Abstract
The effect of a homologue series of nonionic surfactants, namely poly(ethylene glycol) (PEG) fatty acid esters, differing in oxyethylene (PEG 8, PEG 12, and PEG 40) and fatty acid (stearate, mono and di-laurate, and mono and di-oleate) chain lengths, on in vitro skin permeability of ketoprofen (KTP) vehicled in plasters was investigated. The drug diffusion through hairless mouse skin as well as the effect of the surfactant type and strength was studied by Franz diffusion cells and ATR-FTIR spectroscopy. The use of PEG stearate series revealed that the surfactant with the largest polar head, namely PEG 40, was ineffective in enhancing the skin permeation of KTP, independently of the plaster concentrations. The effect of the hydrophobic chain was investigated only by using the shortest oxyethylene chains. The experimental results revealed that the oxyethylene chain length of surfactants appeared to be more influent than the alkyl chain. The prediction of the absorption enhancing capability of these PEG derivatives appeared related to the vehicle other than the proper combination of the number of ethylene oxide groups and alkyl groups.
KEY WORDS: enhancer, hairless mouse skin, ketoprofen, plaster, polyethylene glycol derivatives
INTRODUCTION
Nonionic surfactants have long been recognized as penetration enhancers due to their ability in modifying the permeability of several biological membranes, including skin (1). Two possible mechanisms are proposed to explain their effects on the drug permeation through the stratum corneum (2,3). The former hypothesizes that the surfactants penetrated into the intercellular regions of stratum corneum may increase fluidity and, eventually, solubilize, and extract lipid components. The latter assumes that possible interactions and bonding of surfactants to keratin filaments may result in a disruption within corneocytes. Both the hypotheses were proven by testing the effect of polysorbate 80 (3–5) and polyoxyethylene ethers (6–8) on permeation of several drugs. These data clearly evidenced the key role of the complex chemical structure of nonionic surfactants in determining the enhancement effect. Indeed, the size as well as the shape of both the alkyl chain (“tails”) and the polar group (“heads”) influence the absorption-enhancing ability (9). Moreover, structure–activity relationships of the hydrophobic chains of various penetration enhancers evidenced that the optimum length for saturated chains ranges between C8 and C14 (mostly from 10 to 12 carbon atoms) (9–12). These effects resulted independently of the number of hydrophobic chains contained in the surfactant structure. Indeed, similarly to one-chain derivatives, the most active enhancers containing two saturated hydrocarbon chains at the same length were those with C12 chains (11,12). Hence, it was suggested that the chain length and not the overall lipophilicity was important for the enhancement action. Compared to saturated chains, the longer the alkyl chain of the unsaturated fatty acids, the higher the fluxes and the shorter the lag times; in particular, the C18 to C20 chain-length derivatives provided the optimal permeation-enhancing effects independently of the differences of position and numbers of double bonds (13).
Despite the number of debates on the effects of fatty acids and fatty alcohols series (12,14,15), few reports dealt with the influence of the volume of the surfactant polar head (16) and the nature of the donor medium (10,16) on the skin permeability of drugs. Surfactants in bulk solution can assemble into micelles which produce different consequences on the skin permeability of drugs with moderate lipophilicity: they could reduce the rate of transport of the drug (17) due to interactions with the drug (3) or determine the opposite effect due to an increase of apparent drug solubility.
Generally speaking, nonionic surfactants have been deeply studied and discussed in simple vehicles, such as solutions. Nevertheless, when drugs and surfactants are loaded in more complex systems, namely medicated plasters, in order to be administered, deviations from the pattern registered using solutions as a vehicle can occur (18). Since the effects of nonionic surfactants as skin penetration enhancers in medicated plasters was scantily debated in literature, the present work aimed to provide a deeper insight into the use of such compounds. In particular, the selectivity and the enhancement effect of a series of polyoxyethylene esters (PEG) derivatives, on the skin permeability of ketoprofen vehicled in medicated plasters made of acrylic copolymer was studied in vitro by using Franz diffusion cells and full-thickness hairless mouse skin, as a membrane. The influence of the polar head group, the oxyethylene chain, and hydrophobic chain was investigated using nine different PEG derivatives loaded in plasters at three different strengths.
