Abstract
Surmounting the constraints of limited solubilization efficiency and prime requisite of antioxidant for conventional lipid formulations, the research work explores an edge over formulation utilizing potential applicability of rice germ oil (RGO) as a multifunctional excipient. Self-microemulsifying drug delivery system (SMEDDS) of tacrolimus (TAC) was formulated with RGO, an indigenous source of gamma-oryzanol. Being the same biological source, RGO and rice bran oil (RBO) were compared and it was found that RGO have more solubilization potential for TAC (2.2-fold) as well as higher antioxidant activity (8.06-fold) than the RBO. TAC-SMEDDS was prepared using RGO/Capmul PG8 (2:3) as an oil phase, Cremophore EL as a surfactant, and Transcutol P as a cosurfactant. The approximate particle size of TAC-SMEDDS was found to be 38 nm by dynamic light scattering and 12 nm by small angle neutron scattering. The in vitro dissolution studies showed complete and rapid drug release in 30 min compared to a plain drug (<5%) and marketed capsule (<50%). AUC and Cmax were found to be 45.05 ± 15.64 ng h/ml and 3.91 ± 1.2 ng/ml for TAC-SMEDDS, 12.59 ± 5.54 ng h/ml and 0.48 ± 0.12 ng/ml for plain TAC, and 30.23 ± 10.34 ng h/ml and 2.31 ± 0.68 ng/ml for marketed formulation, respectively. The improved pharmacokinetic profile of TAC-SMEDDS is correlating to the dissolution results. Thus, gamma-oryzanol-enriched RGO acts as a potential multifunctional excipient for lipid formulations.
KEY WORDS: antioxidant, gamma-oryzanol, pharmacokinetic, rice germ oil, SMEDDS
INTRODUCTION
Rapid progress in design and development of value-added pharmaceutical dosage forms demand quality excipients accomplishing desired multifunctionality and cost-effectiveness. Evaluating future trends in dosage form developments, which are inclined towards a novel drug delivery system (NDDS) demands the identification of multifunctional excipients. NDDS mainly consist of technologies like nanosuspensions (1), liposomes (2), microspheres (3), self-microemulsifying drug delivery system (SMEDDS) (4,5), nanoemulsions (6), solid lipid nanoparticles (7), etc. These technologies have already proven their potential in effective delivery of poorly soluble drugs (BCS class II and IV). Among them, lipid-based drug delivery systems particularly SMEDDS had shown interest after the commercial success of Sandimmune®, Neoral® (cyclosporin A), Norvir® (ritonavir), and Fortovase® (saquinavir) (8).
The SMEDDS are an isotropic mixture of oil, surfactant, cosurfactant, and drug which spontaneously form microemulsion when exposed to gastrointestinal fluid (9). There are some key constraints of SMEDDS such as limited solubilization efficiency of oil and use of antioxidant to prevent autooxidative deterioration of oil. Limited solubilization of drug was overcome by the use of a cosolvent like ethanol but over prolonged storage, evaporation of ethanol resulted in crystallization of drug (10). To prevent lipid autooxidative deterioration, synthetic phenolic compounds such as butylated hydroxytoluene and butylated hydroxyanisole are commonly used. However, the studies showed that they might have mutagenic and carcinogenic properties (11). These limitations of traditional SMEDDS can be overcome by the use of multifunctional excipients having high-solubilization efficiency and inherent natural antioxidant property. Hence, the objective of the present investigation was to evaluate rice germ oil (RGO) as a multifunctional excipient for SMEDDS formulation.
The RGO is obtained from Oryza sativa family Gramineae. RGO is an edible oil having 2.35:1.36:1 proportion of mono-unsaturated, polyunsaturated, and saturated fatty acids. It consists of triglycerides, mainly C16–C18 fatty acids (81.3–84.3% w/w) (12). RGO is one of the richest sources of potent antioxidant gamma-oryzanol (4–5% w/w) (13). Gamma-oryzanol is ferulic acid ester derivatives such as 10-phytosteryl ferulates, cycloartenyl ferulate, 24-methylenecycloartanyl ferulate, and campesteryl ferulate. It has various medicinal applications such as prevention of cancer, maintenance of plasma lipid level, and prevention of platelet aggregation (14). Accelerated oxidation study demonstrated the capability of gamma-oryzanol in preventing lipid peroxidation of oil (15).
Tacrolimus (TAC) is a macrolide antibiotic having potent immunosuppressant activity with very low and variable bioavailability due to its poor solubility, first pass metabolism, and inter-subject variability (16,17). Hence, SMEDDS could be the right strategy to circumvent solubility- and bioavailability-related issues of TAC (18,19). In the present study, TAC-SMEDDS were prepared using gamma-oryzanol-enriched RGO as a multifunctional excipient having inherent antioxidant potential as well as higher solubilization capacity for lipophilic drugs like TAC.
MATERIALS AND METHODS
Materials
TAC was provided by Biocon Pvt Ltd., India. Tamsulosin obtained from Suven Life Sciences, India. RGO was purchased from Oryza Oil and Fat Chemical Co. Ltd., Japan. Gamma-oryzanol was purchased from Cruciani Alberto Crual Products, Italy. 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) was procured from Sigma-Aldrich, India. Capmul PG8, Captex 355, and Captex 300 were provided as gift samples by Abitec Co., USA. Cremophore EL was provided by BASF, India. Transcutol P, Lauroglycol FCC, Gelucire 44/14 were provided by Gatteffosse, India. Corn oil, rice bran oil (RBO), coconut oil, castor oil, mustard oil, and kardi oil were procured from a local vendor. Deuterated water was supplied by BARC, India. All other chemicals used in the study were of analytical grade.
HPLC Analysis of TAC
TAC was quantified by an in house-developed and validated RP-HPLC method using an HPLC system equipped with PU-2080 Intelligent HPLC pump and MD-2015 plus multiwavelength detector (Jasco, Tokyo, Japan). The column used was Zorbax C-8 (4.6 × 250 mm and 5 μ) and mobile phase was a mixture of acetonitrile/buffer [80:20 (v/v)]. The buffer used was a mixture of a 50-mM potassium dihydrogen phosphate, 0.1% w/v triethylamine, 0.25% w/v octane-1-sulphonic acid, and 0.25% w/v heptane-1-sulphonic acid with pH 2.5 ± 0.1. The operating parameters were set at flow rate of 2 ml/min, loop size of 100 μL, column oven temperature at 60°C, and detection wavelength at 210 nm. The data were obtained and processed using ChromPass™ Software (Jasco, Tokyo, Japan).
