Abstract
The aim of the present investigation was to evaluate microemulsion as a vehicle for dermal drug delivery and to develop microemulsion-based gel of terbinafine for the treatment of onychomycosis. D-optimal mixture experimental design was adopted to optimize the amount of oil (X1), Smix (mixture of surfactant and cosurfactant; X2) and water (X3) in the microemulsion. The formulations were assessed for globule size (in nanometers; Y1) and solubility of drug in microemulsion (in milligrams per milliliter; Y2). The microemulsion containing 5.75% oil, 53.75% surfactant–cosurfactant mixture and 40.5% water was selected as the optimized batch. The globule size and solubility of the optimized batch were 18.14 nm and 43.71 mg/ml, respectively. Transmission electron microscopy showed that globules were spherical in shape. Drug containing microemulsion was converted into gel employing 0.75% w/w carbopol 934P. The optimized gel showed better penetration and retention in the human cadaver skin as compared to the commercial cream. The cumulative amount of terbinafine permeated after 12 h was 244.65 ± 18.43 μg cm−2 which was three times more than the selected commercial cream. Terbinafine microemulsion in the gel form showed better activity against Candida albicans and Trichophyton rubrum than the commercial cream. It was concluded that drug-loaded gel could be a promising formulation for effective treatment of onychomycosis.
Key words: D-optimal design, microemulsion, onychomycosis, permeation, terbinafine
INTRODUCTION
Onychomycosis is the fungal infection which affects the nail plate and nail bed. It affects 14% of the total world population, with more prevalence in elders (1). Onychomycosis causes the discoloration, thickening and hardening of the nail. The conventional treatment consists of oral and systemic medication for several months. Approximately, 50% of the nail diseases account for onychomycosis. Onychomycosis is caused by dermatophytes viz. Trichyphyton rubrum or nondermatophytes molds. Yeasts (Candida albicans, Candida parapsilosis) are also responsible in some cases. Toe nails are frequently affected than finger nails, due to their slower growth and more susceptibility to infections (2,3).
Drug delivery systems for fungal infections in nail area have been widely studied during the last few years. The topical therapy for onychomycosis has limited success due to the poor permeability of topically applied drugs through the nail plate. Conventional treatment of onychomycosis consists of (a) topical and/or systemic antifungal drug delivery and (b) surgical or chemical nail avulsion or (c) combination of these. Since, nail avulsion is painful and traumatic, systemic and topical antifungal therapy has remained as the only choice for onychomycosis treatment (4,5). Antifungal drugs like itraconazole, miconazole, ketoconazole, and terbinafine have been used orally but they are not widely accepted due to hepatic side effects, high relapse rate and long duration of treatment. Alternatively, topical therapy by drug permeation into nails by iontophoresis has also been researched (6–9), but it was proved to be a costly process and has limited success.
Diseased nail plate could detach from the nail bed (onycholysis) within few weeks as the disease progresses; which imposes a resistance to drug permeation from formulation to nail bed (10). But, onycholysis creates the cavity between the nail plate and nail bed, where drug formulations could be applied, thus providing a direct contact of drug with the nail bed facilitating the drug delivery on the infected areas over and under the nail bed more easily. The present investigation deals with the formulation of microemulsion-based gel of terbinafine for application in to the detached space for effective treatment of onychomycosis. Terbinafine (TF); ((E)-N-(6,6-dimethyl-2-hepten-4-yn-yl)-N-methyl-1-naphthalenemethanamine hydrochloride) is an antifungal agent of the allylamine class which selectively inhibits fungal squalene epoxidase. It is active against dermatophytes, fungi, molds, and yeasts and is used for both oral and topical treatment of mycoses (11,12).
Microemulsions (ME) are transparent and thermodynamically stable as their droplet size range from 10 to 100 nm and they don’t coalesce (13,14). MEs are composed of oil, surfactant, cosurfactant, and water in specific proportions. It is a promising carrier of drugs for both transdermal and dermal drug deliveries as large amount of drug could be incorporated in the formulation and the ingredients of microemulsion could facilitate the permeation rate of the drug by reducing the diffusion barrier of the stratum corneum (15,16). But, as microemulsion has low viscosity, their retention in the affected part is quite less. Thus, the viscosity of the microemulsion could be increased by addition of gelling agents (17–20).
The present study was undertaken to develop and optimize the formulation of terbinafine microemulsion for topical use using D-optimal design. The secondary objective was to prepare the microemulsion with smaller globule size and moderate drug solubility. Performance-related in vitro tests such as permeation study and ex vivo antifungal study were carried out for the formulated microemulsion in gel form as well as for the market formulation containing the same drug.
MATERIALS AND METHODS
Materials
Terbinafine was obtained as a gift sample from Cadila Pharmaceuticals Ltd. (Ahmedabad, India). Isopropyl myristate (IPM) was received as a gift sample from Bombay Tablets Pvt. Ltd. (Gandhinagar, India). Olive oil, oleic acid, and castor oil were purchased from National Chemicals (Vadodara, India). Tween 20, Tween 80, propylene glycol, and Cremophor EL were purchased from Sigma Aldrich (Mumbai, India). Methanol was purchased from Baroda Chemicals Ltd. (Vadodara, India). Capmul MCM was received as gift from Abitec Corporation (OH, USA). Labrasol, Labrafac, and Transcutol P were obtained as gift samples from Gattefosse (Lyon, France). Carbopol 934P was purchased from Corel Pharma (Ahmedabad, India) T. rubrum (MTCC No. 296) and C. albicans (MTCC No. 3018) were procured from Institute of Microbial Technology (Chandigarh, India). Double distilled water was used throughout the study. All other chemical reagents and solvents used were of analytical grade.
Screening of Components for MEs
The solubility of TF in various oils such as oleic acid, IPM, olive oil, and castor oil; surfactants including Tween 20, Tween 80, Labrasol, Labrafac, and Cremophor EL; and cosurfactants like Capmul MCM, Transcutol P and propylene glycol was determined. Excess amount of TF was added in 3 ml of oil or surfactant or cosurfactant in 5-ml-capacity stoppered vials separately and the resultant mixture was mixed initially by vortex mixer. The vials were then shaken on magnetic stirrer (Remi Instruments, Mumbai) for 72 h followed by centrifugation (Remi centrifuge, Mumbai) at 10,000 rpm for 15 min. The supernatant was filtered through a 0.45 μm filter membrane and the concentration of TF in filtrate was determined by UV spectrophotometer (UV 1800, Shimadzu) after appropriate dilution with methanol at its respective λmax. Appropriately diluted solutions of oil, surfactants and cosurfactants in methanol were taken as blank. The components that showed highest solubility of TF were used for further studies.
