Abstract
The topical application of all-trans retinoic acid (ATRA) is an effective treatment for several skin disorders, including photo-aging. Unfortunately, ATRA is susceptible to light, heat, and oxidizing agents. Thus, this study aimed to investigate the ability of polymeric micelles prepared from polyethylene glycol conjugated phosphatidylethanolamine (PEG-PE) to stabilize ATRA under various storage conditions. ATRA entrapped in polymeric micelles with various PEG and PE structures was prepared. The critical micelle concentrations were 97–243 μM, depending on the structures of the PEG and PE molecules. All of the micelles had particle diameters of 6–20 nm and neutral charges. The highest entrapment efficiency (82.7%) of the tested micelles was exhibited by ATRA in PEG with a molecular weight of 750 Da conjugated to dipalmitoyl phosphatidylethanolamine (PEG750-DPPE) micelles. The PEG750-DPPE micelle could significantly retard ATRA oxidation compared to ATRA in 75% methanol/HBS solution. Up to 87% of ATRA remained in the PEG750-DPPE micelle solution after storage in ambient air for 28 days. This result suggests that PEG750-DPPE micelle can improve ATRA stability. Therefore, ATRA in PEG750-DPPE micelle is an interesting alternative structure for the development of cosmeceutical formulations.
KEY WORDS: all-trans retinoic acid, chemical stability, oxidation, phosphatidylethanolamine polymer, polymeric micelles, polyethylene glycol conjugated
INTRODUCTION
All-trans retinoic acid (ATRA) plays an important role to regulate cell growth and differentiation, especially in epithelial cells. It has been found to be an effective dermatological treatment (e.g., in the treatments of photo-aging, acne vulgaris, psoriasis, and many other skin diseases) (1–4). However, its topical use is limited by poor skin absorption due to preferential partitioning into and retention in the stratum corneum layer of the integument for this highly lipophilic drug (log Po/w = 6.7) (5). It also causes undesirable adverse effects known as retinoid dermatitis on the treated area, resulting in irritation, erythema, stinging, itching, burning, and desquamation (6,7). In addition, ATRA has been reported to be susceptible to degradation by light, heat, and oxidizing agents (8–10). Under light exposure, ATRA is rapidly isomerized, forming 13-cis and 9-cis retinoic acids. Various studies have shown that isomerase enzyme in the epithelial cells can metabolize these isomers of ATRA. Thus, they still have therapeutic activities upon topical application (11,12). However, ATRA can be oxidized into 4-hydroxy-, 4-oxo-, and 5,6-epoxy retinoic acids both in vivo and in vitro (10,13,14). For instance, 5,6-epoxy retinoic acid has only 0.5% as effective as ATRA in promoting growth (15). This evidence clearly shows that the oxidation products of ATRA limit the biological activity of pharmacological doses.
Most of previous researchers have attempted to prevent the light-induced degradation of ATRA by loading various carriers, such as liposomes (9,16,17), niosomes (18), phospholipid-based microemulsions (19), and solid lipid nanoparticles (20). However, none of the published articles mention attempt to protect degradation of ATRA from oxidation.
Amphiphilic micelles prepared from polyethylene glycol conjugated phosphatidylethanolamine (PEG-PE) are an attractive colloidal carrier for poorly soluble and amphiphilic substances (21–23). They can solubilize hydrophobic substances in their inner cores. Importantly, polymeric micelles are considerably more stable than conventional surfactant micelles because the PEG molecules on their hydrophilic surfaces provide steric stabilization in aqueous systems and protect the micelles from interactions with other molecules (24,25). This agrees well with the experiment of Chang and Chu (26), who found that micelle of PEG-poly(valerolactone) in phosphate buffer saline pH 7.4 could maintain its size without aggregation and dissociation at 37°C for 6 weeks. Moreover, the experiment of Koo et al. (27) showed that camptothecin loaded in PEGlyated phospholipids micelle was sufficiently high in stability in acetate buffer solution. This resulted from steric hindrance stabilized by PEGlyated coated particles on its surface against precipitation and remained in nanosize range. Moreover, both PEG and PE are chemical enhancing agents and can enhance drug permeation through the skin (28–31). The PE molecule as phospholipids is able to penetrate and disturb the structure of the stratum corneum lipid bilayer. This leads to increase partitioning of drug into the skin (32,33). The PEG molecule serves as nonionic surfactant which has potentially decreased surface tension of the stratum corneum. Thus, it enables to enhance drug diffusion through the skin (34). A combination of enhancers, including PEG and PE, might synergistically enhance the skin absorption of the drug. Thus, this study aimed to investigate the ability of PEG-PE micelles to reduce ATRA oxidation.
MATERIALS AND METHODS
Materials
A series of polyethylene glycols with MWs of 750 Da (PEG750) were conjugated to various diacyl chains of phosphatidylethanolamine, including dimyristoyl phosphatidylethanolamine (PEG750-DMPE, C14:0, MW = 1,415.76 Da), dipalmitoyl phosphatidylethanolamine (PEG750-DPPE, C16:0, MW = 1,471.87 Da), distearoyl phosphatidylethanolamine (PEG750-DSPE, C18:0, MW = 1,527.97 Da), and dioleoyl phosphatidylethanolamine (PEG750-DOPE, C18:1, MW = 1,523.94 Da). PEG with an MW of 5,000 Da was conjugated to dipalmitoyl phosphatidylethanolamine (PEG5000-DPPE, MW = 5,744.97 Da). These reagents were purchased from Avanti Polar Lipids (Alabama, USA). ATRA, ammonium acetate, sodium hydroxide, sodium chloride, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) were obtained from Sigma-Aldrich (St. Louis, USA). Methanol, chloroform, and acetonitrile were purchased from Lab-Scan (Gliwice, Poland).
