Slightly sweet | Smooth | Flexible and easy to handle | Good | 4 |
| Very sweet | Very sweet | Very smooth | Very easy to handle | Very good | 5 |
Stability Study
The ODF formulation (20 × 20 mm) was stored at 40°C (75%RH) for 6 months. The colour, weight and donepezil HCL content of the ODF formulation were examined.
Fourier Transform Infrared Spectroscopy
Fourier transform infrared (FTIR) spectroscopy (FTIR-Nexus, Thermo Nicolet, USA) was carried out to check the compatibility of the drug and excipients in the final formulation. The drug, excipients and ODF formulation were studied. The IR spectra of the samples were obtained using a KBr pellet that was prepared with hydraulic press after careful grinding of a small amount of each sample with KBr. The spectral width was 400–4,000 cm−1. Each spectrum was acquired by performing 32 scans.
Scanning Electron Microscope
Scanning electron microscope (SEM) images of the ODF surface were obtained using the scanning electron microscope (VE-7300, Keyence). The film was cut into smaller pieces and was mounted on a metal stub with double-sided adhesive tapes. Formulation A (without sweetener and flavour), formulation B (without sweetener) and best formulation determined in palatability study were scanned using SEM.
Statistical Analysis
The results were expressed as mean ± standard deviation (SD). The results obtained from in vitro dissolution study, uniformity test and stability study were analysed statistically using one-way analysis of variance. As for the palatability study, the results were analysed using Kruskal–Wallis test. When there was a statistically significant difference, Mann–Whitney test was performed. A statistically significant difference was considered at p < 0.05.
RESULTS AND DISCUSSION
Preparation of ODF
In this study, the formulation developed is simple, easy to prepare and economical. The ingredients are easily available, safe and widely used in the pharmaceutical industry. HPMC film was too brittle. PEG was then incorporated as plasticizer to enhance the flexibility of the film. Crospovidone was incorporated as superdisintegrant for fast disintegration of film when placed in the buccal cavity (20).
Uniformity of ODF
The result of content uniformity is presented in Table III. The donepezil hydrochloride content in all the eight formulations ranged from 92.13% to 97.92% of the theoretical concentration, with relative standard deviations ranged from 1.13% to 4.71%. There was no statistically significant difference (p > 0.05) in the donepezil hydrochloride content among the formulations. Thus, all the ODF met the criteria for the content uniformity test. Moreover, the acceptance value was found to be in the range of 6.13% to 13.35%, which was also within the 15% limit of the uniformity of dosage units for Japanese Pharmacopoeia15 (23).
Table III.
The Results of Content Uniformity
| Formulation | Mean (%) | SD (%) | Acceptance value (%) |
|---|---|---|---|
| A | 92.13 | 2.02 | 12.31 |
| B | 94.56 | 1.13 | 7.93 |
| C | 93.21 | 2.32 | 11.89 |
| D | 94.34 | 1.45 | 8.85 |
| E | 97.92 | 1.84 | 6.13 |
| F | 95.99 | 2.87 | 10.32 |
| G | 97.01 | 4.71 | 13.35 |
| H | 95.23 | 3.01 | 11.39 |
Mean ± SD, N = 6
Characterization of ODF
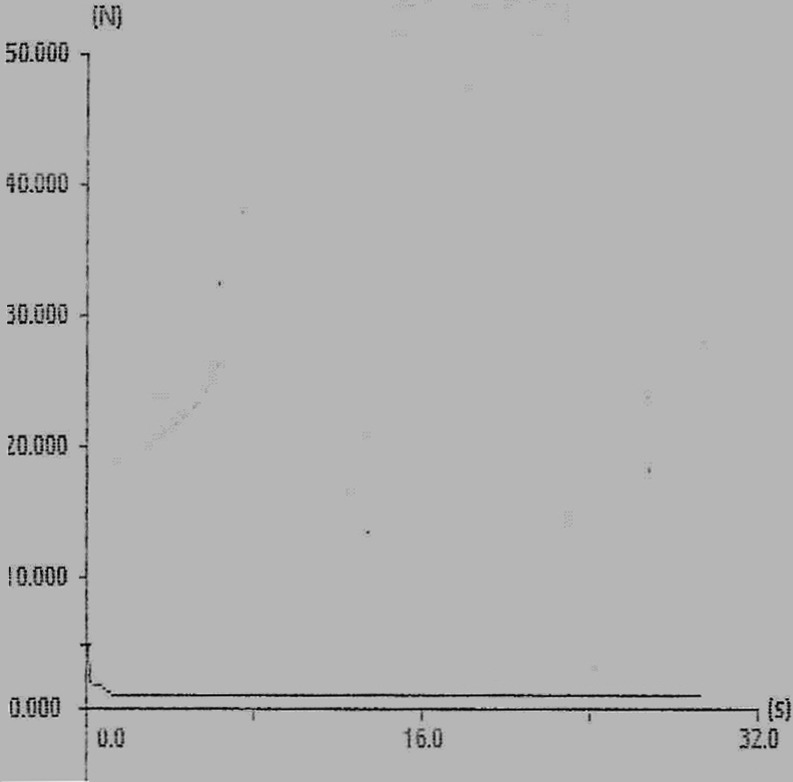
The results of the uniformity of thickness, film flexibility, tensile strength, in vitro disintegration time and in situ disintegration time are presented in Table IV. The force–time plot of formulation E (best formulation determined in the palatability study) is presented in Fig. 2.