EXPERIMENTAL
Materials
Ketoprofen (KTP) was supplied by LCM (Italy). Duro-tak 87-900A (DT 900A) was provided by National Starch (USA). Simulsol M 45, HLB = 12.0 (PEG8-S) was obtained from Seppic (Italy); Cithrol 6MS, HLB = 14.0 (PEG12-S) and Crodet S40LD, HLB = 16.7 (PEG40-S) were obtained from Croda (Italy). PEG 400 monolaurate, HLB = 13.0 (PEG8-ML), PEG 400 mono-oleate, HLB = 12.0 (PEG8-MO), PEG 400 dilaurate, HLB = 11.0 (PEG8-DL), PEG 600 mono-oleate, HLB = 14.0 (PEG12-MO), PEG 600 dioleate, HLB = 10.0 (PEG12-DO), were obtained from Mosselman (Belgium). Cithrol 4DO, HLB = 8.8 (PEG8-DO), was obtained from Croda Chemicals Europe Ltd. (UK). All solvents were of analytic grade, unless specified.
Plaster Preparation and Drug Content
KTP was added to the polymeric solution under stirring at 100 rpm over a 1-h period. The homogeneous blend was let rest to remove air bubbles and then spread onto the siliconized liner by means of a hand-driven laboratory coating machine equipped with a blade coating head. The coating thickness was fixed at 300 μm. The coated mass was dried at 80°C for 20 min and laminated with the backing foil. Plasters were dye-cut out of the laminate in the final size, sealed in airtight pouches, and stored at 20°C until use. The matrix compositions of KTP plasters are reported in Table I.
Table I.
Matrix Composition (%, w/w) and Skin Permeability Parameters (Flux and ER) of the Plasters Containing KTP and PEG derivatives
| Plaster | KTP | DT900A | PEG8-S | PEG12-S | PEG40-S | PEG8-MO | PEG8-DO | PEG8-ML | PEG8-DL | PEG12-MO | PEG12-DO | Flux (μg/cm2/h) | ERa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K1A | 10 | 90 | 8.16 ± 1.41 | – | |||||||||
| K1B | 10 | 90 | 8.55 ± 0.48 | – | |||||||||
| K2 | 10 | 85 | 5 | 8.89 ± 0.61 | 1.12 ± 0.19 | ||||||||
| K3 | 10 | 85 | 5 | 11.70 ± 1.00b | 1.47 ± 0.26 | ||||||||
| K4 | 10 | 85 | 5 | 7.02 ± 0.54 | – | ||||||||
| K5 | 10 | 80 | 10 | 12.74 ± 0.58b | 1.60 ± 0.26 | ||||||||
| K6 | 10 | 80 | 10 | 12.46 ± 1.83b | 1.57 ± 0.31 | ||||||||
| K7 | 10 | 80 | 10 | 6.21 ± 0.55 | – | ||||||||
| K8 | 10 | 70 | 20 | 17.75 ± 2.89b | 2.23 ± 0.47 | ||||||||
| K9 | 10 | 70 | 20 | 13.71 ± 1.39b | 1.73 ± 0.31 | ||||||||
| K10 | 10 | 70 | 20 | 6.41 ± 0.12 | – | ||||||||
| K11 | 10 | 85 | 5 | 10.58 ± 1.58 | – | ||||||||
| K12 | 10 | 85 | 5 | 9.75 ± 1.72 | – | ||||||||
| K13 | 10 | 85 | 5 | 11.41 ± 0.41b | 1.34 ± 0.08 | ||||||||
| K14 | 10 | 85 | 5 | 7.79 ± 0.53 | – | ||||||||
| K15 | 10 | 85 | 5 | 9.13 ± 0.34 | – | ||||||||
| K16 | 10 | 85 | 5 | 13.12 ± 2.02b | 1.54 ± 0.22 | ||||||||
| K17 | 10 | 80 | 10 | 15.07 ± 0.40b | 1.77 ± 0.10 | ||||||||
| K18 | 10 | 80 | 10 | 14.02 ± 2.15b | 1.64 ± 0.23 | ||||||||
| K19 | 10 | 80 | 10 | 12.50 ± 1.03b | 1.47 ± 0.13 | ||||||||
| K20 | 10 | 80 | 10 | 9.59 ± 0.61b | 1.12 ± 0.08 | ||||||||
| K21 | 10 | 80 | 10 | 9.99 ± 1.77 | – | ||||||||
| K22 | 10 | 80 | 10 | 18.73 ± 3.82b | 2.20 ± 0.40 |
aER calculated only for fluxes significantly different from control
bMean values significantly different vs. control
To assess the drug content, a specimen of 50 cm2 was dissolved in 50 mL methanol and the filtered solutions were assayed by the high-performance liquid chromatography (HPLC) method reported below.