HPLC Analysis of Gamma-Oryzanol
The gamma-oryzanol was determined in RGO, RBO, and TAC-SMEDDS formulation by using a RP-HPLC method (20). The HPLC system consisted of a PU-2080 Intelligent HPLC pump (Jasco, Tokyo, Japan) equipped with an MD-2015 plus multiwavelength detector (Jasco, Tokyo, Japan). The gamma-oryzanol was analyzed isocratically with a Nucleosil 4.6 × 250 mm, C-18 column, 5 μ. The mobile phase was methanol/acetonitrile/dichloromethane/acetic acid [50:44:3:3 (v/v)] at a flow rate of 1.6 ml/min at room temperature. The detector was set at a wavelength of 330 nm. The data were obtained and processed using ChromPass™ Software (Jasco, Tokyo, Japan).
Antioxidant Activity of RBO, RGO, and Gamma-Oryzanol
The antioxidant activity of RBO, RGO, and gamma-oryzanol was estimated by DPPH radical scavenging assay. The DPPH assay was done according to the reported method with some modifications (21,22). The ethanolic extract of sample (0.5 ml) was incubated with 0.5 ml of DPPH working solution (0.39 mg/ml) at 25°C for 30 min in the dark. The control sample contained 1 ml of the DPPH solution. The absorbance was measured at wavelength of 517 nm (n = 3). IC50 value was obtained from the plot of percent inhibition of free radical DPPH discoloration versus the concentration of sample. Percent inhibition of free radical DPPH discoloration was calculated according to the formula given as follows:
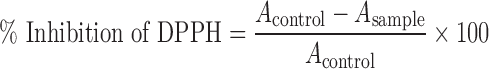 |
1 |
where Acontrol is the absorbance of the control reaction (containing all reagents except the test compound) and Asample is the absorbance of test sample.
Solubility Study of TAC
Solubility of TAC in various oils, surfactants, and cosurfactants was studied. The solubility studies were carried out by adding an excess quantity of TAC into the 2 g sample in a screw-capped vial. The mixtures were subjected for shaking on a mechanical shaker at 100 rpm for 48 h at room temperature followed by centrifugation at 5,000 rpm for 20 min. The supernatant layer was carefully removed and filtered through membrane filter (0.45 μm). The filtered samples were appropriately diluted with ethanol and analyzed for TAC by HPLC method as described earlier.
Construction of Pseudoternary Phase Diagram
The pseudoternary phase diagrams of oil, surfactant/cosurfactant (Smix) were constructed using water titration method to calculate approximate concentration range of components in the formation of microemulsion (4). Each pseudoternary phase diagram was prepared at a fixed Smix (1:1, 2:1, and 3:1) with varied proportion of oil components. Furthermore, these mixtures were titrated with water to form transparent to turbid or turbid to transparent transition. The data were processed by CHEMIX 3.51™ software (Arne Standnes, Norway) to construct a pseudoternary phase diagram.
SMEDDS Formulation of TAC
The TAC-SMEDDS formulation was prepared by using Cremophore EL and Transcutol P as surfactant/cosurfactant combination with RGO and Capmul PG8 as an oil component. SMEDDS was prepared by dissolving TAC (5 mg) in a mixture of oil, surfactant, and cosurfactant at a temperature of 40–45°C.
Microemulsion Globule Size Analysis
The globule size analysis was performed using dynamic light scattering (DLS) and small-angle neutron scattering (SANS). DLS was performed using Malvern 4800 Autosizer using 7132 digital correlator at BARC, India. The light source was argon laser, operated at 514.5 nm with photodiode detector. Double-distilled water was used for dilution.
The SANS study was performed using a small-angle neutron scattering diffractometer at Dhruva Reactor, BARC, India. The diffractrometer was made up of beryllium oxide-filtered beam having a radiation wavelength of 5.2 Å, with distance of 2 m between sample and detector. The length of scattering vector Q was from 0.017 to 0.35 Å−1.
In Vitro Dissolution
In vitro dissolution was carried out at 50 rpm using USP XXIII dissolution apparatus II with 900 ml of hydroxyl propyl solution (1 in 20,000) adjusted to pH 4.5 by phosphoric acid at 37 ± 0.5°C (23). TAC-SMEDDS (capsule size 2), plain TAC (capsule size 4), and marketed capsule (Pangraf® Panacea Biotech, India) formulation were subjected for in vitro dissolution study. TAC was analyzed by using RP-HPLC method as described earlier.
Determination of Emulsification Time
In order to determine the emulsification time (the time required to give the emulsified and homogeneous mixture, upon dilution), 1 g of optimized formulation was added to 250 mL of water at 37°C with gentle agitation using magnetic stirrer (24). The formulation was assessed visually according to the rate of emulsification and the final appearance of the emulsion.
Stability Study of TAC-SMEDDS
The SMEDDS formulations were put into empty hard gelatin capsules (size 2) and subjected to stability studies, 25°C/60% relative humidity (RH), 30°C/65%RH and 40°C/75%RH for 3 months. Samples were charged in stability chambers (Thermolab, Mumbai, India) with humidity and temperature control. The samples were evaluated for appearance, clarity, emulsification time, globule size, assay of TAC, and in vitro dissolution.
In Vivo Pharmacokinetic Study of TAC in Rats
Experimental Design
The animal protocol was approved by the Institutional Animal Ethics Committee (IAEC) at the Institute of Chemical Technology, India (approval no. ICT/PH/IAEC/0909/03). The male Sprague Dawley rats (210 ± 20 g) were used, which were provided by Haffkins Biopharma Corporation Ltd., India. The rats were fasted overnight before experiment with free access to water. The control group (n = 5) was given plain vehicle by oral gavage. Other rats were allocated at random to three treatment groups (n = 5) and they have been given TAC-SMEDDS, suspension of plain TAC in 1% w/v tragacanth, and marketed capsule (Pangraf® Panacea Biotech, India) by oral gavage. After gavage administration of TAC (0.7 mg/kg expressed as TAC equivalent) (25), about 0.5 ml blood sample was collected by retro-orbital puncture into heparinized tubes at 0, 0.5, 1, 2, 4, 6, 8,12, and 24 h. After centrifugation, the plasma (200 μl) was obtained and stored at −20°C until further analysis. The plasma concentration of TAC was determined by LC/MS/MS.