Table IV.
The Results of Uniformity of Thickness, Tensile Strength, Folding Endurance, In Vitro Disintegration Time and In Situ Disintegration Time
| Characterization | Formulation code | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| Thickness (mm) | 0.349 ± 0.012 | 0.374 ± 0.025 | 0.356 ± 0.021 | 0.367 ± 0.014 | 0.35 ± 0.019 | 0.38 ± 0.01 | 0.33 ± 0.014 | 0.358 ± 0.018 |
| Tensile strength (N/cm2) | 106.45 ± 2.41 | 81.46 ± 3.87 | 135.06 ± 5.21 | 114.15 ± 4.93 | 85.40 ± 9.68 | 102.67 ± 7.23 | 135.8 ± 3.94 | 121.31 ± 2.73 |
| Folding endurance (times) | 39 ± 3 | 36 ± 2 | 40 ± 3 | 44 ± 4 | 38 ± 4 | 46 ± 4 | 44 ± 3 | 42 ± 3 |
| In vitro disintegration time (s) | 41 ± 3 | 42 ± 3 | 45 ± 3 | 47 ± 3 | 46 ± 4 | 39 ± 3 | 45 ± 4 | 46 ± 4 |
| In situ disintegration time (s) | 46 ± 3 | 49 ± 3 | 48 ± 3 | 52 ± 4 | 49 ± 3 | 51 ± 3 | 50 ± 3 | 47 ± 3 |
Mean ± SD, n = 6
Fig. 2.
Force–time plot of ODF formulation E
Uniformity of Thickness
The mean thickness of the ODF was 0.358 ± 0.018 mm (range, 0.33–0.38 mm). There was no statistically significant difference (p > 0.05) in thickness among the formulations.
Film Flexibility Determination
The results of the film flexibility study showed that the film cracked after an average of approximately 40 times of folding. The present method is modified from ASTM Bend Mandrel test (D 4338–97). In Bend Mandrel test, the film is bended over a mandrel and examined for cracks over the area of the bend in a strong light. The film is assumed as flexible if no crack is visible at a ×5 magnification (24). In the present study, the ODF did not show any signs of crack when folded 180° at the same place up to 40 times. Hence, the ODF could be termed as flexible.
In Vitro Disintegration Time
The mean in vitro disintegration time was 43.83 ± 2.79 s (range, 39–47 s). There was no statistically significant difference (p > 0.05) in the in vitro disintegration time among the eight formulations.
In Situ Disintegration Time
The mean in situ disintegration time was 49.13 ± 3.18 s (range, 46–52 s), which was in good agreement with the in situ disintegration time of 41.7 ± 3.4 s, reported by Yan et. al. (14) for Aricept ODT®. There was no statistically significant difference (p > 0.05) in the in situ disintegration time results among the eight formulations, indicating that the sweeteners had no significant effect on the in situ disintegration time of the ODF formulations. The images of ODF disintegration process on tongue are shown in Fig. 3. Since ODF formulations disintegrated in less than 1 min upon contact with water, the formulations complied to the requirement of orodispersible tablet as stipulated by BP which stated that the orodispersible tablets are uncoated tablets intended to be placed in the mouth where they disperse rapidly before being swallowed. When run in disintegration tester, orodispersible tablets disintegrate within 3 min.
Fig. 3.
