Abstract
A mature female alpaca was evaluated for weight loss and a 10-day history of anorexia, diarrhea, abdominal distension, and ventral edema. Ultrasonography revealed a hepatic mass, culture of which identified Corynebacterium pseudotuberculosis. This is the first reported case of an internal caseous lymphadenitis lesion resulting in clinical disease in a camelid.
Résumé
Abcès hépatique à Corynebacterium pseudotuberculosis chez un alpaga adulte (Lama pacos). Un alpaga femelle adulte a été évalué pour une perte de poids et une anamnèse de 10 jours d’anorexie, de diarrhée, de distension abdominale et d’œdème ventral. L’échographie a révélé une masse hépatique et la culture a identifié Corynebacterium pseudotuberculosis. Il s’agit du premier cas signalé d’une lésion lymphadénite caséueuse interne produisant une maladie clinique chez un camélidé.
(Traduit par Isabelle Vallières)
An 8-year-old female huacaya alpaca with a 2-month-old cria at foot was presented to the Veterinary Medical Teaching Hospital at Texas A&M University. The alpaca had lost weight since parturition, and had a 10-day history of anorexia, diarrhea, and ventral edema. Treatment with flunixin meglumine (dosage unknown) by the owner briefly improved her appetite 2 d prior to admission. The herd was routinely tested for bovine diarrhea virus and determined to be negative. This alpaca was purchased as an adult with other alpacas several years previously from Oregon.
Case description
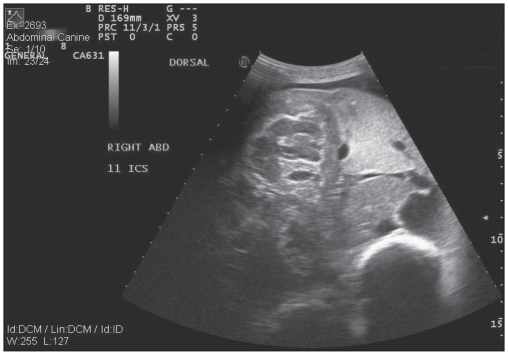
On presentation, the alpaca had a body condition score of 2/10 and weighed 78.6 kg. She was depressed and exhibited weakness during the physical examination with unwillingness to stand for more than a few minutes at a time. Caudal ventral and udder edema was present with poor udder development and minimal milk production. She had a distended abdomen and a fluid thrill was palpable on ballottement of the abdomen. The alpaca passed brown-colored watery feces with no evidence of tenesmus, blood, or mucus. Tachypnea was evident (40 breaths per min) with nostril flare. Bilaterally increased lung sounds were auscultated in the dorsal lung field. Mucous membranes were pale and moist. Ultrasonography of the abdomen was performed using a 3.5 mHz curvilinear probe, which revealed an increased amount of anechoic peritoneal fluid, with minimal fibrinous adhesions cranially. Marked liver enlargement, with a well-defined parenchymal mass that appeared to be adhered to the diaphragm, was visible on the right side at intercostal space 11 (Figure 1).
Figure 1.
Ultrasonographic image of the liver parenchymal mass as viewed from the right 11th intercostal space.
Ultrasound of the thorax revealed anechoic pleural effusion with fibrin deposition on the surface of the diaphragm and parietal surface of the pleura bilaterally. The cranial mediastinum had a similar appearance. No pericardial effusion was present and all structures of the heart appeared to be within normal limits.
A chest tube (24 French, Deknatel, New York, USA) was placed aseptically in the right thorax at intercostal space 7; 2 L of pleural fluid were obtained. The tube was removed after drainage due to aspiration of air into the pleural space. Abdominocentesis was also performed under aseptic conditions using an 18-gauge 1.5-inch needle in the ventral abdomen right of the midline. Samples of the pleural and peritoneal fluid were submitted for cytology and culture. Analysis of the pleural effusion revealed a clear colorless fluid, with total protein concentration of 21 g/L and a total nucleated cell concentration (TNCC) at 336/μL. Cytology identified 57% non-degenerate neutrophils, 42% macrophages, and 1% small lymphocytes, which suggested a transudate with increased numbers of non-degenerate neutrophils and monocytes, indicating a possible inflammatory process. The peritoneal fluid was characterized as a modified transudate: total protein concentration of 31 g/L [reference interval (RI): < 30 g/L] and TNCC of 1180/μL (RI: 2000 to 5000/μL) with 74% large mononuclear cells (macrophages and mesothelial cells), 25% nondegenerate neutrophils, and 1% small lymphocytes.