LC/MS/MS Analysis of TAC in Rat Plasma
Plasma concentration of TAC was quantified by a LC/MS/MS method (26) with some modifications. Tamsulosin was used as an internal standard. The drug and internal standard were extracted by liquid–liquid extraction from plasma. The supernatant layer was separated and evaporated to dryness by nitrogen streaming at 40°C. The dried residue was reconstituted with 200 μl of methanol and injected into LC/MS/MS system for quantification of TAC.
LC/MS/MS was performed using an HPLC system (Thermo Electro-Lab 2000 Series, Germany) connected to a mass spectrometry system (Thermo Electro-Lab 2000, Q-TRAP, Germany). The HPLC analysis was performed by using a C18 column (150 × 4.6 mm, 5 μm, Agilent, USA) kept at 40°C. Mobile phase consisting of methanol (A) and 10 mM ammonium acetate buffer (B) (99:1 v/v) was eluted in an isocratic mode at flow rate of 0.5 ml/min. Mass spectrometry was studied using electrospray ionization with ion source temperature at 350°C, entrance potential at −5 V, shear gas pressure at 26.0 psi, and auxillary gas pressure at 5.0 psi. The quantification of TAC and internal standard was estimated by selective ion monitoring at m/z of 616 and 271, respectively.
Pharmacokinetic Analysis
Cmax and Tmax were determined from a graph of plasma concentration versus time. AUC was determined by using linear trapezoidal rule. The experimental results were analyzed by ANOVA, with the level of statistic significance set at P < 0.05.
RESULT AND DISCUSSION
Determination of Gamma-Oryzanol in RGO and RBO
The content of gamma-oryzanol in the RGO and RBO was found to be 4.3 ± 0.3% (w/w) and 1.5 ± 0.4% (w/w), respectively. The RGO was found to be more enriched with gamma-oryzanol compared to RBO. Though RGO and RBO have the same biological source as O. sativa but higher content of gamma-oryzanol offers higher antioxidant activity to RGO (13).
Antioxidant Activity of RBO, RGO, and Gamma-Oryzanol
The observed antioxidant activity of gamma-oryzanol, RGO, and RBO is ranked in the following sequence: gamma-oryzanol (IC50, 0.0177 ± 0.005 mg/ml) > RGO (IC50, 3.55 ± 0.11 mg/ml) > RBO (IC50, 28.64 ± 1.54 mg/ml) with respect to their IC50. Free radical scavenging activity contributes to the potent antioxidant activity of gamma-oryzanol (15). Hence, RGO shows significantly higher antioxidant potential compared to RBO.
Solubility of TAC
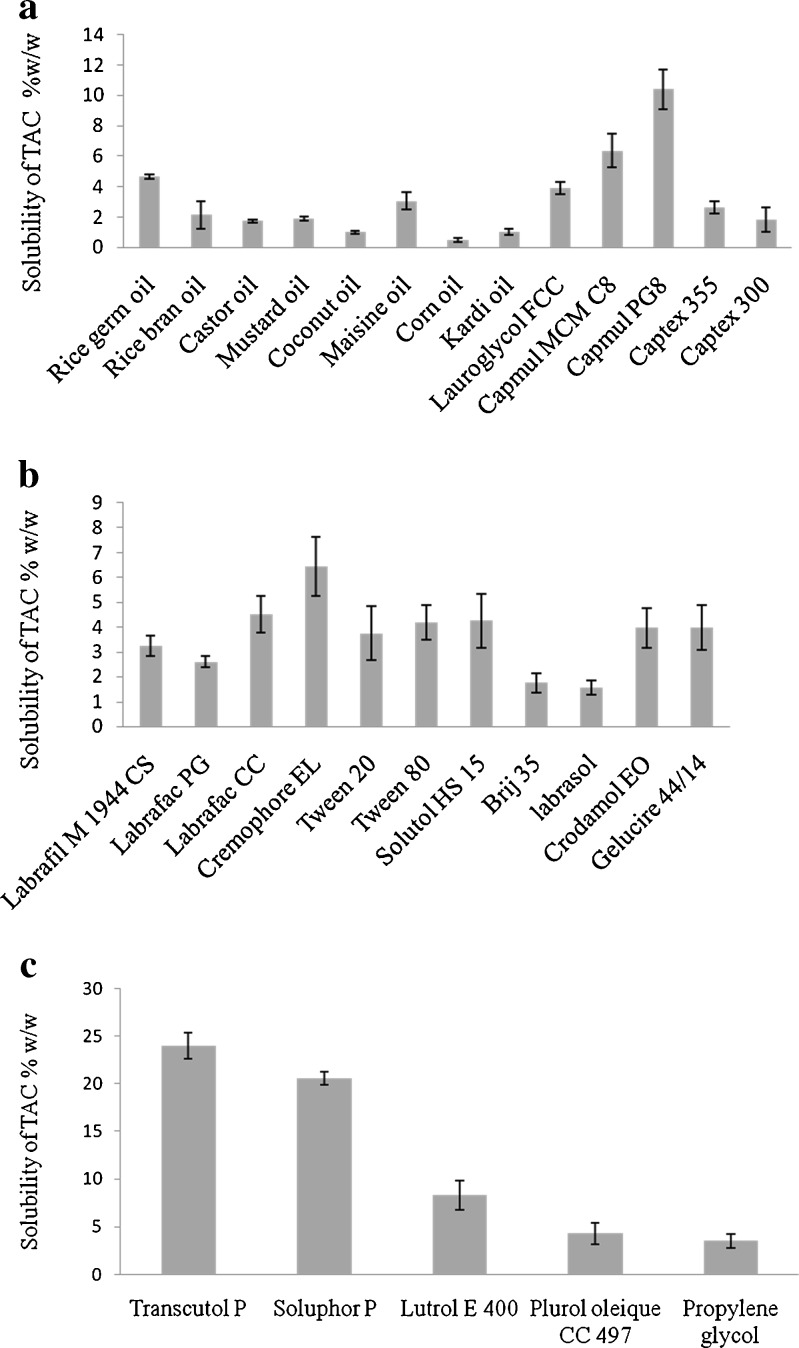
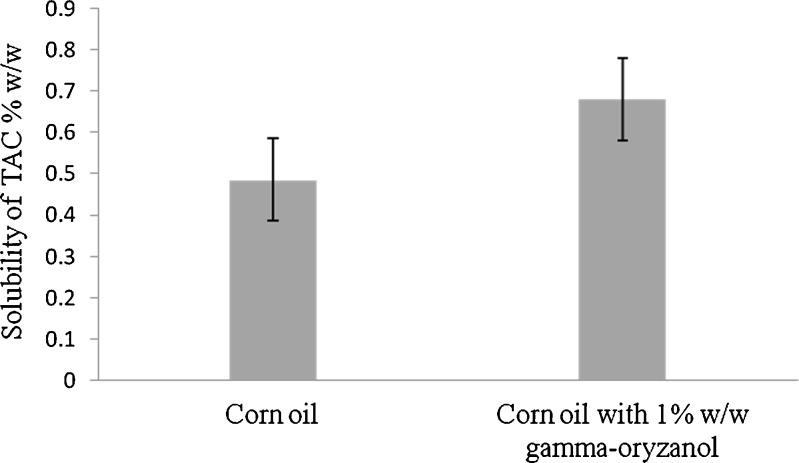