Compatibility Studies
The oil and surfactant blends that showed highest solubility were assessed for the compatibility. The mixtures of chosen oil and surfactant were prepared at 1:3, 1:2, 1:1, 2:1, and 3:1, respectively. The mixtures were mixed for 5 min using a vortex mixer and were evaluated for the physical appearance (clarity and absence of opacity). Similarly, compatibility study between the selected cosurfactant and the mixed system of oil and surfactant was tested by preparing mixtures of cosurfactant and mixed system of oil and surfactant at 1:3, 1:2, 1:1, 2:1, and 3:1, respectively. The blends were assessed for physical appearance (21).
Construction of Pseudo-Ternary Phase Diagrams
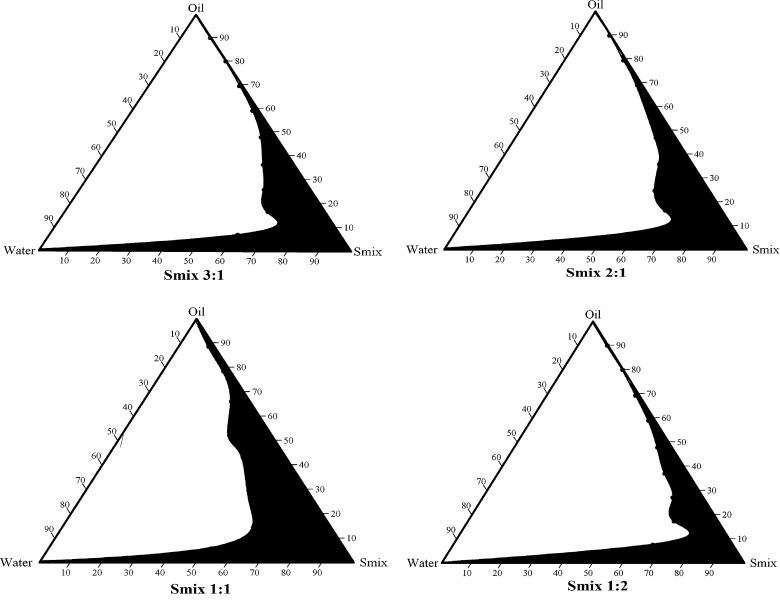
In order to obtain the concentration range of components for the existing range of microemulsions, pseudo-ternary phase diagrams were constructed. The ratio of surfactant to cosurfactant (Smix) was altered at 3:1, 2:1, 1:1, and 1:2. For the construction of pseudo-ternary phase diagram at each Smix ratio, the oily mixtures containing oil, surfactant and cosurfactant were prepared with volume ratio of oil to Smix at 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1, respectively. Double distilled water was added drop by drop to the oil and Smix mixture under magnetic stirring at ambient temperature. Transparent and clear microemulsion was taken as the end point of aqueous titration method. The concentrations of components were then calculated in order to plot the pseudo-ternary phase diagram (Fig. 1).
Fig. 1.
Pseudo-ternary phase diagrams of MEs composed of oil (oleic acid), Smix (surfactant: Labrasol, cosurfactant: Transcutol P) and water at various oil/Smix ratios (3:1, 2:1, 1:1, and 1:2)
Preparation of TF-Loaded Microemulsions
From the pseudo-ternary phase diagrams, Smix ratio with maximum microemulsion region was selected. Different proportions of oil and Smix were mixed depending upon the design points of the D-optimal design space (Table I). Terbinafine was dissolved in the mixture of oil and Smix under magnetic stirring at ambient temperature. Appropriate amount of double distilled water was added drop wise to the oily mixture until clear and transparent liquid was obtained. The mixture was allowed to stabilize and attain the equilibrium with gentle magnetic stirring for 15–20 min. All MEs containing TF were then stored at ambient temperature.
Table I.
Experimental Runs of D-optimal Design and Their Responses
| Experimental runs | X 1 (%) | X 2 (%) | X 3 (%) | Y a1 (nm) | Y a2 (mg/ml) |
|---|---|---|---|---|---|
| 1 | 10.00 | 46.01 | 43.99 | 46.61 ±2.40 | 41.24 ± 1.12 |
| 2 | 10.00 | 60.00 | 30.00 | 35.30 ± 1.06 | 51.89 ± 1.90 |
| 3 | 10.00 | 40.00 | 50.00 | 48.61 ± 1.48 | 38.22 ± 1.13 |
| 4 | 6.49 | 40.00 | 53.51 | 17.78 ± 1.68 | 34.52 ± 0.78 |
| 5 | 10.00 | 60.00 | 30.00 | 39.37 ± 0.79 | 51.35 ± 1.14 |
| 6 | 10.00 | 40.00 | 50.00 | 45.80 ± 1.52 | 37.52 ± 1.88 |
| 7 | 5.00 | 49.92 | 45.08 | 25.80 ± 1.51 | 40.07 ± 1.45 |
| 8 | 10.00 | 55.11 | 34.89 | 39.35 ± 0.84 | 48.63 ± 0.88 |
| 9 | 6.49 | 40.00 | 53.51 | 24.61 ± 1.50 | 34.53 ± 1.40 |
| 10 | 5.00 | 46.25 | 48.75 | 27.27 ± 2.82 | 37.08 ± 0.87 |
| 11 | 7.92 | 51.53 | 40.55 | 22.68 ± 1.48 | 44.24 ± 2.00 |
| 12 | 5.00 | 60.00 | 35.00 | 16.52 ± 0.67 | 47.73 ± 0.47 |
| 13 | 5.00 | 56.08 | 38.92 | 18.41 ± 1.14 | 44.51 ± 1.11 |
| 14 | 7.92 | 51.53 | 40.55 | 22.67 ± 0.57 | 44.16 ± 0.82 |
| 15 | 5.00 | 60.00 | 35.00 | 15.43 ± 0.92 | 47.77 ± 1.48 |
| 16 | 5.00 | 43.40 | 51.60 | 25.23 ± 1.14 | 33.76 ± 1.86 |
aMean of 3 ± SD
Optimization of ME Formulation
The levels of experimental design could not be chosen arbitrarily, where the composition is a factor of interest, because the sum of all the fractions of components equals to unity. Classical experimental designs do not consider specific experimental constraints, and thus they will not have the better prediction power (22,23). For instance, the possible experimental runs are displayed by an equilateral triangle in a three component mixture design, where the real value of the responses could be then represented as distance orthogonal to factor space. Moreover, the range covering the components is limited in the design space, which could be represented by irregular polyhedron delimited by extreme vertices. In such cases, D-optimal design would be appropriate, as maximum prediction power could be obtained in selected set of experimental runs, minimizing the variance associated with the estimates of the coefficients in the model (22–24).