Skin Membrane Preparation
Female CD-1 nude mice, 6 weeks old, (Charles River, Italy), initial weight 20 g, were used. The animals were housed in a conditioned environment (22 ± 1°C, 55 ± 5% relative humidity, 12-h light/12-h dark cycle), with free access to standard laboratory chow and tap water. The animal experiments are approved by the Ethical Committee for animal experimentation (CSEA) as Animal use project no. 02/2009 of 28-01-2009, still active (Responsible for experimental execution: Dr. Giuseppe Rossoni) communicated to The Italian Ministry of Health, having regard to the article 7 of the D.Lvo 116/92. Hairless mouse were anesthetized with thiopentone sodium (Pentothal®; USA) and sacrificed. The full-thickness skin was excised from the abdominal site after removal of the fat and the subdermal tissues by surgical scissor. The skin was washed with saline solution, its integrity verified by means of visual inspection and then it was frozen at −20°C until use.
In Vitro Skin Permeation Study
The hairless mouse skin was carefully mounted on the lower half of the Franz cell with the dermis facing downwards and the stratum corneum side in contact with the plaster. The upper and lower parts of the Franz cell were sealed with Parafilm® and fastened together by means of a clamp, with the membrane acting as a barrier between the donor and receptor compartments. The diffusion area and the volume of the receptor compartment are 1.766 cm2 and about 7 mL, respectively. The receiver volume of each cell was individually calibrated. The receiver compartment was filled with a freshly prepared degassed pH 7.4 phosphate-buffered saline. Before using, the receptor solution was sonicated to remove dissolved air. Special care was taken to avoid the formation of air bubbles between the solution and the membrane in the receptor compartment. The Franz cells were kept at 37°C throughout the experiment, so that the skin surface temperature was 32 ± 1°C. Only the receptor compartment was in contact with the circulating water at 37°C and each Franz cell was equipped with a stirring magnet. At predetermined times, 0.3 mL samples were withdrawn from the receiver compartment and immediately replaced with fresh receiver medium. Sink conditions were maintained throughout the experiment. The withdrawn samples were assayed directly by an HPLC method to determine the concentrations of the compound that had permeated through the membrane. All values are the averages of three parallel experiments.
Permeation Parameters
Permeation parameters were interpreted plotting the cumulative drug per unit skin area (Q/A) versus time. The gradient and x-intercept of the linear portion of the plot yielded steady-state flux (Jss) and lag time (t), accordingly.
The enhancement ratio (ER), namely the ratio between Jss in the presence and absence of penetration enhancers, measured the enhancement in NSAIDs penetration (19):
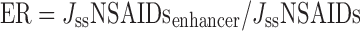 |
Test for significant differences between means were performed by Student’s t test. Differences were considered significant at the P < 0.05 level.
HPLC Analysis
The KTP contents in the plasters and the drug concentrations in the medium were determined by an HPLC method. HPLC system was an HP 1100, Chemstations (Hewlett Packard, USA).
The column was a Xterra, C18, 150 × 4.6 mm, 3.5 μm (Waters, USA) which was kept at 25°C, with a specific precolumn. The mobile phase was 0.05% acetonitrile/trifluoroacetic acid (55:45%, v/v). The flow rate was set to 1 mL/min. Six concentrations of the standard compound were injected at 5 μL (the ranges for UV 233 nm detection are 5–30 μg/mL) The linear regression equation of the standard curve, determined in duplicate, was obtained by plotting the amount of the standard compound injected against the peak area.
ATR-FTIR Spectroscopy
At the end of skin permeation experiments, the full-thickness hairless mouse skin was removed from the Franz cell and the plaster specimens were carefully eliminated. Solution, when used, was completely removed before the cell was disassembled. The skin was wiped by filter paper to remove any traces adhering to the surface and dried over 24–48 h in desiccator to eliminate water. A not-treated membrane was used as such as a control. FTIR spectra of control and all treated membranes were recorded in triplicate on the ATR accessory of the spectrometer SpectrumOne™ (PerkinElmer, USA) equipped with a diamond crystal with the following parameters: resolution of 2 cm−1 and number of 64 scans (n = 3).
RESULTS
In Vitro Skin Permeation Experiment: Influence of the Polar Head
The average drug content of KTP in medicated plaster was 951.14 ± 20.21, indicating that the drug concentration was equivalent in each series (20).