Determination of the Critical Micelle Concentration
To determine critical micelle concentrations (CMCs) of the PEG-PE copolymers, the copolymers were dissolved in chloroform. The solvent was evaporated to form a thin copolymer film. The trace residue of the organic solvent was removed from the film by overnight storage in a desiccator. The copolymer film was then rehydrated by adding 10 mM HEPES with 150 mM sodium chloride (pH 7.4; HBS solution) and then vortexing vigorously. The final concentration of PEG-PE was 5 mM. Next, the PEG-PE solution was diluted with HBS solution to concentrations ranging from 10−3 to 10−7 M. Excess ATRA powder was added to all of the diluted PEG-PE copolymer solutions. Then, the solutions were continually shaken at room temperature for 36 h under light protection and filtered through a 0.45-μm nylon membrane to eliminate any excess ATRA. ATRA that was solubilized in the micelle was quantified by HPLC.
The amount of ATRA in the PEG-PE micelles was determined using a reverse phase HPLC system (35). HPLC was performed using a Shimadzu HPLC system (Shimadzu Corporation, Kyoto, Japan) with a pump (LC-20AT), a UV/VIS detector (SPD-20A), and a system controller (LC-20AT) with a PC control program (LC solution). The C18 reverse phase Phenomenex Gemini 5u C18 (250 × 4.6 mm) equipped with a guard column (Phenomenex Security Guard drop-in guard cartridge holder, 4-mm stacking rings, 3-mm bore, and Phenomenex C18) was used. The mobile phase was a mixture of acetonitrile and 10 mM ammonium acetate (75:25 v/v). The mobile phase was adjusted to pH 3.0 with glacial acetic acid. The solution flowed at 1.5 ml/min. ATRA was detected at wavelength 350 nm. ATRA concentration was calculated from its calibration curve.
Preparation of ATRA Loaded in the PEG-PE Polymeric Micelles
ATRA loaded in PEG-PE micelles and empty PEG-PE micelles were prepared by solvent evaporation (36). In brief, a fixed amount of PEG-PE copolymer at CMC value and ATRA was dissolved in chloroform. It was mixed well together further evaporated to form a thin film of ATRA copolymer. After a trace residue of solvent was eliminated, the film was rehydrated in HBS solution to yield ATRA in PEG-PE polymeric micelles. Excess ATRA was removed by filtrated through 0.45 μm nylon membrane. The supernatant was quantified ATRA solubilized in the micelles by HPLC. The experiment was also performed under light protection for preventing ATRA degradation. In addition, the fixed amount of PEG-PE copolymers at micelles formation without ATRA was prepared in the same fashion as the empty PEG-PE polymeric micelles.
Characterizations of ATRA Loaded in the PEG-PE Polymeric Micelles
Determination of Micelle Size
The average particle diameter and size distribution of the PEG-PE micelles in HBS solution were determined by photon correlation spectroscopy (Brookhaven Instruments, Holtsville, NY).
Zeta Potential Analysis
The zeta potentials of the PEG-PE micelles were measured with a zeta potential analyzer (Brookhaven Instruments, Holtsville, NY).
Entrapment Efficiency of ATRA Loaded in the PEG-PE Polymeric Micelles
A fixed amount of PEG-PE copolymer at CMC was dissolved in chloroform. ATRA (2, 5, and 10 μg) was also dissolved in another volume of chloroform. Both solutions of PEG-PE and ATRA were then well mixed before solvent evaporation. The thin film of ATRA copolymer was eliminated a trace residue overnight. This film was then rehydrated by HBS solution and filtered through 0.45 μm nylon membrane for eliminating excess ATRA. A supernatant of ATRA solubilized in PEG-PE micelles was quantified the amount of ATRA by HPLC. The entrapment efficiency and the loading capacity were calculated using the following (Eqs. 1 and 2):
 |
1 |
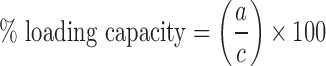 |
2 |
where a is the amount of ATRA solubilized in the PEG-PE micelles, b is the amount of ATRA added initially, and c is the amount of PEG-PE copolymer forming micelles. The chemical stability of the PEG-PE micelle that entrapped the most ATRA was then further studied.
Differential Scanning Calorimetry
The empty PEG-PE and ATRA-loaded PEG-PE micelles were prepared in a same fashion as described above and freeze-dried. The differential scanning calorimetry (DSC) spectra of pure ATRA and freeze-dried micelles (drug-loaded and blank) were recorded by a Mettler Toledo Star System DSC1 in the temperature range of 30 to 250°C (scanning rate 5°C/min).
Stability Study Under Various Storage Conditions
The stability of ATRA loaded in selected PEG-PE micelles was studied under different storage conditions. ATRA solubilized in 75% methanol/HBS solution was used as a control. The samples were kept at room temperature in containers filled with oxygen, nitrogen, or ambient air, with or without light exposure. For the “light exposure” group, the samples were placed 1 m from a fluorescent lamp. Samples were collected after 0, 0.25, 0.5, 1, 2, 3, 5, 7, 9, 12, 18, and 24 h. Another set of the samples was wrapped with aluminum foil (the “light protection” group). These samples were collected at the designated times of 1, 2, 3, 5, 7, 14, 21, and 28 days. The percentage remaining of ATRA in micelle and 75% methanol/HBS solution was quantified by HPLC.
Data and Statistical Analysis
All experiments were performed at least in triplicate. The data were normally expressed as means with standard deviations (±SD). One-way ANOVA was used to evaluate the CMC values and characterize the PEG-PE micelles. The Student’s t test was performed to stability test. A P value of 0.05 or less was considered significant.