In the present study, the levels of the three independent variables; oil (X1), Smix (X2), and water (X3) were chosen on the basis of pseudo-ternary phase diagrams and preliminary experiments. As higher amount of oleic acid causes irritation of skin, its maximum level was restricted to 10%. Hydration effect of stratum corneum has significant effect on the penetration of the drug into skin. But, our objective was to retain the drug into the skin, so the water content was restricted to 55%. The range of the components for the design was selected as follows:
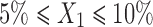 |
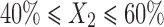 |
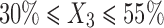 |
The globule size (in nanometers; Y1) and solubility of TF in the MEs (Y2) were selected as the dependent variables (responses). The globule size (Y1) was determined using photon correlation spectroscopy (Zetasizer 1000 HS, Malvern Instruments, UK) at 25°C. The solubility of TF in ME (Y2) was determined by extracting TF in methanol, which was analyzed using UV spectrophotometer. The plain microemulsion without drug with the same composition was taken as blank. The drug-loading capacity of the MEs was estimated on the basis of recorded absorbance using the following equation (25):
 |
1 |
The Design-Expert® software (Version 8.0.4; Stat-Ease, Inc., MN, USA) was employed to treat the responses. The software selected a set of candidate points as a base design consisting of factorial points, constraints on each factor, centers of edges, constraint plane centroids, axial check point, and an overall center point. The base design consisted total 16 runs. Suitable mathematical models of the mixture design such as linear, quadratic and special cubic models were analyzed by the software. Significance of the model was determined by comparisons of statistical parameters like standard deviation (SD), R2, adjusted R2, predicted R2, and predicted residual error sum of squares (PRESS). The best model was decided on the basis of higher values of adjusted R2 and predicted R2. Moreover, adjusted R2 and predicted R2 should be within 0.2 of each other to ensure the validity of the model. PRESS value should be small for the best model. PRESS demonstrates the excellence of model fitting. The optimum formulation was selected, which had the globule size (Y1) less than 20 nm and TF solubility (Y2) between 40 and 45 mg/ml.
Physico-Chemical Characterization of MEs
The structure and morphology of MEs were studied using transmission electron microscopy (Tecnai 20, Philips, Holland). One drop of appropriately diluted sample was directly deposited on holey film grid, was allowed to dry and examined under the microscope. The percentage transmittance of MEs was measured using UV spectrophotometer at 650 nm.
The refractive index of MEs was measured using Abbe refractometer (Bausch and Lomb, New York, USA) by placing one drop of ME on the slide. Isotropic nature of the MEs was verified by placing a drop of it on the glass slide with cover slip on it which was observed under cross polarized light using polarizing microscope (Carl Zeiss, Germany). Electrical conductivity of MEs was determined using a conductivity meter (CM-180 ELICO, India). The viscosities of MEs and microemulsion-based gel (MBG; formulated from optimized ME formulation) were determined as such without dilution using Brookfield DV III Rheometer (Brookfield Engineering Labs, USA) with spindle LV III in 30 g samples using small sample holder. The pH of optimized ME was determined at 25°C temperature using pH meter (Electroquip, Delhi).
Formulation and Characterization of ME-Based Gel of TF
Microemulsions when applied in the cavity between the nail plate and nail bed, tends to drain out from the edges and hence the amount of drug reaching the target site would be quite less. Thus, the viscosity of MEs was required to be increased with suitable gelling agent. Optimized ME formulation was selected to formulate MBG of TF. Carbopol 934P was selected as a gelling agent. Carbopol 934P was allowed to hydrate in sufficient quantity of water for 24 h and optimized ME of TF was gradually added under magnetic stirring. The pH of the mixture was then adjusted to 6–7 with triethanolamine and clear MBG was formed. The final MBG formulations contained 0.5%, w/w TF.
In Vitro Permeation Studies
In onychomycotic nails, infection resides predominantly in nail bed. The nail bed is a skin consisting of non-cornified soft tissue which is structurally similar to the skin of the foot region (26). Thus, human cadaver skin from the foot region was used for the in vitro permeation studies. Full thickness dermatomed human cadaver skin from the foot region of Asian subjects was received from N.H.L. Municipal Medical College (Ahmedabad, India). The skin was stored in 10% glycerin solution at temperature of −20°C till the experiments were completed. The skin was inspected for any holes or irregularities. The skin was prepared by hydrating it in acetate buffer for 1 h at room temperature and then it was cut into 3.3 × 3.3 cm2, for the permeation study.
A Franz diffusion cell with an effective diffusion area of 7.1 cm2 was used for the experiment. The human cadaver skin was placed between the donor and receptor compartments of Franz diffusion cell with the stratum corneum facing donor compartment. Optimized ME formulation of TF (4.44%, w/w TF), MBG (0.5%, w/w TF) and market formulation (MF; containing 1%, w/w TF) were taken equivalent to 5 mg TF and were placed on stratum corneum and the release profiles were taken. The receptor chamber was filled with 25 ml of physiological saline solution (pH 5.5 acetate buffer/methanol (9:1)). The receptor medium was maintained at 37 ± 1°C and was magnetically stirred at 50 rpm. Samples were withdrawn at predetermined time intervals, filtered through 0.45 μm pore size cellulose membrane filter and were analyzed by UV spectrophotometer. Fresh buffer solution was immediately replaced into the receptor chamber after each sampling. Cumulative amount of drug in receptor chamber for the three formulations (ME, MBG, and MF) was plotted as a function of time (t, h). The cumulative amount of TF permeated through the skin was determined as per the following equation:
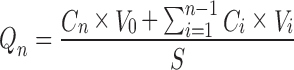 |
2 |
Where, Cn is the drug concentration of receptor medium after each sampling time, Ci is the drug concentration for ith sample, V0 and Vi are the volumes of the receiver solution and sample, respectively, and S is the effective diffusion area (27).
Ex Vivo Antifungal Study
The cylinder plate method was selected for the antifungal study. MF and MBG were evaluated for their antifungal activity using two fungal strains, T. rubrum (MTCC No. 296) and C. albicans (MTCC No. 3018). T. rubrum was cultivated on sabouraud dextrose media (Himedia, India) at 25°C for 7 days, while C. albicans was also cultivated on sabouraud dextrose media at 30°C for 2 days. The spores were harvested with spatula, and were suspended in 20 ml media and finally filtered through sterile gauze. One milliliter of the inoculated media was added to 100 ml of sabouraud dextrose agar at 37 ± 1°C. Ten milliliters of the inoculated media was added to solidified agar media in the petri dish. Three wells in the petridish were bored and filled with 0.1 g each of MBG and MF containing TF and control (distilled water). Two petridishes were used for two different strains. Petridish with T. rubrum was incubated at 25°C for 7 days, while petridish containing C. albicans was incubated at 30°C for 2 days. Zone of inhibition diameter (in millimeters) was then recorded and compared. The experiments were performed in sterilized area.