The prime requirement for formulating SMEDDS is avoiding precipitation of drug on dilution in the gastrointestinal (GI) fluids (27). Thus, the components used in the system should have higher solubilization capacity for TAC ensuring solubilization of drug in resultant microemulsion. The TAC was quantified using HPLC method describe earlier (Fig. 1). The solubility of TAC in various oils, surfactants, and cosurfactants is shown in Fig. 2a–c. As seen from the Fig. 2a, RGO and Capmul PG8 showed highest solubility. Among various surfactants screened, Cremophore EL showed highest solubilization potential (Fig. 2b), whereas among various cosurfactants, Transcutol P showed highest solubilization potential for TAC (Fig. 2c). The selected components for microemulsion system were RGO and Capmul PG8 as an oily phase, Cremophore as a surfactant, and Transcutol P as a cosurfactant. The higher solubility of TAC in RGO may be due to its higher gamma-oryzanol content. This assumption was further confirmed by external addition of gamma-oryzanol [1% (w/w)] in corn oil which has the least solubility for TAC. The external addition of gamma-oryzanol resulted in significant increased in the solubility of TAC (P < 0.05). The solubility of TAC in corn oil was increased by 1.48 times with the addition of gamma-oryzanol (Fig. 3).
Fig. 1.
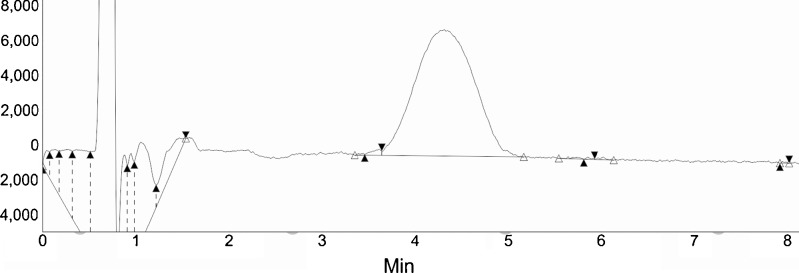
Standard chromatogram of TAC using RP-HPLC method
Fig. 2.
a Solubility studies of TAC in various oils (n = 3; mean ± SD). b Solubility studies of TAC in various surfactants (n = 3; mean ± SD). c Solubility studies of TAC in various cosurfactants (n = 3; mean ± SD)
Fig. 3.
Solubility studies of TAC in corn oil and corn oil with externally spiked (1% w/w) gamma-oryzanol (n = 3; mean ± SD). P < 0.05 when corn oil with externally spiked gamma-oryzanol (1% w/w) is having significantly higher solubility for TAC than corn oil
Construction of Pseudoternary Phase Diagram
The pseudoternary phase diagrams were constructed using RGO and Capmul PG8 as oil components, Cremophore EL as a surfactant, and Transcutol P as a cosurfactant. Initially, pseudoternary phase diagram was constructed for RGO with Cremophore EL and Transcutol P but it revealed lesser region for microemulsion (Fig. 4). The maximum fields of self-microemulsion were obtained when the mixture of RGO and Capmul PG 8 in the ratio of 2:3 was used as oil phase (Fig. 5). Hence, the mixture of RGO and Capmul PG 8 in the ratio of 2:3 was selected as an oil phase. It may be correlated with unique characteristics of Capmul PG 8 to promote water penetration and improve self-emulsifying capacities of the formulations (5). The Smix at 2:1 and 3:1 showed approximately same microemulsion region but higher than Smix at 1:1. Hence, the optimal ratio of surfactant to cosurfactant was selected to be 2:1.
Fig. 4.
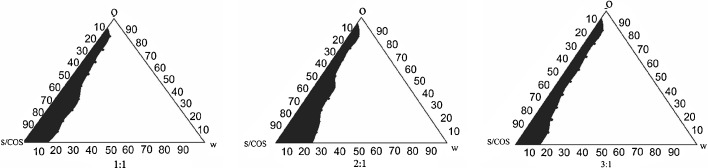
Pseudoternary phase diagram of system with following components: oil (O) = RGO, surfactant (S) = Cremophore EL, and cosurfactant (COS) = Transcutol P. S mix of 1:1, 2:1, and 3:1. The field area represents the microemulsion region. S mix and W indicate surfactant/cosurfactant and water, respectively
Fig. 5.