RESULTS AND DISCUSSION
Determination of the Critical Micelle Concentration
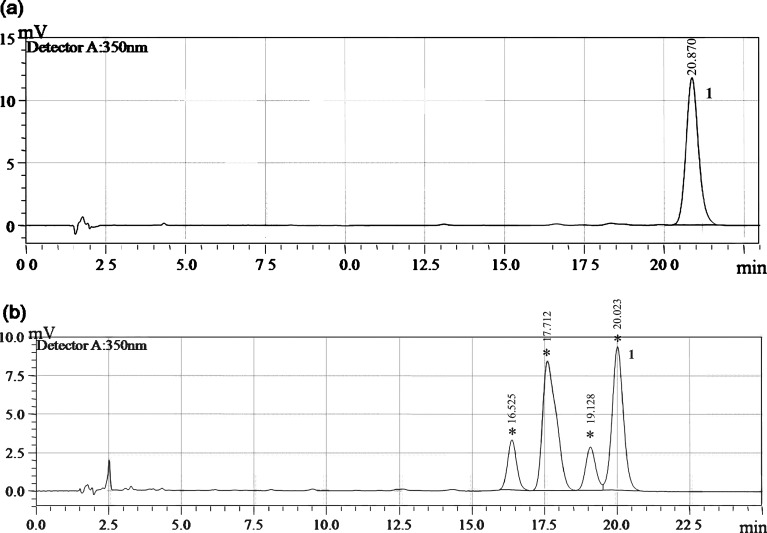
The amount of ATRA loaded in the micelles was quantified using a reverse phase HPLC system. The chromatogram was calculated based on peak height against ATRA concentration. Peak purity of ATRA was also investigated by studying the photodiode array data; no indications for impurities could be found. This method gave a good separation of ATRA (Fig. 1a) and its isomers whose quantitative identification was not included in the aim of this study (Fig. 1b).
Fig. 1.
Chromatograms of ATRA in methanol solution a under light protection and b under light exposure. 1 ATRA. Asterisks represent ATRA isomers
Both hydrophilic and hydrophobic fragments influence the micelle CMC; therefore, various fragment lengths were studied. The CMCs of PEG-PE copolymers in HBS solution are shown in Table I. Increasing the length of the hydrophobic fragment (with the hydrophilic fragment kept at a constant length) resulted in a low CMC. This can be explained by the fact that the increased hydrophobic fragment length influences the hydrophobic interactions among these fragments, forming a micellar core that rapidly reaches a micellization equilibrium state (37). These results are consistent with the findings of Chang and Chu (26), who found that the CMC value of PEG-poly(valerolactone) micelle decreased with increasing length of hydrophobic fragment. It indicated that the long length hydrophobic fragment helped to form micelle. The experiment of Kim et al. (38) also studied an identical PEG fragment with various caprolactone fragments. Result showed that length of caprolactone fragment increased, the CMC value decreased. Additionally, monounsaturated bonds were also observed on the PE chains; these bonds affected the CMC. The CMC of PEG750-DOPE was 6.98 × 10−5 M, which is slightly lower than that of saturated polymeric micelles (PEG750-DSPE). Based on these results, the length and the structure of the hydrophobic fragment affect the CMC.
Table I.
Characteristics of a Series of PEG-PE Micelles in HBS Solution
| PEG-PE copolymers | Critical micelle concentration (M) | Micelle size (nm) | Polydispersity | Zeta potential (mV) |
|---|---|---|---|---|
| PEG5000-DPPE | 2.81 × 10−5 | 17.3 ± 3.4 | 0.368 ± 0.001 | −0.84 ± 2.67 |
| PEG750-DMPE | 2.43 × 10−4 | 7.2 ± 0.9 | 0.295 ± 0.005 | 4.29 ± 1.87 |
| PEG750-DPPE | 2.49 × 10−4 | 6.6 ± 0.9 | 0.415 ± 0.020 | 1.49 ± 1.27 |
| PEG750-DSPE | 9.72 × 10−5 | 8.2 ± 0.5 | 0.337 ± 0.028 | 0.53 ± 0.38 |
| PEG750-DOPE | 6.98 × 10−5 | 7.6 ± 0.1 | 0.235 ± 0.012 | 0.38 ± 0.88 |
DMPE dimyristoyl-PE, DPPE dipalmitoyl-PE, DSPE distearoyl-PE, DOPE dioleoyl-PE
Increasing the hydrophilic fragment length (while keeping the hydrophobic fragment length constant) also resulted in a low CMC. The CMC of PEG5000-DPPE polymeric micelles was significantly decreased compared with that of PEG750-DPPE polymeric micelles. This can be explained in the fact that micelle formation requires the presence of two opposing forces. One is an attractive force between hydrophobic fragments leading to aggregation as described above. The other is a repulsive force that prevents an unlimited growth of micelles into a distinct macroscopic phase (39).
Characterizations of ATRA Loaded in the PEG-PE Polymeric Micelles
Determination of Micelle Size
Dynamic light scattering was used to measure the hydrodynamic diameters of PEG-PE polymeric micelles in HBS solution (Table I). The sizes of the PEG-PE polymeric micelles were all in the range of 6 to 20 nm. The polymeric micelle prepared using short hydrophilic fragments (PEG750) yielded micelles with diameters of less than 10 nm, whereas those with long hydrophilic fragments (PEG5000) produced micelles with an average diameter of 17 nm. In addition, the size distributions of the PEG-PE polymeric micelles were found to be uniform.
Zeta Potential Analysis
The zeta potential determines the charge on the micellar surface. All PEG-PE micelles had neutral charges (Table I) due to the neutral charges of the PEG molecules. This study is in agreement with experimental of Georgiev et al. (40) which showed that the zeta potential of PEG-PE micelles approaches a zero value with increased lateral density of PEG fragment. Although PEG-PE micelles present neutral charge, the high density of the PEG leading leads to decreasing coagulation and increasing stability of PEG-PE micelles due to steric repulsion.