Statistical Analysis
Data were represented as mean ± SD (n = 3). Data were analyzed statistically by Student’s t test at 5% significance level using GraphPad Prism 5 program (GraphPad Inc., USA)
RESULTS AND DISCUSSION
Screening of Components for MEs
The solubility of TF in various oils, surfactant and cosurfactants was estimated for screening the components for MEs containing TF. Solubility of TF was highest in oleic acid (94.13 ± 2.42 mg/ml) among the four oils, followed by olive oil (82.25 ± 3.13 mg/ml) and castor oil (75.64 ± 1.98 mg/ml); while in isopropyl myristate, TF has the lowest solubility (37.5 ± 2.56 mg/ml). Oleic acid was selected for further study owing to its solubility profile. Moreover, oleic acid has the potential to enhance the dermal permeation, as it renders increase in fluidity of the lipid portion of stratum corneum (28–30).
Terbinafine showed highest solubility in Labrasol (58 ± 0.37 mg/ml). It has been reported that surfactants could cause skin irritation, when applied topically. Hence, it is vital to use the surfactant as minimum as possible. Toxicity levels are relatively low for non-ionic surfactants like Labrasol (31), hence it was selected as a surfactant for further studies. Transcutol P demonstrated higher solubility of TF (85.4 ± 1.96 mg/ml) among the tested cosurfactants; hence it was selected for further study. The blends of oil and surfactant and blend of oil, surfactant and cosurfactant showed miscibility and transparency. Globule separation and/or precipitate formation was not noticed.
Construction of Pseudo-Ternary Phase Diagrams
In order to obtain the appropriate components and their concentration ranges for MEs, the pseudo-ternary phase diagrams were constructed for different Smix ratio, 3:1, 2:1, 1:1, and 1:2. The ratio which provided stable and clear solutions was selected. With the help of phase diagram, relationship between the phase behavior of its mixture and components could be explained. From the four phase diagrams (Fig. 1), the largest ME region was observed in Smix 1:1. The ME region showed the expansion as the proportion of Labrasol was decreased, while in the phase diagram of 1:2, the ME region has contraction. This is due to the fact that reduction of o/w interface is sometimes not possible by single-chain surfactants alone. The combination of short to medium chain length alcohols (e.g. Transcutol P) with single-chain surfactants could result in lowering of interfacial tension, which is attributed to increased fluidity at the interface. Miscibility of the aqueous and oily phases could also be increased by medium chain length alcohols due to their partitioning behavior between the phases (13,31).
Optimization of ME Formulation
Table I shows the composition of 16 experimental runs. The responses (Y1, globule size (in nanometers) and Y2, solubility (in milligrams per milliliters) were determined after 1 h of the ME formation, in order to stabilize them. As shown in the table, lowest globule size (Y1) was obtained with run 15 (X1, 5%; X2, 60%; X3, 35%), where proportion of oil was lowest while that of Smix was highest. It implies that as the proportion of oil decreases, the globules tend to constrict and get stabilized with maximum amount of Smix. The highest globule size was 48 nm. The wide range of globule size in the design space implies the effect of selected independent variables on the globule size. On the contrary, solubility (Y2) was highest when proportion of oil was at high level (run 2; X1, 10%; X2, 60%; X3, 35%). This is due to the increased amount of solvent necessary to solubilize the TF in the system. Graphical presentation of the data is easy to interpret and hence Figs. 2a and b were drawn to display the results.
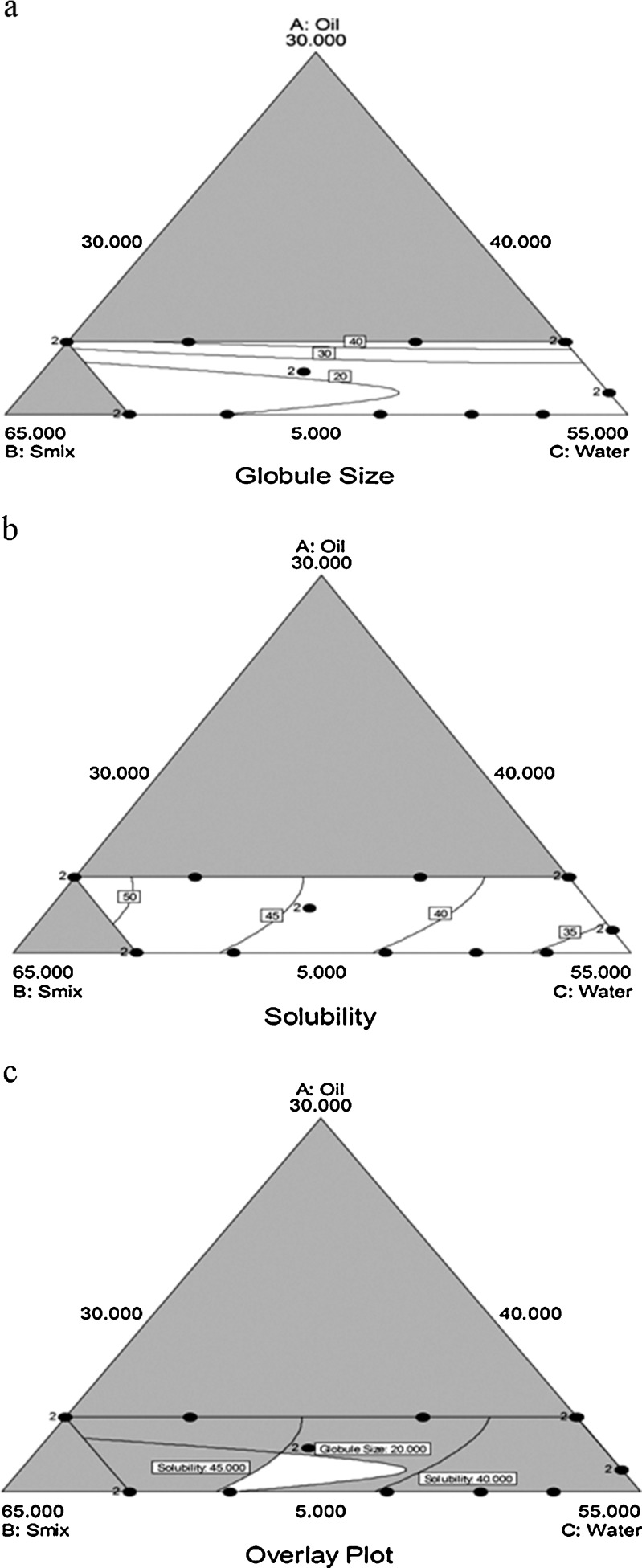
Fig. 2.