Serum biochemical abnormalities included hyperglycemia (10.5 mmol/L, RI: 4.5 to 8.7 mmol/L), increased blood urea nitrogen (24.6 mmol/L, RI: 4.6 to 13.6 mmol/L), hypoproteinemia (43 g/L, RI: 52 to 74 g/L) characterized by hypo-albuminemia (17 g/L, RI: 29 to 52 g/L), increased gamma glutamyl transferase (GGT) (113 U/L, RI: 8 to 30 U/L). Electrolyte abnormalities included hyponatremia (130 mmol/L, RI: 148 to 158 mmol/L), hypochloremia (96 mmol/L, RI: 97 to 117 mmol/L) and hypocalcemia (7.0 mmol/L, RI: 7.6 to 10.1 mmol/L).
Hematological abnormalities included leukocytosis (white blood cells 30 200/μL, RI: 7800 to 19 900/μL) with a neutrophilia (22 348/μL RI: 3650 to 13 826/μL) and toxic left shift (absolute bands 1208/μL, RI: 0 to 145/μL) and monocytosis (absolute monocytes 4228/μL, RI: 0 to 977/μL). The interpretation was that there was a chronic inflammatory process. There was also a non-regenerative anemia (packed cell volume 14%, RI: 26% to 47%).
Treatment was initiated with flunixin meglumine (Banamine; Intervet/Schering-Plough Animal Health, Union, New Jersey, USA), 0.5 mg/kg body weight (BW), IV, q12h, prior to a 256-mL IV bolus of llama plasma (Triple J Farms, Bellingham, Washington, USA). Following the plasma transfusion, intravenous lactated ringer’s fluids (Baxter, Deerfield, Illinois, USA) with an additional 50 mL 23% calcium gluconate (Vedco, St. Joseph, Missouri, USA) per 5 L and 10 mL B Vitamin Complex (Vedco) per 5 L were administered at 1.6 mL/kg BW per hour. Antimicrobial therapy included procaine penicillin G (Pen-aqueous; Agripharm, Westlake, Texas, USA) 22 000 IU/kg BW, SC, q12h and ceftiofur sodium (Naxcel; Pfizer, New York, USA) 5 mg/kg BW, IV, q6h.
During the first night of hospitalization the alpaca became hypothermic (temperature 35.4°C). A heat lamp and blankets were added and her temperature returned to normal range in 8 h. The cause of the hypothermia was unclear. On the second day, repeated serum biochemistry revealed a more severe hypoproteinemia (33 g/L) with a further decrease in albumin concentration (12 g/L). Gamma glutamyl transferase activity had decreased to 66 U/L. Abdominal radiographs did not reveal evidence of a metallic foreign body. Thoracic radiographs were consistent with bicavitary effusion, with an alveolar pattern present in the right cranial lung lobe. There were several ovoid gas opacities within the plane of the heart and lungs ventrally, which suggested pulmonary abscessation or bullae. An abdominal drain (22 French, Deknatel) was placed in the right cranial abdomen and 12 L of fluid were recovered. Hetastarch (Texas Parenteral Medicine, Irvine, California, USA), 10 mL/kg BW, IV was added as further treatment following drainage of the abdominal fluid. Abdominal ultrasound was repeated and aspirates and biopsies were obtained from the liver mass under ultrasound guidance. Cytology of the aspirate revealed a mixed cell inflammation with bacterial sepsis and many extracellular bacteria. The cell population consisted largely of intermediate to large lymphocytes. Interpretation of the cytology was inconclusive but differentials included a neoplastic lymphoid cell population with secondary necrosis and sepsis, or a primary inflammatory process (abscess). Samples were also submitted for culture.