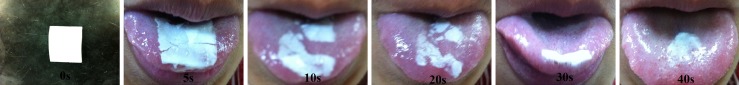
Disintegration of ODF on tongue
In Vitro Drug Dissolution Study
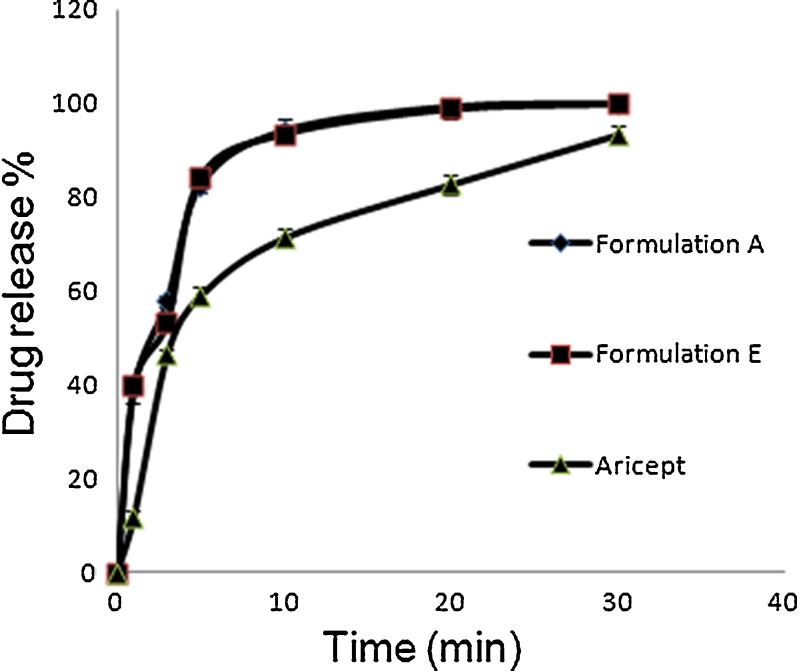
The drug dissolution profiles of ODF formulations A, E and Aricept® are presented in Fig. 4. The two ODF formulations dissolution profiles were closely similar. The addition of sweetener and flavouring agent in the ODF base did not significantly affect the release of donepezil. Chambin et al. (13) showed that the addition of microcrystalline cellulose into oral disintegrating tablet increased the dissolution rate. Shimoda et al. (11) showed that by incorporating more than 50% of microcrystalline cellulose in dexamethasone containing ODF, 90% of the drug was released within 5 min. In the present study, an addition of 40% of microcrystalline cellulose resulted in 80% of drug released in 5 min. On the other hand, Aricept ODT® released 80% of the drug within 20 min. Hence, ODF is far more superior in terms of dissolution rate when compared to conventional tablet.
Fig. 4.
Dissolution profile of ODF formulation A, E and Aricept
Palatability Evaluation
The compliance of Aricept ODT® was reported to be affected by its bitterness in taste (14). As such, development of a taste-masking formulation is highly favourable. The results of the palatability evaluation and statistical analysis results are presented in Table V. Formulation E containing 7 mg sucralose was slightly sweet in taste. Formulation E had the highest score and was significantly different from the other formulations. The effectiveness of sucralose in masking bitter taste can be explained by its powerful sweetness, which is 600–1,000 times sweeter than sucrose (25, 26). The taste of formulation H (contained only flavouring agent), was not significantly different when compared with formulation A (control). The presence of flavouring agent alone showed no taste-masking effect. Formulations B, C, F and G, containing aspartame and saccharin sodium were slightly bitter (score = 3). Aspartame is known to be 200 times (27, 28), while sodium saccharin is 300 to 500 times sweeter than sucrose (29). Nevertheless, at 5 and 7 mg, both sweeteners were insufficient to mask the bitter taste of donepezil HCl.
Table V.