Contour plots: a the effect of variables on globule size (Y 1) b effect of variables on solubility (Y 2) c Overlay plot for the effect of variables on globule size (Y 1) and solubility (Y 2)
The relationship between responses (dependent variables) and factors (independent variables) was established using quadratic equation through statistical analysis of the software, for determining the composition, which yields ME formulation with ideal attributes. The equation that fitted to the data is as follows:
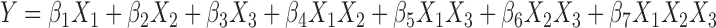 |
3 |
Where, β1 to β7 are the coefficients computed from the observed experimental values of Y. Coefficients with one factor represents the effect of that particular factor while the coefficients with more than one factor represents the interaction between those factors. A positive sign in front of the terms indicates synergistic effects while the negative sign indicates antagonistic effect of the factors. Model equations were calculated, after converting the actual constrains into the L pseudo levels (0 ≤ X1 ≤ 0.2, 0 ≤ X2 ≤ 0.8, 0 ≤ X3 ≤ 1). The conversion was done to overcome the complexity of the non-simplex models, where one of the components (X1) relatively varied lesser than other two components (X2 and X3) (24).
Table II represents the model summary statistics of the responses. Quadratic model showed a superior fit, as adjusted R2 and predicted R2 of it were comparatively higher than the other models, while PRESS for the quadratic model of both the responses were smallest. In Table III, factor effects of D-optimal design and associated p values for the responses are presented. A factor is considered to influence the response, if the effects significantly deviate from zero and the p value is less than 0.05. For response Y1, all the factors were significant, while for response Y2, the factors X1X2 and X2X3 were not significant, as their p values were greater than 0.05. Refined regression equations shown in Table III could be used to calculate the predicted values for other formulations in the design space.
Table II.
Model Summary Statistics of the Measured Responses
| Response | Model | SDa | R 2 | Adjusted R 2 | Predicted R 2 | PRESS |
|---|---|---|---|---|---|---|
| Y 1 | Linear | 5.87 | 0.7682 | 0.7325 | 0.6776 | 623.06 |
| Quadratic | 2.23 | 0.9742 | 0.9613 | 0.9309 | 133.46 | |
| Special Cubic | 2.27 | 0.9761 | 0.9602 | 0.9263 | 142.42 | |
| Y 2 | Linear | 0.59 | 0.9919 | 0.9906 | 0.9872 | 7.09 |
| Quadratic | 0.45 | 0.9963 | 0.9945 | 0.9909 | 5.05 | |
| Special Cubic | 0.45 | 0.9966 | 0.9944 | 0.9888 | 6.18 |
Table III.
Summary of each Factor Effect and its p Values for the Measured Responses
| Factor | Y 1 | Y 2 | ||
|---|---|---|---|---|
| Factor effect | p value | Factor effect | p value | |
| X 1 | 1200.19 | <0.0001 | −0.072 | <0.0001 |
| X 2 | 9.38 | <0.0001 | 52.07 | <0.0001 |
| X 3 | 26.61 | <0.0001 | 32.01 | <0.0001 |
| X 1 X 2 | −1318.06 | <0.0001 | 62.83 | 0.0647 |
| X 1 X 3 | −1339.66 | <0.0001 | 76.13 | 0.0345 |
| X 2 X 3 | 19.14 | 0.0473 | −1.59 | 0.3733 |
| Refined regression equations of the fitted model in terms of L Pseudo Components a | ||||
| Y 1 = 1200.19X 1 + 9.38X 2 + 26.61X 3−1318.06X 1 X 2−1339.66X 1 X 3 + 19.14X 2 X 3 | ||||
| Y 2 = −0.072X 1 + 52.07X 2 − 32.01X 3 + 76.13X 1 X 3 | ||||
aOnly the terms with statistical significance are included
The globule size of ME should be as small as possible to ease penetration into the deeper layers of nail bed and kill the fungus. The contour plot of globule size (Y1; Fig. 2a) proved that oil proportion needs to be lesser in amount to have the minimum globule size of ME. The contour plot of solubility (Fig. 2b) and the refined equation for solubility (Y2; Table III) shows that Smix has a very significant effect on the solubility. It has been reported that retention of drug in the skin of nail bed depends on the distribution of drug between the vehicle of ME and layers of stratum corneum (29,30,32). Thus, the composition of ME showing highest solubility of drug would probably result in reduced partitioning of drug into the nail bed, and majority of the drug would be retained in the vehicle after application of the formulation. So, the composition of ME providing the globule size (Y1) lesser than 20 nm and the solubility of TF in ME system (Y2) between 40 and 45 mg/ml was considered as optimum. The optimum composition was selected on the basis of overlaid contour plot (Fig. 2c). The two responses were plotted collectively by the software to estimate an overall optimum region. The factors X1, X2 and X3 at 5.75%, 53.75%, and 40.5% provided the optimum response of Y1: 18.14 nm and Y2: 43.71 mg/ml. In order to assess the reliability of the developed mathematical model, optimized ME formulation was formed corresponding to above mentioned factor levels. The magnitude of percentage prediction error was significantly lower for Y1 and Y2 (Table IV) which demonstrates robustness of the mathematical model and high prognostic ability of the experimental design.
Table IV.
Experimental and Predicted Values for the Optimized ME Formulation
| Response | Experimental valuea | Predicted value | Percent prediction errorb |
|---|---|---|---|
| Y 1 | 19.15 ± 0.257 | 18.14 | 5.27 |
| Y 2 | 42.65 ± 0.482 | 43.71 | −2.48 |
aMean of 3 ± SD
bPercentage error was calculated using the formula [(Experimental value − Predicted value)/Experimental value] × 100
Physico-Chemical Characterization of Optimized ME Formulation and its Gel (MBG)
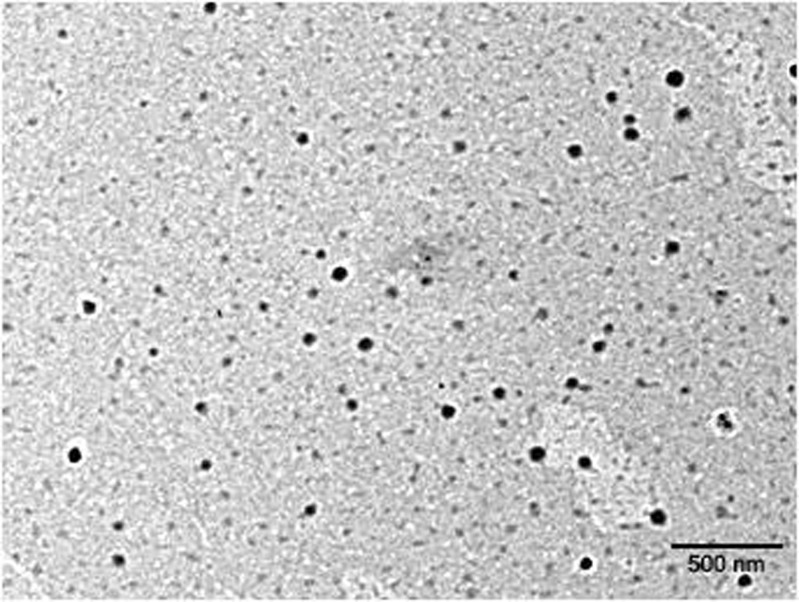
The globules of optimized ME appeared to be almost round in shape in transmission electron microscope. The globule appeared dark in bright surroundings (Fig. 3). The average droplet size of optimized ME was 18.54 nm with polydispersity index (PDI) of 0.15. The PDI value close to zero shows that ME globules were homogenous and had narrow size distribution. The transparency of ME was proved by refractive index 1.45 and >99% transmittance. Further, optimized ME formulation was observed as completely dark under cross polarizer, which implies that ME was optically isotropic and was colloidal dispersion. The conductivity of the formulation was 147.3 ± 2.1 μs cm−1, which confirmed the o/w nature of ME.