Entrapment Efficiency of ATR Loaded in the PEG-PE Polymeric Micelles
To study the ATRA entrapment efficiencies of various types PEG-PE micelles, various amounts of ATRA were added in micelle preparations. The amount of ATRA that was added influenced the amount of ATRA that was entrapped in the PEG-PE micelles (Table II). The entrapment efficiencies and loading capacities of ATRA in all of the polymeric micelles increased when the amount of ATRA that was added increased from 2 to 5 μg. A decrease in the entrapment efficiency was observed when the amount of ATRA added was 10 μg but not in the loading capacity of ATRA.
Table II.
Entrapment of ATRA in Various Types of PEG-PE Micelles Solutions
| Copolymer types | Initial amount of ATRA (μg) | Solubilized ATRA in micelles (μg) | Entrapment efficiency (%) |
|---|---|---|---|
| PEG5000-DPPE | 2 | 0.31 ± 0.01 | 11.46 ± 0.60 |
| 5 | 0.52 ± 0.03 | 9.32 ± 0.45 | |
| 10 | 1.18 ± 0.52 | 11.98 ± 5.32 | |
| PEG750-DMPE | 2 | 1.07 ± 0.04 | 48.15 ± 1.80 |
| 5 | 2.43 ± 0.26 | 77.52 ± 3.13 | |
| 10 | 3.22 ± 0.80 | 62.62 ± 2.82 | |
| PEG750-DPPE | 2 | 0.89 ± 0.05 | 40.10 ± 4.93 |
| 5 | 4.46 ± 0.21 | 82.68 ± 1.86 | |
| 10 | 8.00 ± 0.25 | 69.19 ± 1.59 | |
| PEG750-DSPE | 2 | 1.08 ± 0.04 | 48.60 ± 1.62 |
| 5 | 2.22 ± 0.10 | 43.06 ± 1.42 | |
| 10 | 2.44 ± 0.34 | 52.76 ± 2.31 | |
| PEG750-DOPE | 2 | 0.21 ± 0.03 | 13.64 ± 2.23 |
| 5 | 0.31 ± 0.14 | 13.06 ± 0.74 | |
| 10 | 1.62 ± 0.27 | 22.85 ± 0.60 |
DMPE dimyristoyl-PE, DPPE dipalmitoyl-PE, DSPE distearoyl-PE, DOPE dioleoyl-PE, ATRA all-trans retinoic acid
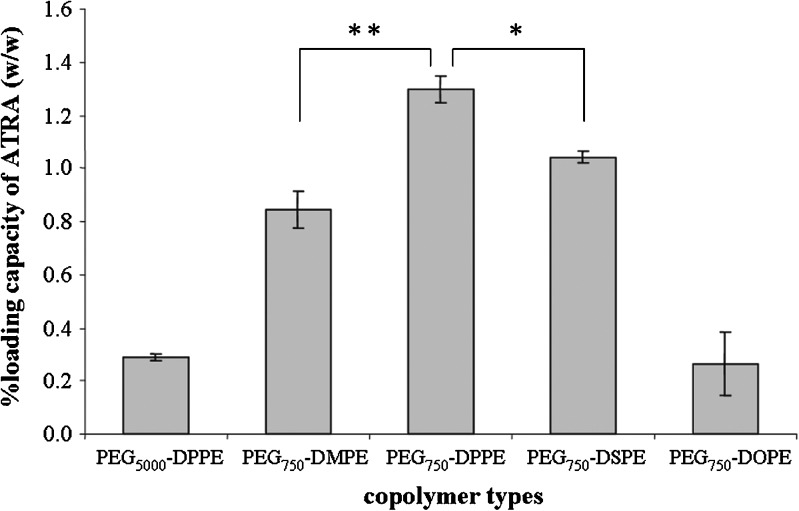
Because PE fragments served as the micellar core for ATRA entrapment, the lengths of the PE fragments strongly affected the ATRA entrapment efficiency. Among the PEG750-PE copolymers, the PEG750-DPPE micelles produced the highest ATRA entrapment efficiency (approximately 83%). An increase in the length of PE fragment from C14 to C16 led to the increase loading capacity of ATRA in the micellar core (P ≤ 0.05, Fig. 2). In addition, the increase in the length of PE from C16 to C18 also influenced the loading capacity of ATRA (P ≤ 0.01). A possible explanation is the compatibility between ATRA and the DPPE fragments (41). Therefore, ATRA entrapped in PEG750-DPPE micelles was selected for further studied.
Fig. 2.
The amount of ATRA loading capacity in various types of PEG-PE micelles when the initial ATRA amount was 5 μg. **P ≤ 0.05 comparison between PEG750-DMPE and PEG750-DPPE micelles, *P ≤ 0.01 comparison between PEG750-DPPE and PEG750-DSPE micelles
Differential Scanning Calorimetry
PEG750-DPPE was selected to prepare polymeric micelles DSC study as it gave the highest entrapment efficiency of ATRA. The DSC thermogram of ATRA powder showed two endothermic peaks at 147°C and 183°C [Fig. 3 (a)] corresponding to the melting point of ATRA and 13-cis RA, respectively, as also suggested by Berbenni et al. (42). The thermogram of freeze-dried empty PEG750-DPPE micelles showed only one endothermic peak at 96°C [Fig. 3 (b)]. For ATRA-loaded PEG750-DPPE micelles, the DSC thermogram showed endothermic peaks at 97°C and 154°C [Fig. 3 (c)]. The lower endothermic peak at 97°C presents melting temperature of DPPE which was similar to the thermogram of the PEG750-DPPE micelles. Interestingly, the thermogram peak of retinoic acid both ATRA and 13-cis RA disappeared and found new peak at 154°C. This result is in agreement with the previous studies showed that mixture of DPPE with ATRA gave a slight broadening of DPPE (43,44). In addition, the endothermogram of DPPE was slightly shifted to lower temperature.
Fig. 3.