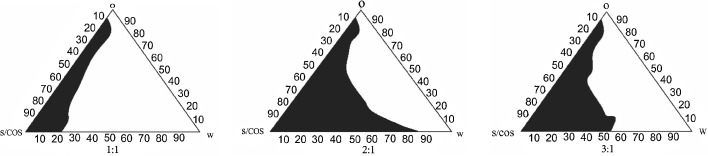
Pseudoternary phase diagram of system with following components: oil (O) = RGO: Capmul PG8, surfactant (S) = Cremophore EL, and cosurfactant (COS) = Transcutol P. S mix of 1:1, 2:1, and 3:1. The field area represents the microemulsion region. S mix and W indicate surfactant/cosurfactant and water, respectively
Formulation of TAC-SMEDDS
Based on the pseudoternary phase diagram and solubilization potential for TAC, a suitable composition was optimized with TAC concentration of 5 mg/capsule (Table I). This optimized TAC-SMEDDS was further subjected to various evaluation parameters.
Table I.
Composition of Optimized TAC-SMEDDS
| Ingredients | Quantity (% w/w) |
|---|---|
| TAC | 2.07 |
| RGO | 8.30 |
| Capmul PG8 | 12.45 |
| Cremophore EL | 51.45 |
| Transcutol P | 25.73 |
TAC tacrolimus, RGO rice germ oil
Determination of Emulsification Time
The emulsification time is an important parameter for the evaluation of self-emulsification efficiency (4,18). The optimized TAC-SMEDDS showed desired emulsification time (<1 min) with no precipitation of drug. Thus, the selected combination of surfactant and cosurfactant is capable of emulsifying the selected oil combination effectively.
Micromemulsion Droplet Size Analysis
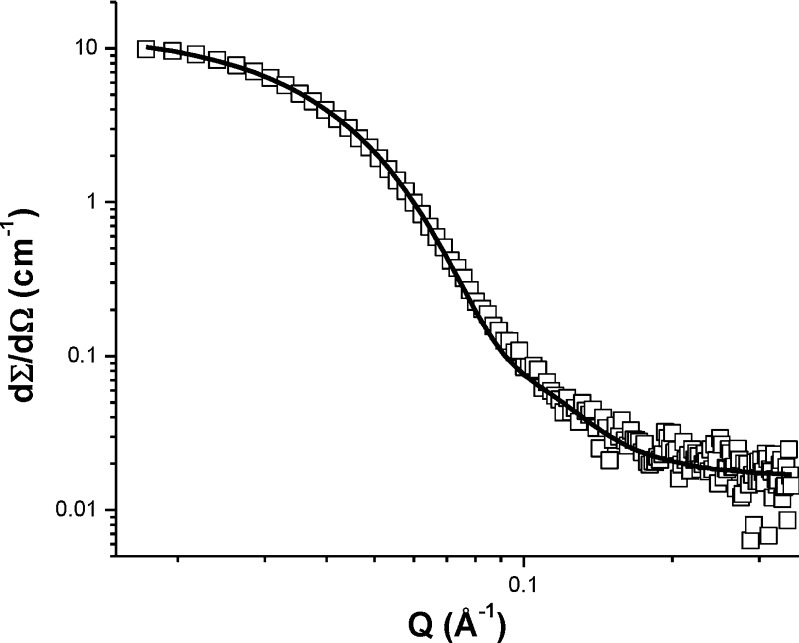
Microemulsion droplet size distribution following self-emulsification is a decisive parameter to study the self-emulsifying systems in vitro because it determines the rate and extent of drug release as well as absorption of drug from GIT (9). In the present work, microemulsion droplet size was analyzed by DLS and SANS on the basis of laser diffractometry. The average droplet size of TAC-SMEDDS was found to be 39 ± 6 nm by DLS and 12 ± 1 nm by SANS at 1:100 aqueous dilution. The SANS study has shown characteristic scattering pattern which was best fitted in the polydisperse spherical model with polydispersity index of 0.2, signifying the spherical shape of particles (28) (Fig. 6).
Fig. 6.
Experimental neutron scattering data of TAC-SMEDDS fitting in polydispersed spherical model at polydispersity index 0.2
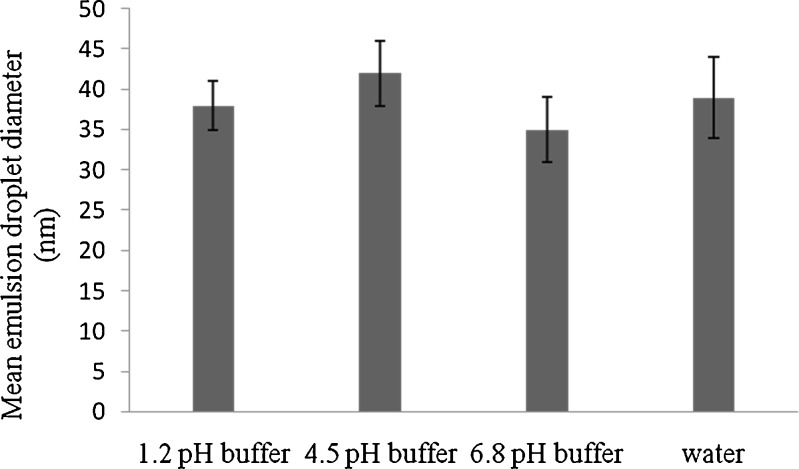
There was no significant change in the particle size of TAC-SMEDDS with respect to different media (Fig. 7). This finding simulates the stability of microemulsion in the GI environment.
Fig. 7.
Effect of various dilution fluids on globule size of TAC-SMEDDS using DLS technique (n = 3; mean ± SD)
In Vitro Dissolution
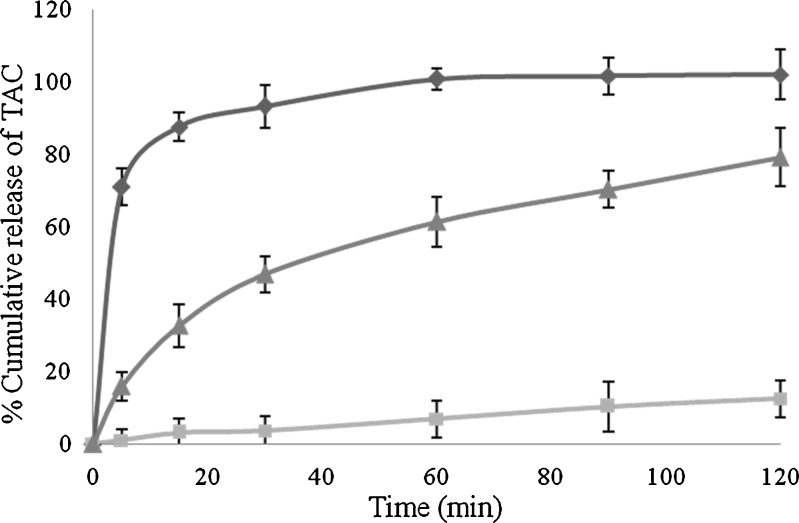
In vitro dissolution study showed the complete release of TAC from SMEDDS formulation within 30 min. Whereas, the plain TAC and marketed capsule (Pangraf® Panacea Biotech, India) showed <5% and <50% drug release in 30 min, respectively (Fig. 8). It could be proposed that SMEDDS formulation resulted in spontaneous formation of microemulsion with nanosized droplets, which gives faster release of drug into aqueous media compared to plain TAC and marketed capsule. When SMEDDS subjected to an aqueous media, the drug existed in different forms such as a free molecular form, micellar form, or in microemulsion droplets (29). This solublised form of TAC from SMEDDS formulation could lead to higher absorption and higher bioavailability.