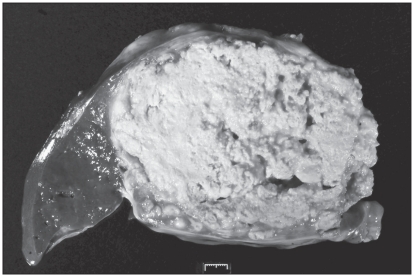
A fecal Wisconsin sugar floatation examination revealed thousands of Eimeria punoensis oocysts. On the third day, the alpaca was markedly weaker and continued to be anorexic. Due to the poor prognosis the owners elected humane euthanasia and necropsy. The results of histopathology of the liver mass biopsy were obtained just prior to necropsy and identified a severe chronic suppurative hepatitis with necrosis, consistent with liver abscessation. No neoplastic cells were identified. Pre-mortem culture of the liver biopsy obtained a pure growth of Corynebacterium pseudotuberculosis. Gross necropsy revealed marked subcutaneous edema, pleural effusion, and ascites. There were numerous 2 to 4 cm diameter firm nodules with caseous material on cut section (abscesses) in the lungs. The liver had numerous similar nodules ranging from 2 cm to a single large abscess measuring 13 × 9 × 7 cm. The largest abscess involved the caudal vena cava, resulting in 5 cm of the vena cava exiting the liver with coarsely nodular fluctuant thickened wall containing caseous material (Figure 2). Multiple lymph nodes exhibited hyperplasia, congestion, and edema. Histopathology and culture from the liver abscess at necropsy confirmed caseous lymphadenitis caused by C. pseudotuberculosis.
Figure 2.
Cross section of the liver abscess at necropsy.
Discussion
Liver disease in camelids is commonly reported in the USA and is associated with vague clinical signs including weight loss, inappetance, lethargy, colic, depression, and recumbency (1,2). The most common presentation of liver disease is hepatic lipidosis secondary to decreased protein intake and stress (3). Unlike the situation in cattle, the diagnosis of primary liver abscessation is rarely reported in the literature. Pathogens that have been isolated from liver lesions at necropsy include Rhodococcus equi in 4 camels from the same farm, all of whom exhibited primary lung lesions (4), Listeria monocytogenes from the liver and ileum in an adult male alpaca with histopathological evidence of necrosis in these organs (5), and Mycobacterium microti in caseous nodules of multiple lymph nodes and organs, including the liver (6). Initial ultrasound images of the hepatic mass could not differentiate between neoplasia and abscessation. Hepatic neoplasias documented in camelids include lymphosarcoma (7–9), congenital hepatoblastoma (10), biliary carcinoma (11), adenocarcinoma (11) and liver metastases from a primary pulmonary carcinoma (12). Hepatoma and hemangiosarcoma have each been reported in 1 llama (8). In this alpaca, the presence of a large liver abscess obstructing the caudal vena cava resulted in bicavitary effusion, ventral edema, and diarrhea due to increased hydrostatic pressure. Eimeria punosensis has been associated with clinical coccidiosis in alpacas (13) and may have contributed to the mild diarrhea and low albumin and total protein in this alpaca.