Fig. 3.
TEM image of optimized formulation
MBG was obtained by addition of 0.75% carbopol 934P, to render it suitable for application in to the cavity between nail plate and nail bed. The concentration of carbopol 934P was selected from the preliminary experiments, which were carried out with a view to get the appropriate viscosity for application in ventral edges of nail, so that the gel could not be drained out. The viscosity of MBG (18.46 ± 0.19 Pa s) increased significantly as compared to ME (35.49 ± 0.25 mPa s). The pH values of ME and MBG were 5.46 and 6.75 respectively, which implies that MBG has less stimulating capacity than ME (18,19). The optimized ME formulation and MBG had TF loading of 4.44%, w/w and 0.5%, w/w, respectively.
The optimized ME and MBG containing TF were also subjected to stability studies for 3 months. The formulations were stable as no degradation, precipitation or change in globule size was evident during their storage at 2–8°C and 40–45°C for 3 months.
In Vitro Permeation Studies
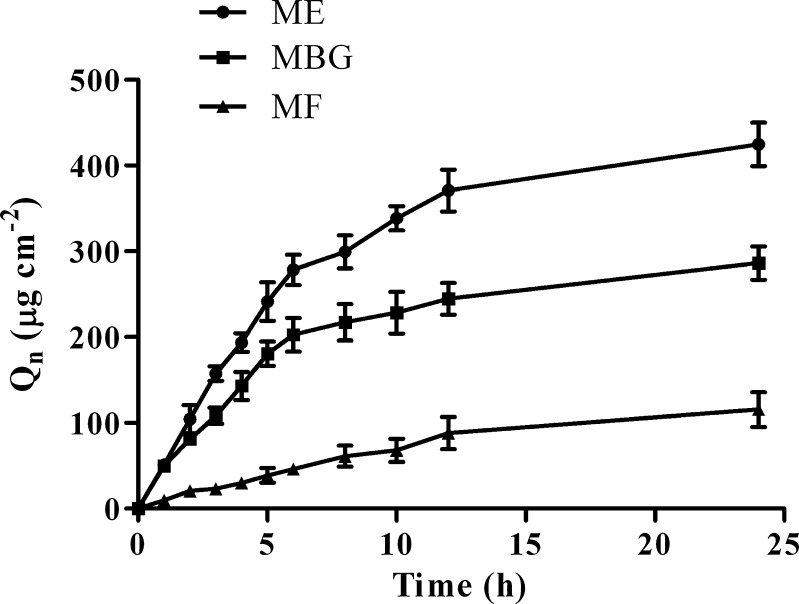
The permeation profile (Fig. 4) was obtained by placing optimized ME formulation, MF and MBG (each containing 5 mg TF) on the human cadaver skin in the Franz diffusion cell. It was observed that TF concentration steadily increased in the receptor chamber with increase in time, where the permeation profile generally followed Fick’s diffusion law. The cumulative amount of TB permeated (Qn) from ME, MBG and MF at 12 h after application were 370.92 ± 24.29, 244.65 ± 18.43 and 88.09 ± 18.65 μg cm−2, respectively. The comparatively higher Qn of optimized ME and MBG could be due to increased diffusion coefficient of drug (33,34). Moreover, smaller globule size of optimized ME provides larger area for permeation of drug in to skin and high drug concentration on the affected area results in a larger concentration gradient, which is a necessity for efficient dermal drug delivery . The amount of drug lost in the sample inserted during the permeation study was considered while calculating percentage drug permeated.
Fig. 4.
Permeation profiles of tested formulations (mean ± SD, n = 3)
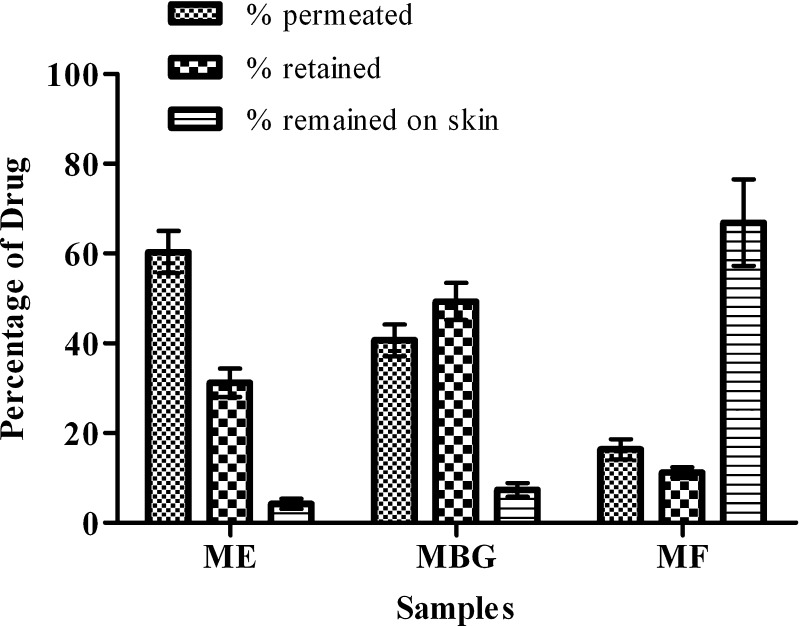
After the permeation study of 12 h, the percentage drug present on the skin was determined by washing the skin three to four times with the physiological saline solution. The solution was filtered through 0.22 μm filter and was analyzed spectrophotometrically for the drug content. For estimating the drug retained in the skin, the skin was cut into small pieces and was kept in the physiological saline solution (pH 5.5 acetate buffer/methanol (9:1)) for 24 h, which was then sonicated in the ultrasonicator for 10 min followed by vortex mixing for 15 min. The sample was then centrifuged at 6,000 rpm for 15 min. After centrifugation, supernatant was taken, filtered through 0.22 μm filter and was analyzed for the drug content. Figure 5 shows the partitioning of drug into different compartments of skin. The permeation was observed maximum with ME (60.33 ± 4.67%) followed by MBG (40.67 ± .53%). The commercial cream which was used as a control had large amount of the drug on the skin (66.9 ± 9.65%). The retention of drug was observed maximum with MBG (49.3 ± 4.12%), followed by ME (31.19 ± 3.16%), which is due to increased viscosity of the MBG, converting the optimized ME into lamellar structure or a highly ordered microstructure (35,36). The killing of nail fungus is possible only if larger amount of drug retains into the skin layers. Thus, MBG could be a promising drug delivery vehicle for the treatment of onychomycosis.