DSC thermograms of ATRA powder (a), freeze-dried form of the empty PEG750-DPPE micelles (b), and freeze-dried form of ATRA-loaded PEG750-DPPE micelles (c)
Stability Study Under Various Storage Conditions
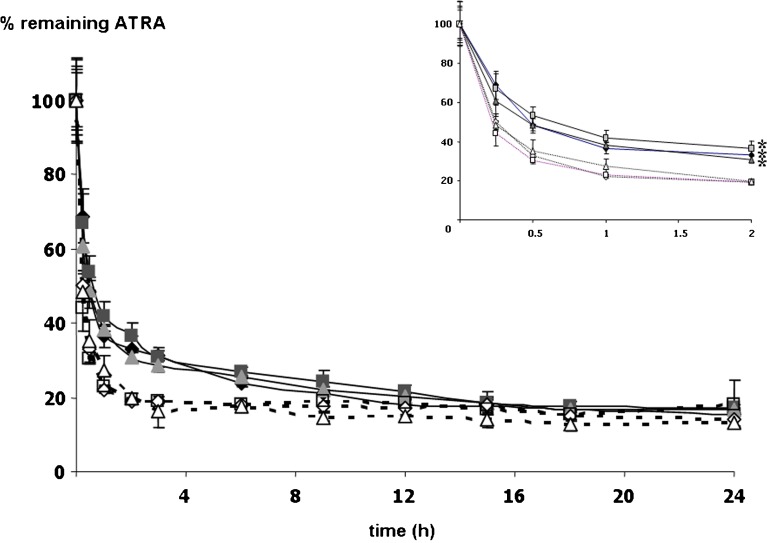
Because ATRA was not dissolved in an aqueous medium, ATRA solution used as a control was prepared by dissolving in 75% methanol/HBS solution. Degradation profiles of ATRA loaded in PEG750-DPPE micelles and 75% methanol/HBS solution with and without light exposure were studied under different storage conditions; the results are shown in Figs. 4 and 5, respectively. Under light exposure, ATRA in all formulations of the PEG750-DPPE micelle storage under oxygen, nitrogen, and ambient air showed degradation reached a plateau by 2 h where as that of ATRA dissolved in 75% methanol/HBS solution reached a plateau by 1 h (Fig. 4, insert). In addition, the amount of ATRA in the micelle solutions decreased exponentially with time in a manner similar to that of ATRA in 75% methanol/HBS solution. Thus, ATRA degradation by light in PEG750-DPPE micelle formulations follows first-order kinetics. This result agrees with previous finding in the literatures (16,18,45). The amount of ATRA in micelles storage in oxygen, nitrogen, and ambient air-filled environments was 36.4%, 41.9%, and 38.3%, respectively, while ATRA in 75% methanol/HBS solutions remained 22.2%, 22.8%, and 27.4% of initial amount when stored in oxygen, nitrogen, and ambient air-filled environments, respectively. These results showed that the stability of ATRA loaded in micelles was superior to that of ATRA solubilized in 75% methanol/HBS (P ≤ 0.01). It is noteworthy that an appearance of PEG750-DPPE micelle formulation was transparent. Thus, light can penetrate through a micelle solution and lead to isomerization. However, as discussed previously, isomerization of ATRA has therapeutic activity on topical application (11,12).
Fig. 4.
The percentage remaining of ATRA loaded in PEG750-DPPE micelles (close symbols) and ATRA in 75% methanol/HBS solutions (open symbols) stored in oxygen, nitrogen, and ambient air under light exposure. Black diamonds and white diamonds represent amount of ATRA in oxygen-filled environment, black squares and white squares represent amount of ATRA in nitrogen-filled environment, and gray triangles and white triangles represent amount of ATRA in ambient air. *P ≤ 0.01 compared with ATRA in 75% methanol/HBS solutions
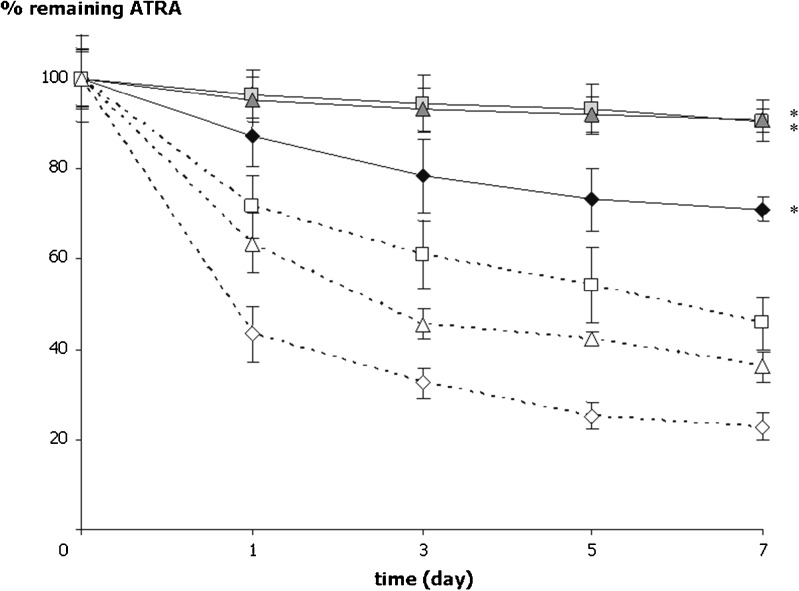
Fig. 5.
