Abstract
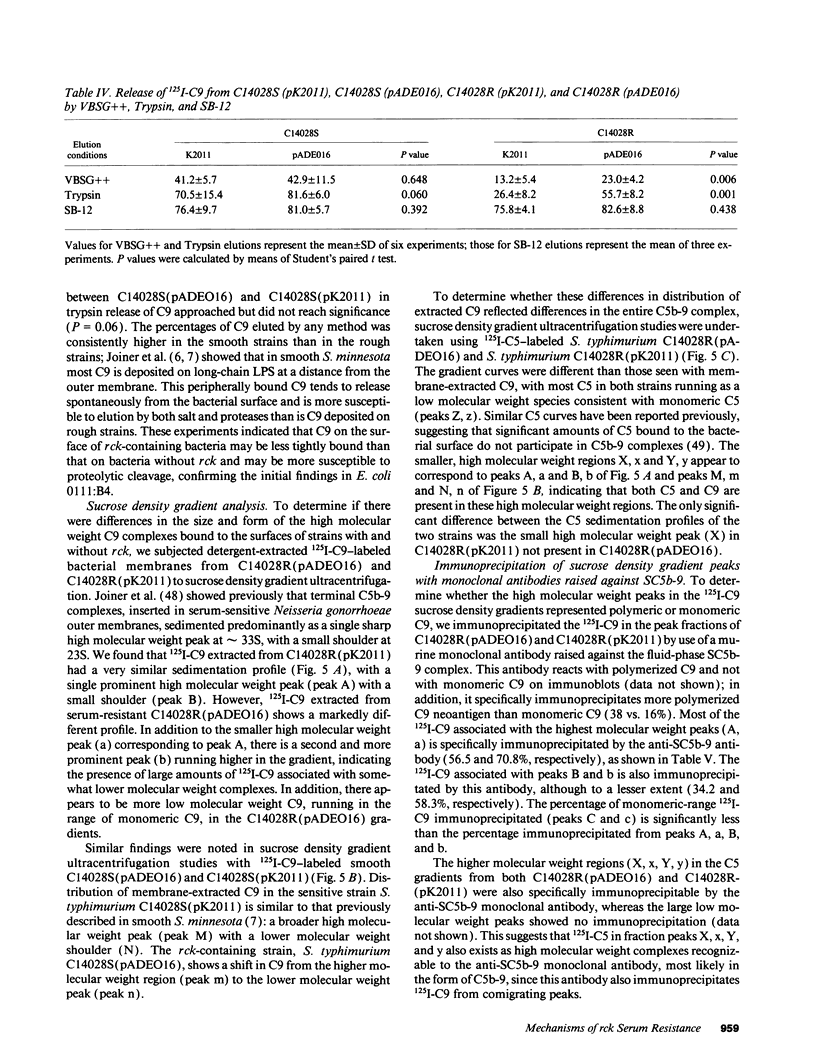
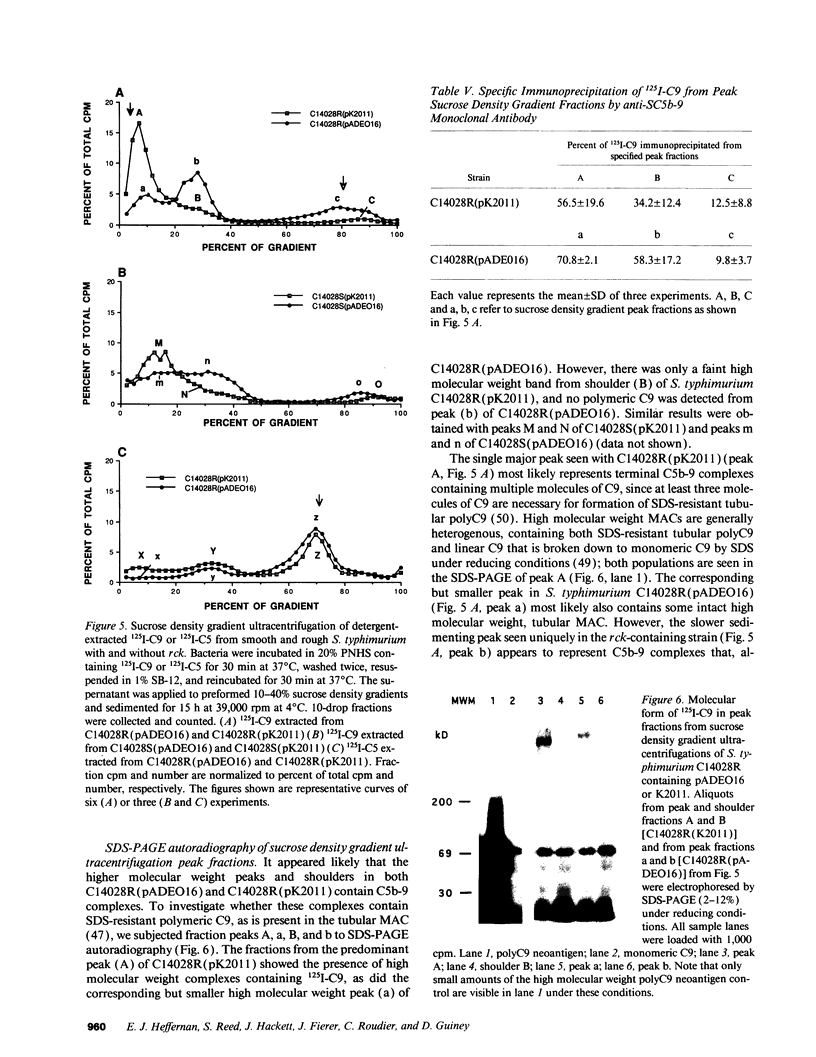
We find that pADEO16, a recombinant cosmid carrying the rck gene of the Salmonella typhimurium virulence plasmid, when cloned into either rough or smooth Escherichia coli and Salmonella strains, confers high level resistance to the bactericidal activity of pooled normal human serum. The rck gene encodes a 17-kD outer membrane protein that is homologous to a family of virulence-associated outer membrane proteins, including pagC and Ail. Complement depletion, C3 and C5 binding, and membrane-bound C3 cleavage products are similar in strains with and without rck. Although a large difference in C9 binding was not seen, trypsin cleaved 55.7% of bound 125I-C9 counts from rough S. typhimurium with pADEO16, whereas only 26.4% were released from S. typhimurium with K2011, containing a mutation in rck. The majority of C9 extracted from rck strain membranes sediments at a lower molecular weight than in strains without rck, suggesting less C9 polymerization. Furthermore, SDS-PAGE analysis of gradient peak fractions indicated that the slower sedimenting C9-containing complexes in rck strains did not contain polymerized C9 typical of the tubular membrane attack complex. These results indicate that complement resistance mediated by Rck is associated with a failure to form fully polymerized tubular membrane attack complexes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barondess J. J., Beckwith J. A bacterial virulence determinant encoded by lysogenic coliphage lambda. Nature. 1990 Aug 30;346(6287):871–874. doi: 10.1038/346871a0. [DOI] [PubMed] [Google Scholar]