The skin permeation profiles of KTP from medicated plasters were linear and the fluxes, J, are reported in Table I. As an example, the permeation profiles obtained by KTP-medicated plasters containing 10% w/w surfactants are reported in Fig. 1.
Fig. 1.
Permeation profiles of KTP in plasters containing 10% w/w PEG8-S (plaster K5), PEG12-S (plaster K6), and PEG40-S (plaster K7)
The addition of PEG40-S never increased KTP flux with respect to the control plasters, plaster K1A.
The KTP fluxes (Table I) obtained from formulations including PEG8-S or PEG12-S were significantly higher (p < 0.05) than that obtained from the control formulation (plaster K1A) with the exception of plaster K2 containing 5% w/w PEG8-S. Moreover, the KTP flux linearly increased according to the amount of enhancer loaded in the plaster (PEG8-S: r2 = 0.9871; PEG12-S: r2 = 0.9976).
Generally speaking, the permeated amounts of KTP were not deeply increased and the enhancing effects of PEG8-S and PEG12-S were slightly evident on the KTP permeability (Table I). Indeed, ER ranged from 1.12 (plaster K2) to 2.23 (plaster K8).
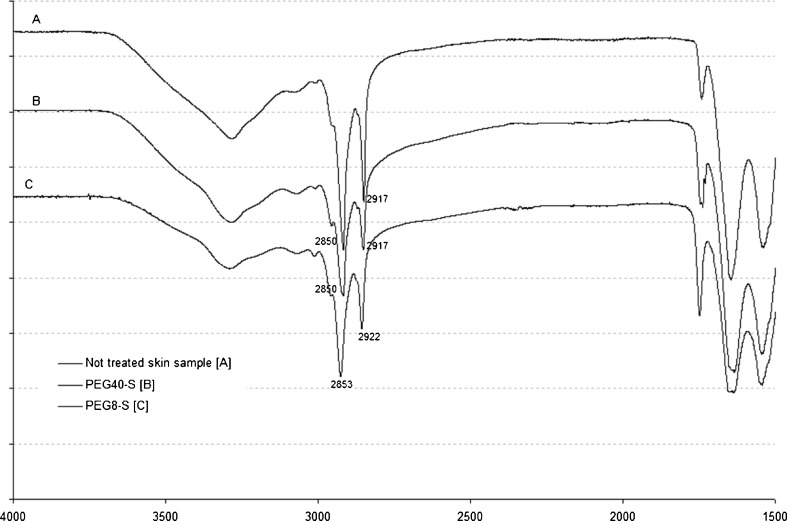
To prove the presence of the three PEG stearates into hairless mouse skin, an ATR-FTIR analysis was carried out on the membranes kept in contact with plasters containing 20% w/w enhancers during the skin permeation test (Table II). Significant shifts in the peak position of the CH asymmetric stretching absorbance at 2,917 cm−1 and the CH symmetric stretching absorbance at 2,850 cm−1 were evident when PEG8-S or PEG12-S were used. PEG40-S did not cause any peak shifts, both when loaded in the plaster or in aqueous solution, suggesting that this surfactant did not enter into the stratum corneum neither modified the conformation of skin lipids (Fig. 2).
Table II.
Effect of PEG-S on Asymmetric or Symmetric C–H Stretching Frequencies ATR-FTIR Spectroscopy
| Content (%) | Asymmetric stretching (2,920 cm−1) | Asymmetric stretching (2,850 cm−1) | |
|---|---|---|---|
| Control | 0 | 2,917.08 ± 0.16 | 2,849.65 ± 0.09 |
| PEG8-S | 20 | 2,922.41 ± 0.58a | 2,852.78 ± 0.61a |
| PEG12-S | 20 | 2,923.06 ± 0.19a | 2,853.44 ± 0.15a |
| PEG40-S | 20 | 2,917.11 ± 0.48 | 2,849.73 ± 0.12 |
Mean values significantly different vs. not treated membrane used as control
Fig. 2.
ATR-FTIR spectra of SCE after skin permeation test
In Vitro Skin Permeation Experiment: Influence of the Hydrophobic Chain
To assess the influence of the hydrophobic chain of PEG derivatives, the skin permeation experiments of the control plaster were carried out twice and the results were not statistically different (Table I, plasters K1A and K1B).