The percentage remaining of ATRA loaded in PEG750-DPPE micelles (close symbols) and ATRA in 75% methanol/HBS solutions (open symbols) stored in oxygen, nitrogen, and ambient air under light protection. Black diamonds and white diamonds represent amount of ATRA in oxygen-filled environment, black squares and white squares represent amount of ATRA in nitrogen-filled environment, and gray triangles and white triangles represent amount of ATRA in ambient air. *P ≤ 0.01 compared with ATRA in 75% methanol/HBS solutions
Interestingly, the micelle of PEG750-DPPE could significantly reduce ATRA degradation caused by oxygen under light protected (P ≤ 0.01). The remaining of ATRA in the PEG750-DPPE micelles was 87.3% while it was only 43.3% in 75% methanol/HBS solution for a day in oxygen-filled environment (Fig. 5). It was also found that under light protected, ATRA in all conditions of the PEG750-DPPE micelle degraded slower than that of ATRA in 75% methanol/HBS solution (P ≤ 0.01). Comparing among three conditions, the amount of ATRA loaded in PEG750-DPPE micelles significantly decreased in oxygen-filled environment (P ≤ 0.01). It remained about 78.4%, 94.4%, and 93.3% for 3 days of incubation in oxygen, nitrogen, and ambient air-filled environments, respectively. The remaining of ATRA in the PEG750-DPPE micelles stored in ambient air was about 90% for 7 days of incubation.
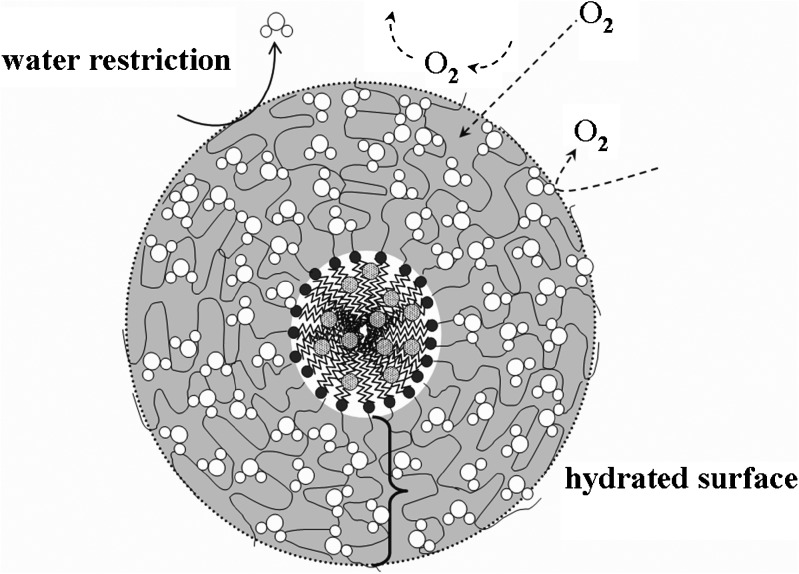
Furthermore, up to 87% of the initial ATRA in the PEG750-DPPE micelles still remained after 28 days of incubation under ambient air and light protected. The PEG moieties have a high affinity to water molecules and may create a hydrated surface surrounding the micellar core. In the presence of this hydrated surface, the minimal interfacial free energy may reduce the attractive force and protecting the colloidal carriers from interacting with other molecules (46,47). In addition, the PEG moieties are well hydrated and flexible. We postulated that hydrated surface of the PEG moieties surround the micellar core may retard oxygen diffusion leading to the reduction of ATRA oxidation (Fig. 6). The degree of water disturbance by the PEG moieties is correlated with the PEG’s flexibility. Frequent PEG movements reflect its high flexibility and can temporarily squeeze out of water molecules; this may restrict the diffusion of other molecules (48,49). In particular, it may restrict oxygen diffusion through the hydrated layer of PEG moieties and retard ATRA degradation in the PEG750-DPPE micelles.
Fig. 6.
A proposed PEG750-DPPE micelle structure that retards degradation of ATRA by oxygen
CONCLUSION
This study demonstrated that PEG750-DPPE micelles significantly slowed the degradation of ATRA in the presence of atmospheric oxygen. We postulate that the PEG plays an important role in the reduction of oxidation by creating hydrophilic hydrated layer surrounding the micellar core and thus retarding oxygen diffusion. Our results suggest that PEG750-DPPE micelles are promising carriers for ATRA. Further stability improvement was made by freeze-drying. However, only ATRA in micelle formula seems not to be good enough for producing a commercial product with acceptable shelf-life. We recommend ATRA in the PEG750-DPPE micelles will be further prepare to powder form by freeze-drying. Cross-linking corona shell of the PEG750-DPPE micelles producing nanospheres particle is also an alternative recommend.
Acknowledgments
The authors are grateful for financial support from Naresuan University, Thailand.
References
- 1.Fisher GJ, Esmann J, Griffiths CEM, et al. Cellular, immunologic and biochemical characterization of topical retinoic acid-treated human skin. J Invest Dermatol. 1991;96:699–707. doi: 10.1111/1523-1747.ep12470632. [DOI] [PubMed] [Google Scholar]
- 2.Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 3.Gilchrest BA. Treatment of photodamage with topical tretinoin: an overview. J Am Acad Dermatol. 1997;36:S27–S36. doi: 10.1016/S0190-9622(97)70058-6. [DOI] [PubMed] [Google Scholar]
- 4.Cho S, Lowe L, Hamilton TA, Fisher GJ, Voorhees JJ, Kang S. Long-term treatment of photoaged human skin with topical retinoic acid improves epidermal cell atypia and thickens the collagen band in papillary dermis. J Am Acad Dermatol. 2005;53:769–764. doi: 10.1016/j.jaad.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 5.Sigma-Aldrich Knowledgebase. Generic EU MSDS. Material safety data sheet of all-trans retinoic acid, Singapore. 2006. http://www.sigma-aldrich.com. Accessed 26 Jun 2007.