Result of In Situ Palatability Study
| Palatability parameters | |||||
|---|---|---|---|---|---|
| Formulation code | Taste | Aftertaste | Mouthfeel | Ease of handling | Acceptance |
| A | 2.0 ± 0.1 | 2.0 ± 0.4 | 2.0 ± 0.1 | 4.0 ± 0.1 | 2.0 ± 0.1 |
| B | 3.0 ± 0.3 | 3.0 ± 0.5 | 3.0 ± 0.2 | 4.0 ± 0.1 | 3.0 ± 0.1 |
| C | 3.0 ± 0.5 | 3.0 ± 0.3 | 3.0 ± 0.4 | 4.0 ± 0.1 | 3.0 ± 0.1 |
| D | 3.0 ± 0.6 | 3.0 ± 0.3 | 3.0 ± 0.4 | 4.0 ± 0.2 | 3.0 ± 0.2 |
| E | 4.0 ± 0.4 | 4.0 ± 0.4 | 4.0 ± 0.4 | 4.0 ± 0.1 | 4.0 ± 0.2 |
| F | 3.0 ± 0.4 | 3.0 ± 0.6 | 3.0 ± 0.4 | 4.0 ± 0.1 | 3.0 ± 0.2 |
| G | 3.0 ± 0.1 | 3.0 ± 0.2 | 3.0 ± 0.3 | 4.0 ± 0.2 | 3.0 ± 0.2 |
| H | 2.0 ± 0.2 | 2.0 ± 0.2 | 3.0 ± 0.2 | 4.0 ± 0.2 | 3.0 ± 0.2 |
| Statistical analysis (Kruskal–Wallis test) | p < 0.05 | p < 0.05 | p < 0.05 | p > 0.05 | p < 0.05 |
| Post hoc test (Mann–Whitney test) | E and A (p < 0.05) | E and A (p < 0.05) | E and A (p < 0.05) | – | E and A (p < 0.05) |
| E and B (p < 0.05) | E and B (p < 0.05) | E and B (p < 0.05) | E and B (p < 0.05) | ||
| E and C (p < 0.05) | E and C (p < 0.05) | E and C (p < 0.05) | E and C (p < 0.05) | ||
| E and D (p < 0.05) | E and D (p < 0.05) | E and D (p < 0.05) | E and D (p < 0.05) | ||
| E and F (p < 0.05) | E and F (p < 0.05) | E and F (p < 0.05) | E and F (p < 0.05) | ||
| E and G (p < 0.05) | E and G (p < 0.05) | E and G (p < 0.05) | E and G (p < 0.05) | ||
| E and H (p < 0.05) | E and H (p < 0.05) | E and H (p < 0.05) | E and H (p < 0.05) | ||
Mean ± SD, n = 16
Aftertaste is another important factor to be considered in the development of ODF formulation. Formulations B, C, F and G containing aspartame and sodium saccharin, had slightly bitter aftertaste. Bitter aftertaste of preparations containing aspartame and sodium saccharin have been reported (28–31). In contrast, bitter aftertaste was not reported for sucralose (26). Formulation E which contained 7 mg of sucralose had a slightly sweet aftertaste (score = 4). There was a statistically significant difference in the aftertaste among the formulations.
In the present study, mouthfeel was defined as the ability of the formulation to cause a sense of irritation and/or grittiness when the ODF was placed on the tongue. Formulation E gave a good smooth mouthfeel (score = 4). The mouthfeel of this formulation was significantly different from those of the other formulations. The taste-masking effect of sucralose at 7 mg might contribute to the smooth mouthfeel.
All the eight formulations were noted to be flexible and easy to handle. This is because the film base of all the eight ODF formulations was comprised of the same ingredients, except the sweeteners and flavouring agent. The results show that incorporation of sweeteners and flavouring agent has negligible effect on the flexibility of the ODF.
In short, formulation E had the highest acceptance and the difference was significant compared to the other formulations.
Stability Study
The hygroscopic nature of ODT makes it unstable, which is a major disadvantage (32, 33). Special packaging is needed to protect the product, which increases the production cost (33–35). Development of a stable formulation would help to reduce the packaging cost. Formulation E was selected for the stability test. The appearance of the film after storage for 6 months remained unchanged. There was no statistically significant change observed in the weight of ODF. The average donepezil content of formulation E after 6-month storage was 9.51 ± 0.17 mg (range, 91.88–96.80%). Therefore, the drug was stable up to 6 months at 40°C (75%RH).
