Abstract
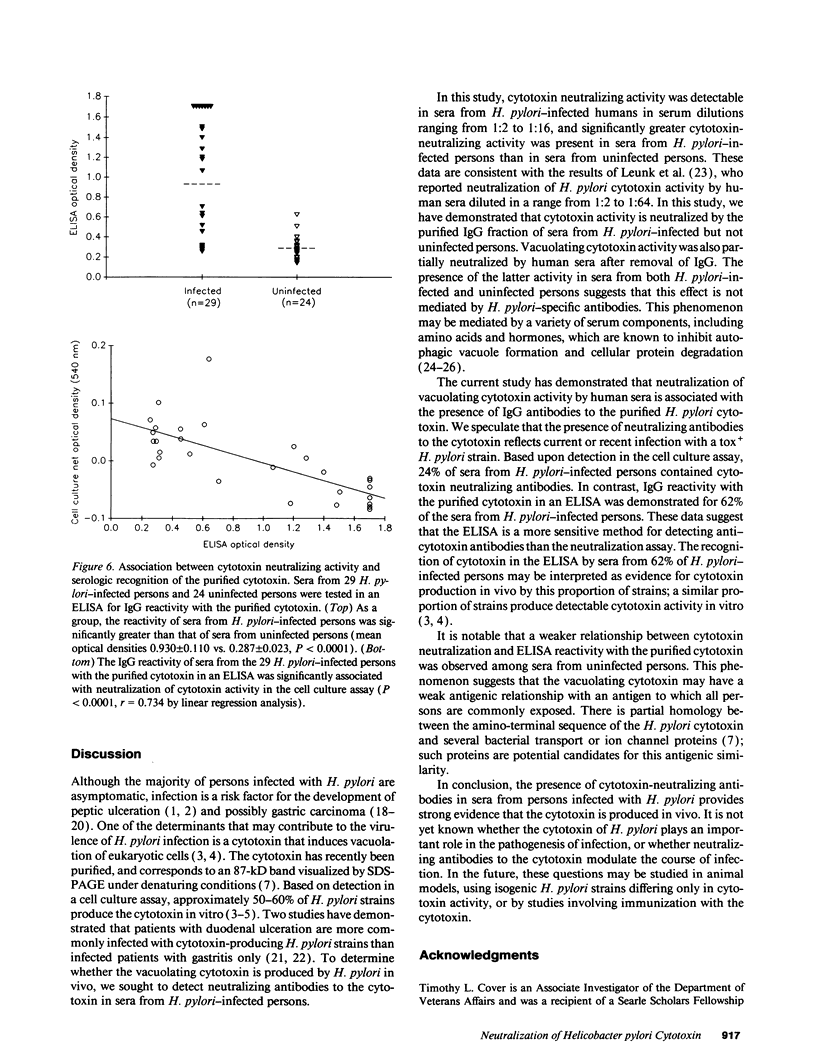
Approximately 50% of Helicobacter pylori isolates produce a cytotoxin in vitro that induces vacuolation of eukaryotic cells. To determine the in vivo relevance of this phenomenon, we sought to detect cytotoxin-neutralizing antibodies in sera from H. pylori-infected persons. As a group, sera from 29 H. pylori-infected patients neutralized the activity of the purified cytotoxin to a significantly greater extent than sera from 24 uninfected persons (P = 0.007). The cytotoxin neutralizing activity in sera from H. pylori-infected persons was mediated predominantly by the purified IgG fraction. Sera from H. pylori-infected persons neutralized the cytotoxins produced by multiple H. pylori strains, but failed to neutralize trimethylamine-induced cell vacuolation. Neutralization of cytotoxin activity by human or immune rabbit sera was associated with immunoblot IgG recognition of an 87-kD H. pylori protein. Similarly, neutralization of the toxin by sera was associated with IgG recognition of the purified cytotoxin in an enzyme-linked immunosorbent assay (P less than 0.0001). The presence of cytotoxin-neutralizing antibodies in sera from H. pylori-infected persons indicates that the cytotoxin is synthesized in vivo.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J., Knowles S. E., Wong S. S., Bodner J. B., Wood C. M., Gunn J. M. Inhibition of protein breakdown in cultured cells is a consistent response to growth factors. FEBS Lett. 1980 Jun 2;114(2):209–212. doi: 10.1016/0014-5793(80)81116-1. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Blaser M. J. Helicobacter pylori and gastroduodenal disease. Annu Rev Med. 1992;43:135–145. doi: 10.1146/annurev.me.43.020192.001031. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Blaser M. J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992 May 25;267(15):10570–10575. [PubMed] [Google Scholar]
- Cover T. L., Dooley C. P., Blaser M. J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990 Mar;58(3):603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Puryear W., Perez-Perez G. I., Blaser M. J. Effect of urease on HeLa cell vacuolation induced by Helicobacter pylori cytotoxin. Infect Immun. 1991 Apr;59(4):1264–1270. doi: 10.1128/iai.59.4.1264-1270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton K. A., Morgan D. R., Krakowka S. Campylobacter pylori virulence factors in gnotobiotic piglets. Infect Immun. 1989 Apr;57(4):1119–1125. doi: 10.1128/iai.57.4.1119-1125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessey S. J., Spencer J., Wyatt J. I., Sobala G., Rathbone B. J., Axon A. T., Dixon M. F. Bacterial adhesion and disease activity in Helicobacter associated chronic gastritis. Gut. 1990 Feb;31(2):134–138. doi: 10.1136/gut.31.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunk R. D., Ferguson M. A., Morgan D. R., Low D. E., Simor A. E. Antibody to cytotoxin in infection by Helicobacter pylori. J Clin Microbiol. 