Fig. 8.
Comparative result of in vitro drug release of TAC from (diamond) TAC-SMEDDS, (square) plain TAC, and (triangle) marketed capsule of TAC in pH 4.5 buffer (n = 6; mean ± SD)
Antioxidant Activity
The antioxidant activity of developed TAC-SMEDDS was determined by DPPH assay, and it was found to be 70.52 ± 2.56%. The indigenous antioxidant property of natural RGO protects TAC-SMEDDS from autooxidative deteriotation. The accelerated stability study of TAC-SMEDDS shows no significant change in gamma-oryzanol content and antioxidant activity which illustrate the stability of developed formulation. Gamma-oryzanol acts as an antioxidant interfering with the organic radicals by free radical scavenging action. It protects oils from lipoperoxidation which make it an ideal candidate for use as natural antioxidant (15). Moreover, RGO, naturally rich in gamma-oryzanol and tocopherols, could be suggested as a novel excipient for SMEDDS like lipid drug delivery system.
Stability Study of Optimized TAC-SMEDDS
The formulation was found to be stable for 3-month period. There was no significant change in the drug content, drug release (t 90%), or particle size of the resultant microemulsion which proves the stability of developed formulation (Table II). In addition, there was no significant change in the appearance, clarity after dilution, and emulsification time. Furthermore, the formulation showed no phase separation, drug precipitation, or capsule leaks. Thus, these studies substantiated the stability of the developed formulation and its compatibility with hard gelatin capsules.
Table II.
Evaluation Data of TAC-SMEDDS Formulation Subjected to Stability Studies (n = 10)
| Time (month) | Drug content (%) | Average particle size by DLS (nm) | Average particle size by SANS (nm) | t 90% (min) |
|---|---|---|---|---|
| A. 25°C/60% RH | ||||
| 0 | 100.34 ± 0.67 | 34.78 ± 3 | 11.21 ± 0.32 | <15 |
| 1 | 99.23 ± 0.23 | 40.2 ± 4 | 11.75 ± 0.21 | <15 |
| 2 | 101.67 ± 0.52 | 36.7 ± 2 | 11.45 ± 0.30 | <15 |
| 3 | 100.19 ± 1.56 | 38.5 ± 3 | 11.60 ± 0.42 | <15 |
| B. 30°C/65% RH | ||||
| 0 | 101.34 ± 0.34 | 34.67 ± 4 | 11.60 ± 0.31 | <15 |
| 1 | 100.78 ± 0.78 | 38.90 ± 5 | 11.20 ± 0.24 | <15 |
| 2 | 99.67 ± 0.34 | 41.20 ± 2 | 11.89 ± 0.36 | <15 |
| 3 | 101.89 ± 0.45 | 37.80 ± 3 | 10.98 ± 0.23 | <15 |
| C. 40°C/75% RH | ||||
| 0 | 101.34 ± 0.65 | 37.9 ± 4 | 11.34 ± 0.34 | <15 |
| 1 | 100.67 ± 0.46 | 36.89 ± 5 | 11.89 ± 0.26 | <15 |
| 2 | 100.12 ± 0.65 | 40.23 ± 4 | 11.20 ± 0.10 | <15 |
| 3 | 99.30 ± 0.78 | 34.89 ± 3 | 11.43 ± 0.20 | <15 |
DLS dynamic light scattering, SANS small-angle neutron scattering, RH relative humidity, t90% time taken for 90% drug release
Pharmacokinetic Study of TAC-SMEDDS on Rat
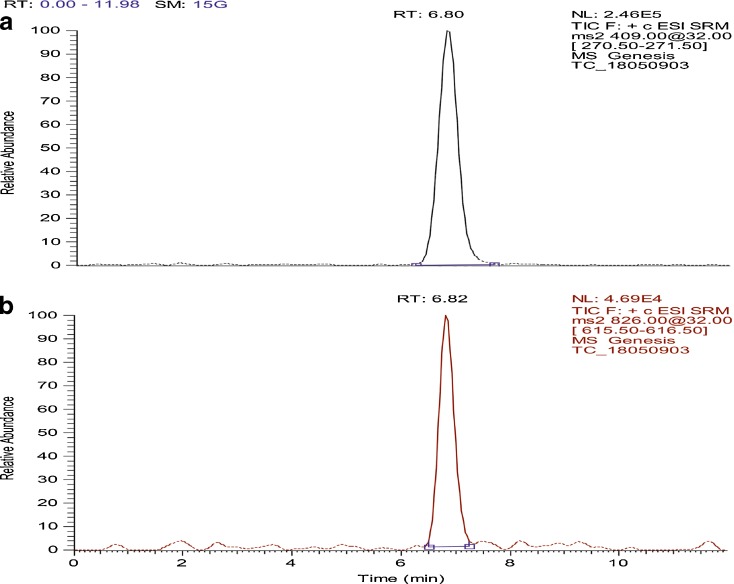
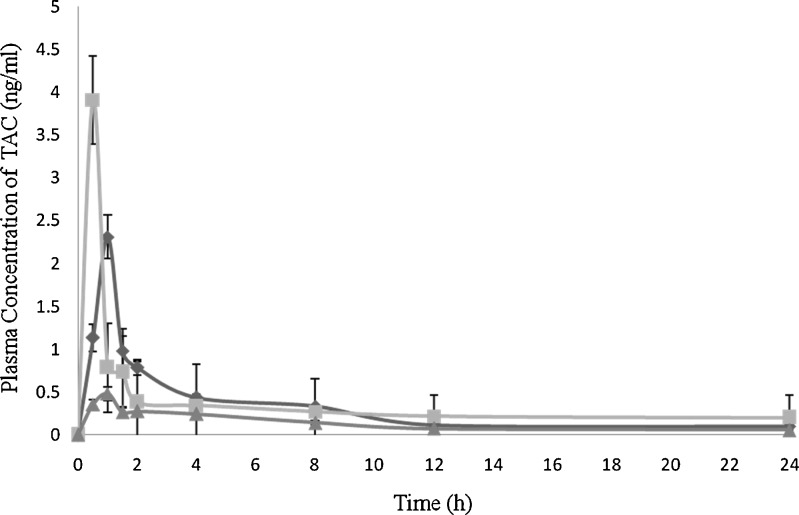