- 6.Kochhar DM, Christian MS. Tretinoin: a review of the nonclinical developmental toxicology experience. J Am Acad Dermatol. 1997;36:S47–S59. doi: 10.1016/S0190-9622(97)70060-4. [DOI] [PubMed] [Google Scholar]
- 7.Kim BH, Lee YS, Kang KS. The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol Lett. 2003;146:65–73. doi: 10.1016/j.toxlet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Brisaert MG, Everaerts I, Plaizier-Vercammen JA. Chemical stability of tretinoin in dermatological preparations. Pharm Acta Helv. 1995;70:161–166. doi: 10.1016/0031-6865(95)00016-3. [DOI] [Google Scholar]
- 9.Ioele G, Cione E, Risoli A, Genchi G, Ragno G. Accelerated photostability study of tretinoin and isotretinoin in liposome formulations. Int J Pharm. 2005;293:251–260. doi: 10.1016/j.ijpharm.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Panzella L, Manini P, Napolitano A, D’Ischia M. Free radical oxidation of (E)-retinoic acid by the Fenton reagent: competing epoxidation and oxidative breakdown pathways and novel products of 5,6-epoxy retinoic acid transformation. Chem Res Toxicol. 2004;17:1716–1724. doi: 10.1021/tx049794b. [DOI] [PubMed] [Google Scholar]
- 11.Elbaum DJ. Comparison of the stability of topical isotretinoin and topical tretinoin and their efficacy in acne. J Am Acad Dermatol. 1988;19:486–491. doi: 10.1016/S0190-9622(88)70202-9. [DOI] [PubMed] [Google Scholar]
- 12.Lovat PE, Irving H, Malcolm AJ, Pearson ADJ, Christopher PFR. 9-cis retinoic acid-a better retinoid for the modulation of differentiation, proliferation and gene expression in human neuroblastoma. J Neurooncol. 1997;31:85–91. doi: 10.1023/A:1005785431343. [DOI] [PubMed] [Google Scholar]
- 13.Duell EA, Astrom A, Griffiths CEM, Chambon P, Voorhees JJ. Human skin levels of retinoic acid and cytochrome P-450 derived 4-hydroxy retinoic acid after topical application of retinoic acid in vivo compared to concentrations required to stimulate retinoic acid receptor-mediated transcription in vitro. J Clin Invest. 1992;90:1269–1274. doi: 10.1172/JCI115990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samokyszyn VM, Freyaldenhoven MA, Chang HC, Freeman JP, Compadre RL. Regiospecificity of peroxyl radical addition to (E)-retinoic acid. Chem Res Toxicol. 1997;10:795–801. doi: 10.1021/tx970045m. [DOI] [PubMed] [Google Scholar]
- 15.Zile MH, Inhorn RC, Deluca HF. The biological activity of 5,6-epoxy retinoic acid. J Nutr. 1980;110:2225–2230. doi: 10.1093/jn/110.11.2225. [DOI] [PubMed] [Google Scholar]
- 16.Brisaert M, Gabiels M, Matthijs V, Plaizier-Vercammen J. Liposomes with tretinoin: a physical and chemical evaluation. J Pharm Biomed Anal. 2001;26:909–917. doi: 10.1016/S0731-7085(01)00502-7. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu K, Tamagawa K, Takahashi N, Takayama K, Maitani Y. Stability and antitumor effects of all-trans retinoic acid loaded liposome contained sterylglucoside mixture. Int J Pharm. 2003;258:45–53. doi: 10.1016/S0378-5173(03)00151-0. [DOI] [PubMed] [Google Scholar]
- 18.Manconi M, Valenti D, Sinico C, Lai F, Loy G, Fadda MA. Niosomes as carriers for tretinoin II. Influence of vesicular incorporation on tretinoin photostability. Int J Pharm. 2003;260:261–272. doi: 10.1016/S0378-5173(03)00268-0. [DOI] [PubMed] [Google Scholar]
- 19.Hwang SR, Lim SJ, Park JS, Kim CK. Phospholipid-based microemulsion formulation of all-trans retinoic acid for parenteral administration. Int J Pharm. 2004;276:175–183. doi: 10.1016/j.ijpharm.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Lim SJ, Lee MK, Kim CK. Altered chemical and biological activities of all-trans retinoic acid incorporated in solid lipid nanoparticle powders. J Control Release. 2004;100:53–61. doi: 10.1016/j.jconrel.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 21.Gao Z, Lukyanov AN, Singhal A, Torchilin VP. Diacyllipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2002;2:979–982. doi: 10.1021/nl025604a. [DOI] [Google Scholar]
- 22.Lukyanov AN, Gao Z, Torchilin VP. Micelles from polyethylene glycol/phosphatidylethanolamine conjugates for tumor drug delivery. J Control Release. 2003;91:97–102. doi: 10.1016/S0168-3659(03)00217-7. [DOI] [PubMed] [Google Scholar]
- 23.Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev. 2004;56:1273–1289. doi: 10.1016/j.addr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Kwon GS, Kataoka K. Block copolymer micelles as long-circulating drug vehicles. Adv Drug Deliv Rev. 1995;16:295–309. doi: 10.1016/0169-409X(95)00031-2. [DOI] [Google Scholar]
- 25.Kwon GS, Okanob T. Polymeric micelles as new drug carriers. Adv Drug Deliv Rev. 1996;21:107–116. doi: 10.1016/S0169-409X(96)00401-2. [DOI] [Google Scholar]
- 26.Chang YC, Chu IM. Methoxy poly(ethylene glycol)-b-poly(valerolactone) diblock polymeric micelles for enhanced encapsulation and protection of camptothecin. Eur Pol J. 2008;44:3922–3930. doi: 10.1016/j.eurpolymj.2008.09.021. [DOI] [Google Scholar]