The average KTP content of the medicated plasters containing PEG derivates was 930.14 ± 20.00 μg/cm2. The KTP fluxes significantly higher (p < 0.05) than that of the control (namely plaster K1B) were measured for plasters K13 and K19, i.e., 5% and 10% w/w PEG8-ML, K17 and K20, i.e., 10% w/w PEG8-MO, PEG8-DO, and PEG8-DL, K16 and K22, i.e., 5% and 10% w/w PEG12-DO (Table I). The lowest surfactant concentration (5% w/w) resulted suitable to significantly increase the KTP permeation only in presence of PEG8-ML and PEG12-DO contained in plasters K13 and K16, respectively. Increasing the enhancer concentration from 5% to 10%, the flux significantly increased only by using PEG8-MO, PEG8-DO, and PEG8-DL. PEG8 laurate or oleate showed fluxes significantly different at the highest concentration and only in the case of the monoesters. Comparing mono- or di-substituted nonionic surfactants, only plaster K13 vs. K14 and plaster K21 vs. K22 had fluxes significantly different. In all cases, the KTP-permeated amounts were not deeply increased, as ER resulted in the range 1.07 (plaster K15)–2.20 (plaster K22) (Table I). Significant shifts in the peak position of the CH asymmetric stretching absorbance at 2,917 cm−1 and the CH symmetric stretching absorbance at 2,850 cm−1 were evident in case of PEG derivatives with fluxes different from control (data not shown).
DISCUSSION
The current results underlined the relevance of the polar head structure of PEG stearates in the enhancement process of a lipophilic compound, namely KTP. PEG40-S was unable to increase the fluxes of this compound. On the contrary, PEG12-S and PEG8-S were able to promote the skin penetration of KTP according to its concentrations in the medicated plasters. At the highest surfactant concentration the fluxes resulted higher in the case of PEG8-S than in that of PEG12-S.
In accordance with literature data, the enhancement effect of PEG stearates could be mainly due to their ability to penetrate into the intercellular regions of the stratum corneum, increase fluidity, and eventually solubilize and extract lipid components. After treating skin by PEG40-S, both loaded in plaster and used in solution, the lack of modifications on the diagnostic bands in the ATR-FTIR spectra with respect to the untreated samples confirmed its absence into the skin. In the case of PEG8-S and PEG12-S, the modifications in the ATR-FTIR spectra (Table II) were in agreement with the results of the skin permeability. Shin et al. evidenced similar correlation between the enhancer effectiveness, expressed as ER, of three nonionic polyoxyethylene ethers in poloxamer gels and the thermal behavior of rat skin samples (21).
The lack of the enhancement effect of PEG40-S on skin permeability of KTP could be due to the extremely high hydrophilicity (HLB ≅ 16.0) or to the large volume associated with the hydrophilic head group, a polyoxyethylene chain of n = 40, which inhibited the penetration of the surfactant into the skin as supported by the results obtained from the solution. This data is consistent with those reported by Lopez A. et al. (22) which reported more effective enhancing properties of Span®20 than Tween®20 on 5-FU skin penetration. The authors suggested that the large volume associated with the hydrophilic head group of Tween®20 could impede the penetration into the tails of the lipid bilayers of the stratum corneum. The dependency of the nonionic surfactants efficacy in improving skin penetration and the ethylene oxide chain length (E) was also described by Walters K.A. et al. (23) studying the influence of a series of polyethoxylated nonionic surfactants upon the transport of methyl nicotinate, a freely soluble molecule, across HMS as a membrane. The series of surfactants having the constant alkyl chain of C16 and an E chain from E6 to E14 increased the methyl nicotinate permeability coefficient up to 50%, while a minor influence on the permeability coefficient was measured in the case of ethylene oxide chain longer than E20.
The influence of a series of nonionic surfactants varied in the length of both alkyl chain and E chain, on the transport of ibuprofen across rat skin was also investigated by Park et al. (9). At a constant alkyl chain length of 18, E(2)oleyl ether and E(10)stearyl ether showed a similar enhancing effect (3.20 and 3.14-fold, respectively), that are the highest within this series. In the stearyl ether series, the effect of the enhancers was in the order E(2)stearyl ether (1.61-fold) < E(100)stearyl ether (2.73-fold) ≅ E(20)stearyl ether (2.75-fold) < E(10)stearyl ether, while in the oleyl ether series, the order was E(20)oleyl ether (2.78-fold) < E(2)oleyl ether. In the case of the saturated chain (stearyl alcohol), very short or high E chain had the tendency to be less effective than E(10), as observed in our experiment (stearic acid) of KTP and at 5% w/w enhancers (Table I). Meanwhile, in all other experiments the effect was similar to that of the oleyl series (9): the shortest chain surfactants seemed to perform better than the longest one, that means fluxes obtained by using PEG8-S were greater than or equal to PEG12-S and the effect of E40 was always deeply lower than the others (Table I).