Caseous lymphadenitis (CLA) caused by the gram-positive facultative intracellular pathogen Corynebacterium pseudotuberculosis is an important disease of small ruminants worldwide (14), leading to economic losses due to carcass trimming and clean wool loss (15). C. pseudotuberculosis has been cultured from multiple species including sheep, goats, cattle, buffalo, horses, zebras (16), and deer (17). The prevalence in small ruminants in the western USA has been documented to be as high as 42.5% at slaughter (15). The disease prevalence is higher in female small ruminants, possibly due to females being kept until older ages (18). The prevalence of CLA in South American camelids has not been determined but infection has been documented in both North and South America (19,20). Braga et al (19) cultured C. pseudotuberculosis from 45 of 84 animals with abscesses from 2 herds in the Peruvian highlands. Of those culture-positive animals with external abscessation of the superficial lymph nodes or mammary glands (or both) that had postmortem examinations performed, 4% had evidence of liver abscessation. External abscessation is more common in goats, in particular of the lymph nodes of the head and neck (15), whereas internal (21) and superficial lymph node abscesses of the torso occur more frequently in sheep (15,21). In alpacas, abscessation of renal lymph nodes and superficial lymph nodes in adults and juveniles, respectively, has been observed (15). Superficial lymph nodes in 5 young alpacas from a single farm in North America had external abscesses positive for C. pseudotuberculosis on culture (20). None of these alpacas showed signs of systemic disease.
In sheep, inoculation in natural disease is thought to occur trans-cutaneously via wounds and abrasions, but has been induced experimentally via intravenous, intratracheal, intra-vaginal, and intralymphatic routes (15). Corynebacterium pseudotuberculosis survives and replicates in macrophages, which transport the organism to locally draining lymph nodes, allowing for systemic dissemination (15). Intra-dermal experimental infection of C. pseudotuberculosis in alpacas induced a febrile response locally 96 h after inoculation and subsequently caused purulent drainage at the inoculation site (22). There were internal abscesses (primarily of the renal lymph node and liver) in all but 1 alpaca euthanatized after day 58. Experimental infection induced an increase in antibody titer against a cell wall antigen on day 16 of infection (22); however, titers were not correlated to disease severity. Unlike in sheep, pulmonary lesions have not been associated with CLA in South American camelids (23).
This herd had no previous history of external abscessation, and no other herd members have been identified with C. pseudotuberculosis either internally or externally.
To our knowledge, this is the first report of internal abscessation caused by C. pseudotuberculosis in South American camelids in North America. Caseous lymphadenitis should be considered among other causes of weight loss and internal abscessation in alpacas.
Acknowledgments
We acknowledge assistance from Drs. Tracy Norman, M. Keith Chaffin, Sara D. Lawhon, Erin Quist, and Katrin Burke. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Fowler ME. 2nd ed. Vol. 43. Ames, Iowa: Iowa State Univ Pr; 1989. Medicine and Surgery of South American Camelids; pp. 263–267. [Google Scholar]
- 2.Anderson DE. Liver disease, metabolism and digestion in llamas and alpacas. J Camel Pract Res. 1999;6:163–169. [Google Scholar]