Fourier Transform Infrared Spectroscopy
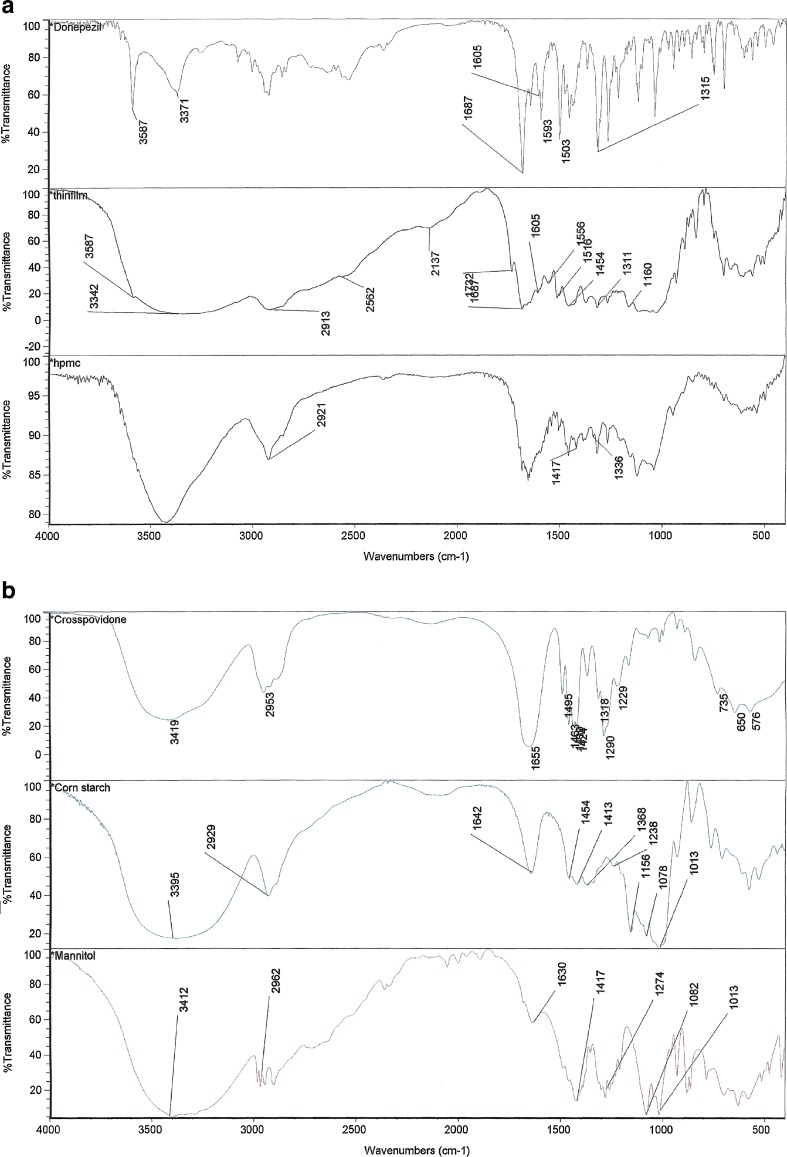
The FTIR spectra of the donepezil hydrochloride (pure drug), ODF formulation, hydroxypropyl methylcellulose, corn starch, microcrystalline cellulose and Crospovidone are presented in Fig. 5a, b. From the spectra of the donepezil hydrochloride, it was observed that the main functional groups of the compound are aromatic phenone and para-substituted aromatic hydrocarbon. The most intensive absorption band around 1,683 cm−1 in the spectra was attributed to the stretching vibrations of C=O group in the structure of donepezil hydrochloride (36). This sharp peak indicated the presence of aromatic phenone ring in the compound. Absorption band around 1,601 cm−1 indicated the presence of C=C stretching in the compound. The sharp absorption band at 1,315 cm−1 indicated the presence of C–N bond in the structure (37). From the spectrum of ODF formulation, it was observed that the intensive absorption bands were noted around 1,687 cm−1, 1,605 cm−1 and 1,311 cm−1 in the structure. All the functional groups in donepezil hydrochloride were maintained in the spectrum of ODF formulation. The results indicate that no chemical interaction occurred between donepezil hydrochloride and excipients in the ODF formulation.
Fig. 5.