The experimental results obtained by using KTP plasters containing nonionic surfactants differing in alkyl-chain length showed that the enhancer concentration was relevant for the effect of the PEG derivatives; in most of the cases, the enhancement effect was measured at the percentage of 10% w/w while the KTP percutaneous absorption was rarely enhanced at the lowest concentration (Table I).
It was confirmed that small E chain could contribute to the enhancement effect of the surfactant, according to literature data (24) but the dependence of the enhancement effect on the lipophylic portion was not so marked. Two different patterns were found considering the E chain equal to 8 or 12 and mono- or di-derivatives; the PEG8 monoderivatives permitted to obtain higher ER values than PEG12, while for dioleate trend resulted opposite (Table I).
By comparing the fluxes obtained from plasters containing nonionic surfactants having the same number of hydrophobic chain, polar head group, i.e. PEG8, and percentage, plasters K17, namely monoleate, showed values significantly different from those obtained by using plasters K19 (p = 0.04), but also K5 (p = 0.01). The difference between the two saturated fatty acid were not significant. The trend of the fluxes of plasters containing PEG8 was always unsaturated C18 > saturated C12, even if the difference between unsaturated C18 and saturated C12 fatty acid was significant only in few cases. In the case of the PEG12 monoester, the opposite trend was observed. In general, the enhancer potency, expressed as ER values, of the laurate and stearate esters was lower than those of the oleate esters (Table I).
If the effect of the corresponding one and two hydrophobic chain enhancers was considered, only in a limited number of cases significant differences were measured in the flux values by doubling the same hydrophobic chain, namely plasters K13 vs. K14 (p = 0.02) and plasters K21 vs. K22 (p = 0.02). In this cases, the order between the fluxes obtained from analogues differing in number of hydrophobic chain was not similar, as plasters K13 > K14 and K21 < K22. This confirm that the size and number of the alkyl chains seemed to be less influent on the enhancing ability of the PEG derivatives when combined with a relatively small E chain.
In the PEG stearate series, the HLB value seemed to be a reliable predictor of the absorption enhancing capability. The less hydrophilic the PEG stearate (PEG8-S = 11.7; PEG12-S = 13.5), the higher the enhancement effect on the skin permeability. But enlarging the set of molecules this resulted quite controversial. Therefore, it seems that the best balance to have the enhancement effect on the KTP skin permeability was in the range 10–14. Our results were different with respect to those of other authors. Park et al. (9) concluded that the nonionic surfactants resulted effective as enhancers of ibuprofen flux contained the E chain length of 2–5, HLB value of 7–9 and an alkyl chain length of C16–C18. Myoung and Choi (25) studied the effect of various vehicles on the skin permeability of isosorbide dinitrate (ISDN). The maximum flux of ISDN was indicated when the HLB value of vehicles was about 7. Furuishi T. et al. (26) investigated the enhancing effect on the percutaneous absorption of pentazocine from the isopropyl myristate solution system improved with glyceryl monocaprylate (GEFA-C8), which is a type of glycerol ester of fatty acid (GEFA). They revealed that the HLB value of GEFAs of about 8 seemed optimal for the enhancing effect on the percutaneous absorption of pentazocine.
It seems clear that a relevant variable is the vehicle. Nonionic surfactant at HLB values ranging 7–9 or lower (27) can penetrate into the skin resulting effective in enhancing the drug penetration when were in solution. In our study, the effect measured by using more hydrophilic surfactant, having higher HLB values, could be due to a best release from the lipophilic matrix of the plaster that is composed of hydrophobic side groups and had a relatively low content of water-soluble monomers. The escaping tendency of a molecule from a vehicle into the stratum corneum increased when decreased its interaction or affinity with the vehicle (28). This can justify the highest HLB values of the PEG derivatives requested to have the optimal performance in these plasters.