The TAC was quantified from plasma using LC/MS/MS method described earlier and chromatogram of TAC was depicted in Fig. 9. The pharmacokinetic study was carried out to determine the effect of enhanced solubility and in vitro dissolution of TAC-SMEDDS on the GI absorption of drug after oral administration in rats. The plasma profiles of TAC in Sprague Dawley rats after oral administration of the plain TAC, TAC-SMEDDS, and TAC marketed capsule (Pangraf® Panacea Biotech, India) were compared (Fig. 10). The pharmacokinetic parameters of TAC-SMEDDS was significantly (P < 0.05) higher than plain TAC and marketed capsule (Pangraf® Panacea Biotech, India) at the same dose. The bioavailability and pharmacokinetic parameters of TAC after the oral administration of TAC-SMEDDS, plain TAC, and marketed capsule (Pangraf® Panacea Biotech, India) are illustrated in Table III. The results showed that Cmax of TAC-SMEDDS was 1.69-fold and 8.14-fold higher than marketed capsule (Pangraf® Panacea Biotech, India) formulation and plain TAC, respectively. The relative bioavailability of the TAC-SMEDDS was 1.5-fold and 3.5-fold higher than marketed capsule (Pangraf® Panacea Biotech, India) and plain TAC, respectively. TAC-SMEDDS shows enhanced oral bioavailability with higher Cmax and lower Tmax which contributed by its higher drug solubilization potential and spontaneous formation of microemulsion. Furthermore, it may be possible that a higher bioavailability of SMEDDS resulted from enhanced absorption through the lymphatic pathway due to presence of long chain triglyceride in RGO (30,31). The low oral bioavailability of plain TAC and marketed formulation may be due to the low solubility of TAC with incomplete drug release. Thus, SMEDDS appear to be an alternative dosage form, which increase the bioavailability of TAC.
Fig. 9.
Standard chromatogram of (a) internal standard (Tamsulosin) and (b) TAC using LC/MS/MS method
Fig. 10.
Plasma concentration–time profiles (mean values, n = 5) following oral administration of (diamond) marketed capsule of TAC (Pangraf®), (square) TAC-SMEDDS, and (triangle) plain TAC to Sprague Dawley rats (n = 5; mean ± SD)
Table III.
Pharmacokinetic Parameters of Plain TAC, Marketed Capsule, and TAC-SMEDDS Following Oral Administration at Dose of 0.7 mg/kg Equivalent to TAC in Fasted Rats (n = 5)
| Parameters | Plain TAC | Marketed capsule | TAC-SMEDDS |
|---|---|---|---|
| AUC(0 − 24) (ng h/ml) | 12.59 ± 5.54* | 30.23 ± 10.34* | 45.05 ± 15.64 |
| C max (ng/ml) | 0.48 ± 0.12* | 2.31 ± 0.68* | 3.91 ± 1.2 |
| T max (h) | 1.0 | 1.0 | 0.5 |
| Rel. BA % | 40.03 ± 6.5 | – | 148.72 ± 2.0 |
TAC tacrolimus, SMEDDS self-microemulsifying drug delivery system, Rel. BA relative bioavailability, AUC area under curve
*P < 0.05 when compared marketed capsule formulation and plain drug with TAC-SMEDDS using one-way ANOVA
CONCLUSIONS
The present study explores the multifunctionality of RGO in SMEDDS formulation of lipophilic drug such as TAC. The antioxidant potential and solubilization capacity of RGO was significantly higher than RBO. The TAC-SMEDDS formulation using RGO showed significantly higher dissolution profile as well as improvement in oral pharmacokinetic parameters of TAC in comparison with plain TAC and marketed capsule. Hence, it can be concluded that gamma-oryzanol-enriched natural RGO is a multifunctional excipient for lipid drug delivery system like SMEDDS.
ACKNOWLEDGMENTS
The authors would like to thank UGC for providing financial assistance during the research work. The authors would like to thank AICTE/NAFETIC for providing the facility to conduct research. Authors are very pleased to thank Dr. V. K. Aswal and Dr. P. A. Hassan for conducting SANS and DLS study at BARC, India.