a FTIR spectra of (1) donepezil hydrochloride, (2) ODF formulation E and (3) HPMC. b FTIR spectra of (1) Crospovidone, (2) corn starch and (3) mannitol
Scanning Electron Microscopy
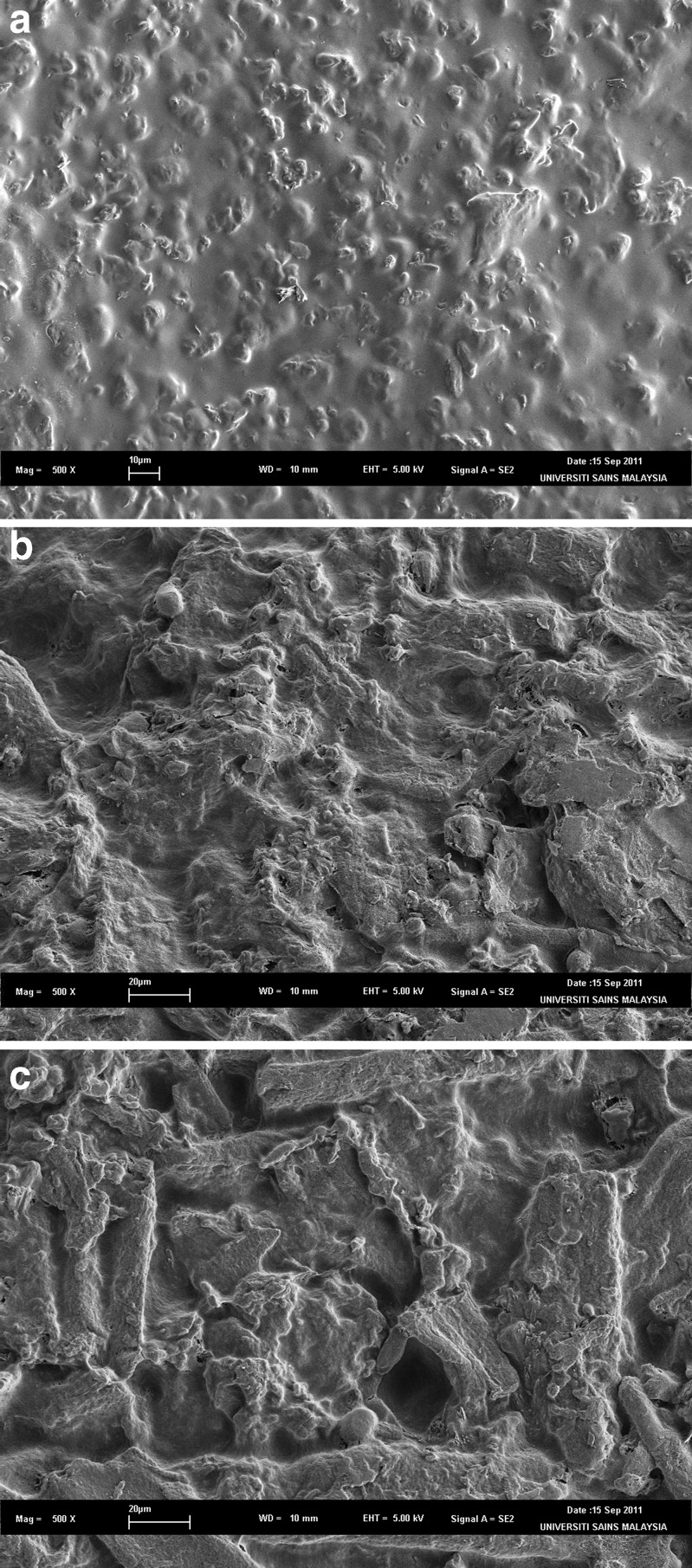
The scanning electron micrographs are presented in Fig. 6. It can be seen that the surface of ODF was more coarse and rough with the incorporation of flavour (Fig. 6b) when compared with ODF without sweetener and flavour (Fig. 6a). With incorporation of sweetener and flavour (Fig. 6c), the coarseness and roughness of ODF surface was more obvious.
Fig. 6.
SEM images of ODF
CONCLUSION
A flexible donepezil ODF formulation with fast disintegration time, acceptable palatability and stable over a period of 6 months was successfully developed. The findings suggest that doneprezil ODF has the potential as an alternative dosage form in treating Alzheimer’s disease.
ACKNOWLEDGEMENT
This author would like to thank Institute of Postgraduate Studies, Universiti Sains Malaysia for providing Fellowship.