Fig. 5.
Mass balance of terbinafine (TF) in different compartments of skin after the in vitro permeation study (mean ± SD, n = 3)
Ex Vivo Antifungal Study
Ex vivo antifungal study was carried with optimized MBG, MF containing TF and control. For both the strains (C. albicans and T. rubrum), mean zone of inhibition was calculated, which was taken as an indicator for the antifungal activity. It was observed that MBG was more effective in killing the fungus as compared to MF (Table V). Significant difference was observed at 5% level of significance between zone of inhibition diameters of MBG and control, MF and control, MBG and MF (P < 0.05). The smaller globule size of optimized ME results in better diffusion of the drug through the barriers. Moreover, the optimized ME loaded in MBG has very low interfacial tension and large interfacial area; so larger amount of drug could be concentrated and localized within the same isotropic medium, thus providing enhanced antifungal action as compared to commercial cream.
Table V.
Zone of Inhibition Diameter for the MBG and MF
| Samples | Mean zone of inhibition diameter (mm) | |
|---|---|---|
| C. albicans | T. rubrum | |
| MBG | 29.67 ± 2.08 | 26.67 ± 251 |
| MF | 17.33 ± 1.53 | 16.67 ± 2.52 |
aMean of 3 ± SD
CONCLUSIONS
In this research paper, the microemulsion components were optimized using D-optimal design to get the optimum globule size and solubility. The results showed that microemulsion components had significant effect on the responses. The formulation containing 5.75% oleic acid, 26.87% Labrasol, 26.87% Transcutol P, and 40.5% water was selected as an optimized formulation. The drug-loaded gel showed better retention in the skin and an enhanced antifungal activity as compared to market formulation, which could be due to special characteristics of microemulsion. Thus, the drug-loaded gel could be a promising formulation to reduce the symptoms and to cure onychomycosis faster than the conventional therapies.
Acknowledgments
The authors are thankful to Cadila Pharmaceuticals Ltd. (Ahmedabad, India) for providing terbinafine; Abitec Corporation (OH, USA) for providing Capmul MCM; and Gattefosse (Lyon, France) for providing the free samples of Labrasol, Labrafac, and Transcutol P. The authors are also thankful to Institute of Microbial Technology (Chandigarh, India) for providing the fungal strains. The authors are grateful to Mrs. Mallika Babu for proof-reading the manuscript for grammatical and spelling errors. This study is a part of research project, carried out at Kadi Sarva Vishwavidyalaya (Gandhinagar, India).
References
- 1.Hui X, Shainhouse Z, Tanojo H, Anigbogu A, Markus GE, Maibach HI, Wester RC. Enhanced human nail drug delivery: nail inner drug content assayed by new unique method. J Pharm Sci. 2002;91(1):189–195. doi: 10.1002/jps.10003. [DOI] [PubMed] [Google Scholar]
- 2.Baran R, Gupta AK, Pierard GE. Pharmacotherapy of onychomycosis. Expert Opin Pharmacother. 2005;6(4):609–624. doi: 10.1517/14656566.6.4.609. [DOI] [PubMed] [Google Scholar]
- 3.Arrese JE, Pierard GE. Treatment failures and relapses in onychomycosis: a stubborn clinical problem. Dermatology. 2003;207(3):255–260. doi: 10.1159/000073086. [DOI] [PubMed] [Google Scholar]
- 4.Murdan S. Drug delivery to the nail following topical application. Int J Pharm. 2002;236(1–2):1–26. doi: 10.1016/S0378-5173(01)00989-9. [DOI] [PubMed] [Google Scholar]
- 5.Murdan S. 1st meeting on topical drug delivery to the nail. Expert Opin Drug Deliv. 2007;4(4):453–455. doi: 10.1517/17425247.4.4.453. [DOI] [PubMed] [Google Scholar]
- 6.Amichai B, Nitzan B, Mosckovitz R, Shemer A. Iontophoretic delivery of terbinafine in onychomycosis: a preliminary study. Br J Dermatol. 2010;162(1):46–50. doi: 10.1111/j.1365-2133.2009.09414.x. [DOI] [PubMed] [Google Scholar]
- 7.Sachdeva V, Siddoju S, Yu YY, Kim HD, Friden PM, Banga AK. Transdermal iontophoretic delivery of terbinafine hydrochloride: quantitation of drug levels in stratum corneum and underlying skin. Int J Pharm. 2010;388(1–2):24–31. doi: 10.1016/j.ijpharm.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Oon HH, Tan HH. Iontophoretic terbinafine delivery in onychomycosis: questionable nail growth. Br J Dermatol. 2010;162(3):699–700. doi: 10.1111/j.1365-2133.2009.09605.x. [DOI] [PubMed] [Google Scholar]
- 9.Nair AB, Vaka SR, Sammeta SM, Kim HD, Friden PM, Chakraborty B, Murthy SN. Trans-ungual iontophoretic delivery of terbinafine. J Pharm Sci. 2009;98(5):1788–1796. doi: 10.1002/jps.21555. [DOI] [PubMed] [Google Scholar]
- 10.Murdan S. Enhancing the nail permeability of topically applied drugs. Expert Opin Drug Deliv. 2008;5(11):1267–1282. doi: 10.1517/17425240802497218. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Kimball AB. New antifungal therapies for the treatment of onychomycosis. Expert Opin Investig Drugs. 2009;18(6):727–734. doi: 10.1517/13543780902810352. [DOI] [PubMed] [Google Scholar]
- 12.Nair AB, Vaka SRK, Murthy SN. Transungual delivery of terbinafine by iontophoresis in onychomycotic nails. Drug Dev Ind Pharm. 2011;37(10):1253–1258. doi: 10.3109/03639045.2011.568946. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45(1):89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh PK, Murthy RS. Microemulsions: a potential drug delivery system. Curr Drug Deliv. 2006;3(2):167–180. doi: 10.2174/156720106776359168. [DOI] [PubMed] [Google Scholar]