CONCLUSION
The experimental results revealed that the surfactant alkyl chain seemed to be less influent than oxyethylene chain length in enhancing KTP permeability. The penetration into the skin and partitioning behavior from the vehicle, that means the potency as enhancer, resulted not so strongly dependent on the hydrophobic portion of the surfactant structure even if the presence of a double bond in the alkyl chain could lead to more efficient enhancers as the fluidity of the stratum corneum is altered (27). The prediction of the absorption enhancing capability of these PEG derivatives appeared therefore related to the vehicle other than the proper combination of the number of ethylene oxide groups and alkyl groups.
REFERENCES
- 1.Florence T, Tucker IG, Walters KA. Interactions of nonionic alkyl and aryl ethers with membranes and other biological systems. In: Rosen MJ, editor. Structure: performance relationships in surfactants. ACS Symp. Ser; 1984; 253 p. 189–207
- 2.Breuer MM. The interaction between surfactant and keratinous tissue. J Soc Cosmet Chem. 1979;30:41–64. [Google Scholar]
- 3.Moser K, Kriwet, Naik A, Kalia YN, Guy RH. Passive skin penetration enhancement and its quantification in vitro. Eur J Pharm Biopharm. 2001;52(2):103–112. doi: 10.1016/S0939-6411(01)00166-7. [DOI] [PubMed] [Google Scholar]
- 4.Nokhodchi A, Shokri J, Dashbolaghi A, Hassan-Zadeh D, Ghafourian T, Barzegar-Jalali M. The enhancement effect of surfactants on the penetration of lorazepam through rat skin. Int J Pharm. 2003;250(2):359–369. doi: 10.1016/S0378-5173(02)00554-9. [DOI] [PubMed] [Google Scholar]
- 5.Shokri J, Nokhodchi A, Dashbolaghi A, Hassan-Zadeh D, Ghafourian T, Barzegar Jalali M. The effect of surfactants on the skin penetration of diazepam. Int J Pharm. 2001;228(1–2):99–107. doi: 10.1016/S0378-5173(01)00805-5. [DOI] [PubMed] [Google Scholar]
- 6.Krasowska H. Solubilities of certain anti-inflammatory compounds in nonionic surfactant solutions. Pharm Ind. 1978;40:1381–1384. [Google Scholar]
- 7.Sarpotdar PP, Zatz JL. Percutaneous-absorption enhancement by nonionic surfactants. Drug Dev Ind Pharm. 1987;13(1):15–37. doi: 10.3109/03639048709040153. [DOI] [Google Scholar]
- 8.Bialik W, Walters KA, Brain KR, Hadgraft J. Some factors affecting the in vitro penetration of ibuprofen through human skin. Int J Pharm. 1993;92(1–3):219–223. doi: 10.1016/0378-5173(93)90283-L. [DOI] [Google Scholar]
- 9.Park E-S, Chang S-Y, Hahn M, Chi S-C. Enhancing effect of polyoxyethylene alkyl ethers on the skin permeation of ibuprofen. Int J Pharm. 2000;209:109–119. doi: 10.1016/S0378-5173(00)00559-7. [DOI] [PubMed] [Google Scholar]
- 10.Kanikkannan N, Kandimalla K, Lamba SS, Singh M. Structure–activity relationship of chemical penetration enhancers in transdermal drug delivery. Curr Med Chem. 2000;7(6):593–608. doi: 10.2174/0929867003374840. [DOI] [PubMed] [Google Scholar]
- 11.Vávrová K, Hrabálek A, Doležal P, Šámalová L, Palát K, Zbytovská J, et al. Synthetic ceramide analogues as skin permeation enhancers: structure–activity relationships. Bioorg Med Chem. 2003;11(24):5381–5390. doi: 10.1016/j.bmc.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Vávrová K, Hrabálek A, Doležal P, Holas T, Zbytovská J. L-serine and glycine based ceramide analogues as transdermal permeation enhancers: polar head size and hydrogen bonding. Bioorg Med Chem Lett. 2003;13(14):2351–2353. doi: 10.1016/S0960-894X(03)00409-8. [DOI] [PubMed] [Google Scholar]
- 13.Morimoto K, Tojima H, Haruta T, Suzuki M, Kakemi M. Enhancing effects of unsaturated fatty acids with various structures on the permeation of indomethacin through rat skin. J Pharm Pharmacol. 1996;8(11):1133–1137. doi: 10.1111/j.2042-7158.1996.tb03908.x. [DOI] [PubMed] [Google Scholar]