1990 Jun;28(6):1181–1184. doi: 10.1128/jcm.28.6.1181-1184.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunk R. D., Johnson P. T., David B. C., Kraft W. G., Morgan D. R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988 Jun;26(2):93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Mikawa M., Nakashio S., Takabatake M., Okado I., Yamakawa K., Serikawa T., Okumura S., Nishida S. Isolation of Clostridium difficile from the feces and the antibody in sera of young and elderly adults. Microbiol Immunol. 1981;25(4):345–351. doi: 10.1111/j.1348-0421.1981.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol. 1981 Sep;90(3):656–664. doi: 10.1083/jcb.90.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Perez-Perez G. I., Dworkin B. M., Chodos J. E., Blaser M. J. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988 Jul 1;109(1):11–17. doi: 10.7326/0003-4819-109-1-11. [DOI] [PubMed] [Google Scholar]
- Perez-Perez G. I., Witkin S. S., Decker M. D., Blaser M. J. Seroprevalence of helicobacter pylori infection in couples. J Clin Microbiol. 1991 Mar;29(3):642–644. doi: 10.1128/jcm.29.3.642-644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson W. L. Helicobacter pylori and peptic ulcer disease. N Engl J Med. 1991 Apr 11;324(15):1043–1048. doi: 10.1056/NEJM199104113241507. [DOI] [PubMed] [Google Scholar]
- Pfeifer U. Inhibition by insulin of the formation of autophagic vacuoles in rat liver. A morphometric approach to the kinetics of intracellular degradation by autophagy. J Cell Biol. 1978 Jul;78(1):152–167. doi: 10.1083/jcb.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B., Jacobs B., Mitra R. Antitoxin responses to infections with enterotoxigenic Escherichia coli. J Infect Dis. 1974 Mar;129(3):330–335. doi: 10.1093/infdis/129.3.330. [DOI] [PubMed] [Google Scholar]
- Schworer C. M., Shiffer K. A., Mortimore G. E. Quantitative relationship between autophagy and proteolysis during graded amino acid deprivation in perfused rat liver. J Biol Chem. 1981 Jul 25;256(14):7652–7658. [PubMed] [Google Scholar]
- Talley N. J., Zinsmeister A. R., Weaver A., DiMagno E. P., Carpenter H. A., Perez-Perez G. I., Blaser M. J. Gastric adenocarcinoma and Helicobacter pylori infection. J Natl Cancer Inst. 1991 Dec 4;83(23):1734–1739. doi: 10.1093/jnci/83.23.1734. [DOI] [PubMed] [Google Scholar]
- Thomsen L. L., Gavin J. B., Tasman-Jones C. Relation of Helicobacter pylori to the human gastric mucosa in chronic gastritis of the antrum. Gut. 1990 Nov;31(11):1230–1236. doi: 10.1136/gut.31.11.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricottet V., Bruneval P., Vire O., Camilleri J. P., Bloch F., Bonte N., Roge J. Campylobacter-like organisms and surface epithelium abnormalities in active, chronic gastritis in humans: an ultrastructural study. Ultrastruct Pathol. 1986;10(2):113–122. doi: 10.3109/01913128609014587. [DOI] [PubMed] [Google Scholar]