- 27.Koo OM, Rubinstein I, Onyuksel H. Camptothecin in sterically stabilized phospholipid micelles: a novel nanomedicine. Nanomedicine. 2005;1:77–84. doi: 10.1016/j.nano.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Maghraby GMME, Campbell M, Finnin BC. Mechanisms of action of novel skin penetration enhancers: phospholipid versus skin lipid liposomes. Int J Pharm. 2005;305:90–104. doi: 10.1016/j.ijpharm.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Hu G. Advances in studies of phospholipids as carriers in skin topical application. JNMU. 2007;21:349–353. [Google Scholar]
- 30.Asbill CS, Michniak BB. Percutaneous penetration enhancers: local versus transdermal activity. PSTT. 2000;3:36–41. doi: 10.1016/s1461-5347(99)00225-4. [DOI] [PubMed] [Google Scholar]
- 31.Karande P, Mitragotri S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim Biophys Acta. 2009;1788:2362–2373. doi: 10.1016/j.bbamem.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Kirjavainen M, Monkkonen J, Saukkosaari M, Valjakka-Koskela R, Kiesvaara J, Urtti A. Phospholipids affect stratum corneum lipid bilayer fluidity and drug partitioning into the bilayers. J Control Release. 1999;58:207–214. doi: 10.1016/S0168-3659(98)00152-7. [DOI] [PubMed] [Google Scholar]
- 33.Yokomizo Y. Effects of phospholipids on the percutaneous penetration of drugs through the dorsal skin of the guinea pig, in vitro. 3. The effects of phospholipids on several drugs having different polarities. J Control Release. 1996;42:217–228. doi: 10.1016/0168-3659(96)01347-8. [DOI] [Google Scholar]
- 34.Vos AMD, Kinget R. Study of the penetration-enhancing effect of two nonionic surfactants (cetiol HE and eumulgin B3) on human stratum corneum using differential scanning calorimetry. Eur J Pharmacol. 1993;1:89–93. doi: 10.1016/0928-0987(93)90022-3. [DOI] [Google Scholar]
- 35.Dimitrova B, Poyre M, Guiso G, Badiali A, Caccia S. Isocratic reversed-phase liquid chromatography of all-trans retinoic acid and its major metabolites in new potential supplementary test systems for developmental toxicology. J Chromatogr B. 1996;681:153–160. doi: 10.1016/0378-4347(95)00573-0. [DOI] [PubMed] [Google Scholar]
- 36.Genevive G, Helene DM, Vinayak PS, Ning K, Dusica M, Jean-Christophe L. Block copolymer micelles: preparation, characterization and application in drug delivery. J Control Release. 2005;109:169–188. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 37.Holmberg K. Handbook of applied surface and colloid chemistry. Chichester: Wiley; 2002. [Google Scholar]
- 38.Kim SY, Shin IG, Lee YM, Cho CS, Sung YK. Methoxy poly(ethylene glycol) and caprolactone amphiphilic block polymeric micelle containing indomethacin. II Micelle formation and drug release behaviours. J Control Release. 1998;51:13–22. doi: 10.1016/S0168-3659(97)00124-7. [DOI] [PubMed] [Google Scholar]
- 39.Astafieva I, Zhong XF, Eisenberg A. Critical micellization phenomena in block polyelectrolyte solutions. Macromolecules. 1993;26:7339–7352. doi: 10.1021/ma00078a034. [DOI] [Google Scholar]
- 40.Georgiev GA, Sarker DK, Al-Hanbali O, Georgiev GD, Lalchev Z. Effects of poly(ethylene glycol) chains conformational transition on the properties of mixed DMPC/DMPE-PEG thin liquid films and monolayers. Colloids Surf B Biointerfaces. 2007;59:184–193. doi: 10.1016/j.colsurfb.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Allen C, Maysinger D, Eisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf B Biointerfaces. 1999;16:3–27. doi: 10.1016/S0927-7765(99)00058-2. [DOI] [Google Scholar]
- 42.Berbenni V, Marini A, Bruni G, Cardini A. Thermoanalytical and spectroscopic characterisation of solid-state retinoic acid. Int J Pharm. 2001;221:123–141. doi: 10.1016/S0378-5173(01)00677-9. [DOI] [PubMed] [Google Scholar]
- 43.Ortiz A, Aranda FJ, Gomez-Fernandez JC. Interaction of retinol and retinoic acid with phospholipid membranes. A differential scanning calorimetry study. BBA. 1992;1106:282–290. doi: 10.1016/0005-2736(92)90007-9. [DOI] [PubMed] [Google Scholar]
- 44.Ortiz A, Aranda FJ, Villalain J, Gomez-Fernandez JC. Influence of retinoids on phosphatidylethanolamine lipid polymorphism. BBA. 1992;1112:226–234. doi: 10.1016/0005-2736(92)90395-3. [DOI] [PubMed] [Google Scholar]
- 45.Brisaert M, Plaizier-Vercammen J. Investigation on the photostability of a tretinoin lotion andstabilization with additives. Int J Pharm. 2000;199:49–57. doi: 10.1016/S0378-5173(00)00366-5. [DOI] [PubMed] [Google Scholar]
- 46.Woodle MC, Lasic DD. Sterically stabilized liposomes. BBA. 1992;1113:171–199. doi: 10.1016/0304-4157(92)90038-c. [DOI] [PubMed] [Google Scholar]
- 47.Woodle MC. Surface-modified liposomes: assessment and characterization for increased stability and prolonged blood circulation. Chem Phys Lipids. 1993;64:249–262. doi: 10.1016/0009-3084(93)90069-F. [DOI] [PubMed] [Google Scholar]
- 48.Tirosh O, Barenholz Y, Katzhendler J, Priev A. Hydration of polyethylene glycol-grafted liposomes. Biophys J. 1998;74:1371–1379. doi: 10.1016/S0006-3495(98)77849-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vonarbourg A, Passirani C, Saulnier P, Benoit JP. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials. 2006;27:4356–4373. doi: 10.1016/j.biomaterials.2006.03.039. [DOI] [PubMed] [Google Scholar]