REFERENCES
- 1.Joseph TD, Robert LT, Gary CY, Gary RM, Barbara GW, Michael LP. Pharmacotherapy: a pathophysiologic approach. 6. New York: McGraw Hill; 2005. pp. 2162–2187. [Google Scholar]
- 2.Martin RF. Effective pharmacologic management of Alzheimer disease. AJM. 2007;120:388–397. doi: 10.1016/j.amjmed.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 3.Hanawa T. Development of a new and kindly oral dosage form for elderly. JPST. 1997;13:251–258. [Google Scholar]
- 4.Yamamoto Y, Fujii M, Watanabe K, Tsukamoto M, Shibata Y, Kondoh M, et al. Effect of powder characteristics on oral tablet disintegration. Int J Pharm. 2009;365:116–120. doi: 10.1016/j.ijpharm.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Sunada H, Bi YX. Preparation, evaluation and optimization of rapidly disintegrating tablets. Powder Technol. 2002;122:188–198. doi: 10.1016/S0032-5910(01)00415-6. [DOI] [Google Scholar]
- 6.Abdelbary G, Eouani C, Prinderre P, Joachim J, Reynier Jp, Piccerelle Ph. Determination of the in-vitro disintegration profile of rapidly disintegrating tablets and correlation with oral disintegration. Int J Pharm. 2005;292:29–41. doi: 10.1016/j.ijpharm.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Dixit RP, Puthli SP. Oral strip technology: overview and future potential. J Control Release. 2009;139:94–107. doi: 10.1016/j.jconrel.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Goel H, Rai P, Rana V, Tiwary AK. Orally disintegrating systems: innovations in formulation and technology. Recent Patents on Drug Delivery and Formulation. 2008;2:258. doi: 10.2174/187221108786241660. [DOI] [PubMed] [Google Scholar]
- 9.Mishra R, Amin A. Formulation development of taste-masked rapidly dissolving films of cetirizine hydrochloride. Pharmtech. 2009;33(2):48–56. [Google Scholar]
- 10.Pillai R, Karatgi P. Orally disintegrating tablets: the path to improved patient compliance and enhanced life cycle management. Pharmtech Europe. 2009;21(5):37–41. [Google Scholar]
- 11.Shimoda H, Taniguchi K, Nishimura M, Matsuura K, Tsukioka T, Yamashita H, et al. Preparation of a fast dissolving oral thin film containing dexamethasone: a possible application to antiemesis during cancer chemotherapy. Eur J Pharm Sci. 2009;73:361–365. doi: 10.1016/j.ejpb.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura M, Matsuura K, Tsukioka T, Yamashita H, Inagaki N, Sugiyama T, et al. In vitro and in vivo characteristics of prochlorperazine oral disintegrating film. Int J Pharm. 2009;368:98–102. doi: 10.1016/j.ijpharm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Chambin O, Champion D, Debray C, Rochat-Gonthier MH, Le Meste M, Pourcelot Y. Effects of different cellulose derivatives on drug release mechanism studied at a preformulation stage. J Control Release. 2004;95:101–108. doi: 10.1016/j.jconrel.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Yan YD, Jong SW, Kang JH, Yong CS, Choi HG. Preparation and evaluation of taste-masked donepezil hydrochloride orally disintegrating tablets. Biol Pharm Bull. 2010;33(8):1364–1370. doi: 10.1248/bpb.33.1364. [DOI] [PubMed] [Google Scholar]
- 15.Borsadia SB, O'Halloran D, Osborne JL. Quick-dissolving films—a novel approach to drug delivery. Drug development and delivery. 2003;3(3). Available online at http://www.drugdeliverytech.com/ME2/dirmod.asp?sid=&nm=&type=Publishing&mod=Publications%3A%3AArticle&mid=8F3A7027421841978F18BE895F87F791&tier=4&id=1462E9E570724362AF256AB9CEC63126.
- 16.Koh N, Sakamoto S, Chino F. Improvement in medication compliance and glycemic control with voglibose oral disintegrating tablet. Tohoku J Exp Med. 2008;216(3):249–257. doi: 10.1620/tjem.216.249. [DOI] [PubMed] [Google Scholar]
- 17.Bruce JK, Angela LH, Liu H, Sara KW. Olanzapine orally disintegrating tablet in the treatment of acutely ill non-compliant patients with schizophrenia. IJNP. 2003;6:97–102. doi: 10.1017/S1461145703003389. [DOI] [PubMed] [Google Scholar]
- 18.Raviraj P, Pradeep K. Orally disintegrating tablets: the path to improve patient compliance and enhanced life cycle management. Pharmtech Europe. 2009;21:5.