- 3.Cebra CK. Disorders of carbohydrate or lipid metabolism in camelids. Vet Clin N Am. 2009;25:339–352. doi: 10.1016/j.cvfa.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Kinne J, Madarame H, Takai S, Jose S, Wernery U. Disseminated Rhodococcus equi infection in dromedary camels (Camelus dromedarius) Vet Microbiol. 2011;149:269–272. doi: 10.1016/j.vetmic.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 5.Seehusen F, Lehmbeker A, Puff C, Kleinschmidt S, Klein S, Baumgärtner W. Listeria monocytogenes septicemia and concurrent clostridial infection in an adult alpaca (Lama pacos) J Comp Path. 2008;139:126–129. doi: 10.1016/j.jcpa.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Zanolari P, Robert N, Lyashchenko KP, et al. Tuberculosis caused by Mycobacterium microti in South American camelids. J Vet Intern Med. 2009;23:1266–1272. doi: 10.1111/j.1939-1676.2009.0377.x. [DOI] [PubMed] [Google Scholar]
- 7.Cebra CK, Garry FB, Powers BE, Johnson LW. Lymphosarcoma in 10 New World camelids. J Vet Intern Med. 1995;9:381–385. doi: 10.1111/j.1939-1676.1995.tb03297.x. [DOI] [PubMed] [Google Scholar]
- 8.Potter K, Young JL. Three cases of hepatic neoplasia in llamas. Vet Med-US. 1994;89:914–916. [Google Scholar]
- 9.Irwin JA. Lymphosarcoma in an alpaca. Can Vet J. 2001;42:805–806. [PMC free article] [PubMed] [Google Scholar]
- 10.Watt BC, Cooley J, Darlen BJ. Congenital hepatoblastoma in a neonatal alpaca cria. Can Vet J. 2001;42:872–874. [PMC free article] [PubMed] [Google Scholar]
- 11.Valentine BA, Martin JM. Prevalence of neoplasia in llamas and alpacas (Oregon State University, 2001–2006) J Vet Diagn Invest. 2007;19:202–204. doi: 10.1177/104063870701900213. [DOI] [PubMed] [Google Scholar]
- 12.Ramos-Vara JA, Loiacono CM, Williams F, III, Pardo I, Lakritz J. Pulmonary neoplasia in two llamas (Lama glama) Vet Pathol. 2004;41:520–523. doi: 10.1354/vp.41-5-520. [DOI] [PubMed] [Google Scholar]
- 13.Ballweber LR. Ecto- and endoparasites on New World camelids. Vet Clin Food Anim. 2009;25:295–310. doi: 10.1016/j.cvfa.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Dorella FA, Pacheco LGC, Oliverira SC, Miyoshi A, Azevedi V. Corynebacterium pseudotuberculosis: Microbiology, biochemical properties, pathogenesis and molecular studies of virulence. Vet Res. 2006;37:201–218. doi: 10.1051/vetres:2005056. [DOI] [PubMed] [Google Scholar]
- 15.Baird GJ, Fontaine MC. Corynebacterium pseudotuberculosis and its role in ovine caseous lymphadenitis. J Comp Path. 2007;137:179–219. doi: 10.1016/j.jcpa.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Aleman MA, Spier SJ. Corynebacterium pseudotuberculosis infection. In: Smith BP, editor. Large Animal Internal Medicine. 4th ed. St Louis, Missouri: Mosby Elsevier; 2009. pp. 1184–1188. [Google Scholar]
- 17.Stauber E, Armstrong P, Chamberlain K. Caseous lymphadenitis in a white-tailed deer. J Wildl Dis. 1973;9:56–57. doi: 10.7589/0090-3558-9.1.56. [DOI] [PubMed] [Google Scholar]
- 18.Al-Gaabary M, Osman SA, Oreiby AF. Caseous lymphadenitis in sheep and goats: Clinical, epidemiological and preventative studies. Small Ruminant Res. 2009;87:116–121. [Google Scholar]
- 19.Braga WU, Chavera A, Gonzalez A. Corynebacterium pseudotuberculosis infection in highland alpacas (Lama pacos) in Peru. Vet Rec. 2006;159:23–24. doi: 10.1136/vr.159.1.23. [DOI] [PubMed] [Google Scholar]
- 20.Anderson DE, Rings DM, Kowalski J. Infection with Corynebacterium pseudotuberculosis in five alpacas. J Am Vet Med Assoc. 2004;225:1743–1747. doi: 10.2460/javma.2004.225.1743. [DOI] [PubMed] [Google Scholar]
- 21.Braga WU, Chavera AE, Gonzalez AE. Clinical, humoral, and pathologic findings in adult alpacas with experimentally induced Corynebacterium pseudotuberculosis infection. Am J Vet Res. 2006;67:1570–1574. doi: 10.2460/ajvr.67.9.1570. [DOI] [PubMed] [Google Scholar]
- 22.Loftstedt J. Progressive bacterial and viral pneumonias of sheep and goats. In: Smith BP, editor. Large Animal Internal Medicine. 4th ed. St Louis, Missouri: Mosby Elsevier; 2009. pp. 658–659. [Google Scholar]
- 23.Braga WU. Protection in alpacas against Corynebacterium pseudotu-berculosis using different bacterial components. Vet Microbiol. 2007;119:297–303. doi: 10.1016/j.vetmic.2006.08.019. [DOI] [PubMed] [Google Scholar]