REFERENCES
- 1.Muller RH, Jacobs C, Kayser O. Nanosuspension as particulate drug formulation in therapy: Rationale of development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47:3–19. doi: 10.1016/S0169-409X(00)00118-6. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Sharma U. Liposomes in drug delivery: Progress and limitations. Int J Pharm. 1997;154:123–140. doi: 10.1016/S0378-5173(97)00135-X. [DOI] [Google Scholar]
- 3.Okada H, Toguchi H. Biodegradable microsphere in drug delivery. Crit. Rev. Ther. Carrier Syst. 1995;12:1–99. doi: 10.1615/critrevtherdrugcarriersyst.v12.i1.10. [DOI] [PubMed] [Google Scholar]
- 4.Patel AR, Vavia PR. Preparation and in vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrate. AAPS J. 2007;9:344–352. doi: 10.1208/aapsj0903041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–182. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Gao F, Zhang Z, Bu H, Huang Y, Gao Z, Shen J, Zhao C, Li Y. Nanoemulsion improves the oral absorption of candesartan cilexetil in rats: Performance and mechanism. J Control Release. 2011;149:168–174. doi: 10.1016/j.jconrel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Muhlen AZ, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery—drug release and release mechanism. Eur J Pharm Biopharm. 1998;45:149–155. doi: 10.1016/S0939-6411(97)00150-1. [DOI] [PubMed] [Google Scholar]
- 8.Grove M, Mullertz A, Nielsen JL, Pedersen GP. Bioavailability of seocalcitol II: Development and characterisation of self-microemulsifying drug delivery systems (SMEDDS) for oral administration containing medium and long chain triglycerides. Eur J Pharm Sci. 2006;28:233–242. doi: 10.1016/j.ejps.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: Physical and biopharmaceutical aspects. Pharm Res. 1995;12:1561–1572. doi: 10.1023/A:1016268311867. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Jiang X, Jiang W, Lina WL, Shi Z. Preparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain. Int J Pharm. 2004;275:85–96. doi: 10.1016/j.ijpharm.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 11.Whysner J, Williams GM. Butylated hydroxyanisole mechanistic data and risk assessment: Conditional species-specific cytotoxicity, enhanced cell proliferation, and tumor promotion. Pharmacol Ther. 1996;71:137–151. doi: 10.1016/0163-7258(96)00066-6. [DOI] [PubMed] [Google Scholar]
- 12.Kim IH, Kim CJ, You JM, Lee KW, Kim CT, Chung SH, Tae BS. Effect of roasting temperature and time on the chemical composition of rice germ oil. JAOCS. 2002;79:413–418. doi: 10.1007/s11746-002-0498-2. [DOI] [Google Scholar]
- 13.Bergman CJ, Xu Z. Genotype and environment effects on tocopherols, tocotrienols and gamma-oryzanol contents of Southern US rice. Cereal Chem. 2003;80:446–449. doi: 10.1094/CCHEM.2003.80.4.446. [DOI] [Google Scholar]
- 14.Garcia ML, Martinez JZ, Alfonso EFS, Mendonça RC, Ramos GR. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem. 2009;115:389–404. doi: 10.1016/j.foodchem.2009.01.063. [DOI] [Google Scholar]
- 15.Juliano C, Cossu M, Alamanni MC, Piu L. Antioxidant activity of gamma-oryzanol: Mechanism of action and its effect on oxidative stability of pharmaceutical oils. Int J Pharm. 2005;299:146–154. doi: 10.1016/j.ijpharm.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Kino T, Hatanaka H, Hashimoto M, Nishiyama M, Goto T, Okuhara M, Kohsaka M, Aoki H, Imanaka H. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot. 1987;40:1249–1255. doi: 10.7164/antibiotics.40.1249. [DOI] [PubMed] [Google Scholar]
- 17.Prasad TN, Stiff DD, Subbotina N, Zemaitis MA, Burckart GJ, Starzl TE, Venkataramanan R. FK 506 (Tacrolimus) metabolism by rat liver microsomes and its inhibition by other drugs. Res Commun Chem Pathol Pharmacol. 1994;84:35–46. [PubMed] [Google Scholar]
- 18.Borhade V, Nair H, Hegde D. Design and evaluation of self-microemulsifying drug delivery system (SMEDDS) of tacrolimus. AAPS PharmSciTech. 2008;9(1):13–21. doi: 10.1208/s12249-007-9014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Sun J, Zhang T, Liu H, He F, He Z. Enhanced oral bioavailability of tacrolimus in rats by self-microemulsifying drug delivery systems. Drug Dev Ind Pharm. 2011;37(10):1225–1230. doi: 10.3109/03639045.2011.565774. [DOI] [PubMed] [Google Scholar]
- 20.Anwar F, Anwer T, Mahmood Z. Methodical characterization of rice (Oryza sativa) bran oil from Pakistan. Grasas Aceites. 2005;56:125–134. doi: 10.3989/gya.2005.v56.i2.120. [DOI] [Google Scholar]
- 21.Apak R, Guçlu K, Demirata B, Ozyurek M, Çelik SE, Bektaşoglu B, Berker KI, Ozyurt D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC Assay. Molecules. 2007;12:1496–1547. doi: 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma OP, Bhat TK. DPPH antioxidant assay revisited. Food Chem. 2009;113:1202–1205. doi: 10.1016/j.foodchem.2008.08.008. [DOI] [Google Scholar]
- 23.http://www.accessdata.fda.gov/scripts/cder/dissolution. Accessed 29 August 11.
- 24.Basalious EB, Shawky N, Badr-Eldin SM. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: Development and optimization. Int J Pharm. 2010;391(1–2):203–211. doi: 10.1016/j.ijpharm.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Nassar T, Rom A, Nyska A, Benita S. Novel double coated nanocapsules for intestinal delivery and enhanced oral bioavailability of tacrolimus, a P-gp substrate drug. J Control Release. 2009;133:77–84. doi: 10.1016/j.jconrel.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Ramakrishna NVS, Vishwottam KN, Puran S, Manoj S, Santosh M, Wishu S, Koteshwara M, Chidambara J, Gopinadh B, Sumatha B. Liquid chromatography–negative ion electrospray tandem mass spectrometry method for the quantification of Tacrolimus in human plasma and its bioanalytical applications. J. Chromat. B. 2004;805:13–20. doi: 10.1016/j.jchromb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Pouton C. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur J Pharm Sci. 2000;11:S93–S98. doi: 10.1016/S0928-0987(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 28.Campo L, Yaghmur A, Garti N, Leser ME, Folmer B, Glatter O. Five-component food-grade microemulsions: structural characterization by SANS. J Colloid Interface Sci. 2004;274:251–267. doi: 10.1016/j.jcis.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Ping Z, Ying L, Nianping F, Jie X. Preparation and evaluation of self-microemulsifying drug delivery system of oridonin. Int J Pharm. 2008;30:2–8. doi: 10.1016/j.ijpharm.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Gershanik T, Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Sci. 2000;50:179–188. doi: 10.1016/s0939-6411(00)00089-8. [DOI] [PubMed] [Google Scholar]
- 31.Nankervis R, Davis S, Day N, Shaw P. Effect of lipid vehicle on the intestinal lymphatic transport of isotretinoin in the rat. Int J Pharm. 1995;119:173–181. doi: 10.1016/0378-5173(94)00390-Q. [DOI] [Google Scholar]