- 19.Emilio MC, Vincente GG, Albert NS, Jose PF, Joan HN, Jordi GC, et al. A study on the subjective compliance and acceptance of oral lanzoprazole in traumatology. The ECOFT-TR Study. Clin Rheumatol. 2009;5(2):49–54. doi: 10.1016/j.reuma.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Gohel MC, Parikh RK, Brahmbhatt BK, Shah AR. Improving the tablet characteristics and dissolution profile of ibuprofen by using a novel coprocessed superdisintegrant: a technical note. AAPS PharmSciTech. 2007;8(1):13. doi: 10.1208/pt0801002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra R, Amin A. Manufacturing techniques of orally dissolving films. J Pharm Sci. 2011;35(1):70–73. [Google Scholar]
- 22.Pappa H, Farrú R, Vilanova PO, Palacios M, Pizzorno MT. A new HPLC method to determine donepezil hydrochloride in tablets. J Pharmaceut Biomed. 2002;27:177–182. doi: 10.1016/S0731-7085(01)00499-X. [DOI] [PubMed] [Google Scholar]
- 23.Japanese Pharmacopoeia 15. General tests, processes and apparatus. Tokyo, Japan: The Society of Japanese Pharmacopoeia. p. 107.
- 24.Cilurzo F, Cupone IE, Minghetti P, Selmin F, Montanari L. Fast dissolving films made of maltodextrins. Eur J Pharm Biopharm. 2008;70(3):895–900. doi: 10.1016/j.ejpb.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 25.Kuno Y, Kojima M, Andoand S, Nakagami H. Effect of preparation method on properties of orally disintegrating tablets made by phase transition. Int J Pharm. 2008;355(1–2):87–92. doi: 10.1016/j.ijpharm.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 26.Rowe RC, Sheskey PJ, Quinn ME. Handbook of pharmaceutical excipients. 6. London: Pharmaceutical Press; 2009. p. 702. [Google Scholar]
- 27.Doe J. 2010 Sucralose—Technological Justification. www.food.gov.uk/multimedia/pdfs/sucraconsult2.pdf. Accessed26 Oct 2010.
- 28.Rowe R, Sheskey PJ, Quinn ME. Handbook of pharmaceutical excipients. 6. London: Pharmaceutical Press; 2009. p. 48. [Google Scholar]
- 29.Bayarri S, Izquierdo L, Costell E. Sweetening power of aspartame in hydrocolloids gels: influence of texture. Food Hydrocolloids. 2007;21:1265–1274. doi: 10.1016/j.foodhyd.2006.09.010. [DOI] [Google Scholar]
- 30.Rowe RC, Sheskey PJ, Quinn ME. Handbook of pharmaceutical excipients. 6. London: Pharmaceutical Press; 2009. [Google Scholar]
- 31.Riera CE, Vogel H, Simon SA, Coutre Jl. Artificial sweeteners and salts producing a metallic taste sensation activate TRPV1 receptors. Am J Physiol. 2007;293:626–634. doi: 10.1152/ajpregu.00286.2007. [DOI] [PubMed] [Google Scholar]
- 32.Okuda Y, Irisawa Y, Okimoto K, Osawa T, Yamashita S. A new formulation for orally disintegrating tablets using a suspension spray-coating method. Int J Pharm. 2009;382(1–2):80–87. doi: 10.1016/j.ijpharm.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Chandrasekhar R, Hassan Z, AlHusban F, Smith AM, Mohammed AR. The role of formulation excipients in the development of lyophilised fast-disintegrating tablets. Eur J Pharm Biopharm. 2009;72:119–129. doi: 10.1016/j.ejpb.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Kearney P, Rathbone MJ, Hadgraft R. The Zydis oral fast-dissolving dosage form. Modified-Release Drug Delivery Technology. 2002;2:191–201.
- 35.Abdelbary G, Prinderre P, Eouani C, Joachim J, Reynier JP, Piccerelle Ph. The preparation of orally disintegrating tablets using a hydrophilic waxy binder. Int J Pharm. 2004;278:423–433. doi: 10.1016/j.ijpharm.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 36.Park TJ, Ko DH, Kim YJ, Kim YG. Polymorphic characterization of pharmaceutical solids, donepezil hydrochloride, by C CP/MAS solid-state nuclear magnetic resonance spectroscopy. B Kor Chem Soc. 2009;30(9):2007–2010. doi: 10.5012/bkcs.2009.30.9.2007. [DOI] [Google Scholar]
- 37.Krishna R, Moses JB, Anil K, Chandrashekar, Vijayavitthal T, Eswaraiah S, et al. Identification and characterization of potential impurities of donepezil. J Pharmaceut Biomed. 2004;35(5):1047–1058. doi: 10.1016/j.jpba.2004.03.022. [DOI] [PubMed] [Google Scholar]