- 14.Shahi V, Zatz JL. Effect of formulation factors on penetration of hydrocortisone through mouse skin. J Pharm Sci. 1978;67:789–792. doi: 10.1002/jps.2600670615. [DOI] [PubMed] [Google Scholar]
- 15.Zaslavsky BY, Ossipov NN, Krivitch VS, Baholdina LP, Rogozhin SV. Action of surface-active-substances on biological membranes. II. Haemolytic activity of nonionic surfactants. Biochim Biophys. 1978;507:1–7. doi: 10.1016/0005-2736(78)90368-1. [DOI] [PubMed] [Google Scholar]
- 16.Walters KA, Dugard PH, Florence AT. Nonionic surfactants and gastric mucosal transport of paraquat. J Pharm Pharmacol. 1981;33(4):207–213. doi: 10.1111/j.2042-7158.1981.tb13759.x. [DOI] [PubMed] [Google Scholar]
- 17.Cilurzo F, Minghetti P, Alberti E, Gennari CGM, Pallavicini M, Valoti E, et al. An investigation into the influence of counterion on the RS-propranolol and S-propranolol skin permeability. J Pharm Sci. 2010;99(3):1217–1224. doi: 10.1002/jps.21891. [DOI] [PubMed] [Google Scholar]
- 18.Cilurzo F, Alberti E, Minghetti P, Gennari CGM, Casiraghi A, Montanari L. Effect of drug chirality on the skin permeability of ibuprofen. Int J Pharm. 2010;386(1–2):71–76. doi: 10.1016/j.ijpharm.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 19.Vaddi HK, Ho PC, Chan SY. Terpenes in propylene glycol as skin-penetration enhancers: permeation and partition of haloperidol, Fourier transform infrared spectroscopy, and differential scanning calorimetry. J Pharm Sci. 2002;91(7):1639–1651. doi: 10.1002/jps.10160. [DOI] [PubMed] [Google Scholar]
- 20.Cilurzo F, Minghetti P, Casiraghi A, Tosi L, Pagani S, Montanari L. Polymethacrylates as crystallization inhibitors in monolayer transdermal patches containing ibuprofen. Eur J Pharm Biopharm. 2005;60(1):61–66. doi: 10.1016/j.ejpb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Shin SC, Cho CW, Oh IJ. Effects of nonionic surfactants as permeation enhancers towards piroxicam from the poloxamer gel through rat skins. Int J Pharm. 2001;222:199–203. doi: 10.1016/S0378-5173(01)00699-8. [DOI] [PubMed] [Google Scholar]
- 22.López A, Llinares F, Cortell C, Herráez M. Comparative enhancer effects of Span®20 with Tween®20 and Azone® on the in vitro percutaneous penetration of compounds with different lipophilicities. Int J Pharm. 2000;202(1–2):133–140. doi: 10.1016/S0378-5173(00)00427-0. [DOI] [PubMed] [Google Scholar]
- 23.Walters KA, Walker M, Olejnik O. Nonionic surfactant effects on hairless mouse skin permeability characteristics. J Pharm Pharmacol. 1988;40(8):525–529. doi: 10.1111/j.2042-7158.1988.tb05295.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim HS, Oh SY. Effect of polyoxyethylene alkyl esters on permeation enhancement and impedance of skin. Biomol Ther. 2011;19(1):109–117. doi: 10.4062/biomolther.2011.19.1.109. [DOI] [Google Scholar]
- 25.Myoung Y, Choi HK. Effects of vehicles and pressure sensitive adhesives on the penetration of isosorbide dinitrate across the hairless mouse skin. Drug Deliv. 2002;9:121–126. doi: 10.1080/10426500290095430. [DOI] [PubMed] [Google Scholar]
- 26.Furuishi T, Oda S, Saito H, Fukami T, Suzuki T, Tomono K. Effect of permeation enhancers on the in vitro percutaneous absorption of pentazocine. Biol Pharm Bull. 2007;30(7):1350–1353. doi: 10.1248/bpb.30.1350. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Lee CH, Choi HK. Transdermal delivery of physostigmine: effects of enhancers and pressure-sensitive adhesives. Drug Dev Ind Pharm. 2002;28(7):833–839. doi: 10.1081/DDC-120005629. [DOI] [PubMed] [Google Scholar]
- 28.Roberts MS, Cross SE, Pellett MA. Skin transport. In: Walters KA, editor. Dermatological and transdermal formulations. New York: Marcel Dekker; 2002. pp. 100–111. [Google Scholar]