- 15.Kreilgaard M. Influence of microemulsions on cutaneous drug delivery. Adv Drug Deliv Rev. 2002;54(Suppl 1):S77–S98. doi: 10.1016/S0169-409X(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 16.Patel MR, Patel RB, Parikh JR, Solanki AB, Patel BG. Effect of formulation components on the in vitro permeation of microemulsion drug delivery system of fluconazole. AAPS PharmSciTech. 2009;10(3):917–923. doi: 10.1208/s12249-009-9286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mou D, Chen H, Du D, Mao C, Wan J, Xu H, Yang X. Hydrogel-thickened nanoemulsion system for topical delivery of lipophilic drugs. Int J Pharm. 2008;353(1–2):270–276. doi: 10.1016/j.ijpharm.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Chang X, Du D, Li J, Xu H, Yang X. Microemulsion-based hydrogel formulation of ibuprofen for topical delivery. Int J Pharm. 2006;315(1–2):52–58. doi: 10.1016/j.ijpharm.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Mou D, Du D, Chang X, Zhu D, Liu J, Xu H, Yang X. Hydrogel-thickened microemulsion for topical administration of drug molecule at an extremely low concentration. Int J Pharm. 2007;341(1–2):78–84. doi: 10.1016/j.ijpharm.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 20.Lapasin R, Grassi M, Coceani N. Effects of polymer addition on the rheology of o/w microemulsions. Rheol Acta. 2001;40:185–192. doi: 10.1007/s003970000151. [DOI] [Google Scholar]
- 21.Kawakami K, Yoshikawa T, Moroto Y, Kanaoka E, Takahashi K, Nishihara Y, Masuda K. Microemulsion formulation for enhanced absorption of poorly soluble drugs. I. Prescription design. J Control Release. 2002;81(1–2):65–74. doi: 10.1016/S0168-3659(02)00049-4. [DOI] [PubMed] [Google Scholar]
- 22.Leucuta SE, Bodea A. Optimization of hydrophilic matrix tablets using a D-optimal design. Int J Pharm. 1997;153(2):247–255. doi: 10.1016/S0378-5173(97)00117-8. [DOI] [Google Scholar]
- 23.Holm R, Jensen IH, Sonnergaard J. Optimization of self-microemulsifying drug delivery systems (SMEDDS) using a D-optimal design and the desirability function. Drug Dev Ind Pharm. 2006;32(9):1025–1032. doi: 10.1080/03639040600559024. [DOI] [PubMed] [Google Scholar]
- 24.Lewis GA, Mathieu D, Phan-Tan-Luu R. Mixtures in a constrained region of interest. In: Swarbrick J, editor. Pharmaceutical Experimental Design. New York: Marcel Dekker; 1999. pp. 413–454. [Google Scholar]
- 25.Raza K, Negi P, Takyar S, Shukla A, Amarji B, Katare OP. Novel dithranol phospholipid microemulsion for topical application: development, characterization and percutaneous absorption studies. J Microencapsul. 2011;28(3):190–199. doi: 10.3109/02652048.2010.546435. [DOI] [PubMed] [Google Scholar]
- 26.Hui X, Wester RC, Maibach HI, Barbadillo S. Nail Penetration—Enhance Topical Delivery of Antifungal Drugs by Chemical Modification of the Human Nail. In: Bronaugh RL, Maibach HI, editors. Percutaneous absorption: drugs–cosmetics–mechanisms–methodology. 4. Boca Raton: Taylor & Francis; 2005. pp. 643–653. [Google Scholar]
- 27.Zhu W, Yu A, Wang W, Dong R, Wu J, Zhai G. Formulation design of microemulsion for dermal delivery of penciclovir. Int J Pharm. 2008;360(1–2):184–190. doi: 10.1016/j.ijpharm.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Larrucea E, Arellano A, Santoyo S, Ygartua P. Combined effect of oleic acid and propylene glycol on the percutaneous penetration of tenoxicam and its retention in the skin. Eur J Pharm Biopharm. 2001;52(2):113–119. doi: 10.1016/S0939-6411(01)00158-8. [DOI] [PubMed] [Google Scholar]
- 29.Hua L, Weisan P, Jiayu L, Ying Z. Preparation, evaluation, and NMR characterization of vinpocetine microemulsion for transdermal delivery. Drug Dev Ind Pharm. 2004;30(6):657–666. doi: 10.1081/DDC-120039183. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Pan WS, Wu Z, Li JY, Xia LX. Optimization of microemulsion containing vinpocetine and its physicochemical properties. Yao Xue Xue Bao. 2004;39(9):681–685. [PubMed] [Google Scholar]
- 31.Shafiq-un-Nabi S, Shakeel F, Talegaonkar S, Ali J, Baboota S, Ahuja A, Khar RK, Ali M. Formulation development and optimization using nanoemulsion technique: a technical note. AAPS PharmSciTech. 2007;8(2):E12–E17. doi: 10.1208/pt0802028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ceschel G, Bergamante V, Maffei P, Borgia SL, Calabrese V, Biserni S, Ronchi C. Solubility and transdermal permeation properties of a dehydroepiandrosterone cyclodextrin complex from hydrophilic and lipophilic vehicles. Drug Deliv. 2005;12(5):275–280. doi: 10.1080/10717540500176563. [DOI] [PubMed] [Google Scholar]
- 33.El Maghraby GM. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: effects of cosurfactants. Int J Pharm. 2008;355(1–2):285–292. doi: 10.1016/j.ijpharm.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Huang YB, Lin YH, Lu TM, Wang RJ, Tsai YH, Wu PC. Transdermal delivery of capsaicin derivative-sodium nonivamide acetate using microemulsions as vehicles. Int J Pharm. 2008;349(1–2):206–211. doi: 10.1016/j.ijpharm.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Peltola S, Saarinen-Savolainen P, Kiesvaara J, Suhonen TM, Urtti A. Microemulsions for topical delivery of estradiol. Int J Pharm. 2003;254(2):99–107. doi: 10.1016/S0378-5173(02)00632-4. [DOI] [PubMed] [Google Scholar]
- 36.Trotta M. Influence of phase transformation on indomethacin release from microemulsions. J Control Release. 1999;60(2–3):399–405. doi: 10.1016/S0168-3659(99)00094-2. [DOI] [PubMed] [Google Scholar]