- Beninger P. R., Chikami G., Tanabe K., Roudier C., Fierer J., Guiney D. G. Physical and genetic mapping of the Salmonella dublin virulence plasmid pSDL2. Relationship to plasmids from other Salmonella strains. J Clin Invest. 1988 May;81(5):1341–1347. doi: 10.1172/JCI113461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Kuller G., Muhly M., Fromm S., Seibert G., Parrisius J. Formation of transmural complement pores in serum-sensitive Escherichia coli. Infect Immun. 1987 Jan;55(1):206–210. doi: 10.1128/iai.55.1.206-210.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch E. F., Schmetz M. A., Foulds J., Hammer C. H., Frank M. M., Joiner K. A. Multimeric C9 within C5b-9 is required for inner membrane damage to Escherichia coli J5 during complement killing. J Immunol. 1987 Feb 1;138(3):842–848. [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R., Müller-Eberhard H. J. The reaction mechanism of human C5 in immune hemolysis. J Exp Med. 1970 Oct 1;132(4):775–793. doi: 10.1084/jem.132.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankert J. R., Esser A. F. Bacterial killing by complement. C9-mediated killing in the absence of C5b-8. Biochem J. 1987 Jun 1;244(2):393–399. doi: 10.1042/bj2440393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. E., Freedman S. D., Douglas H., Braude A. I. Analysis of sugars in bacterial endotoxins by gas-liquid chromatography. Anal Biochem. 1969 Apr 4;28(1):243–256. doi: 10.1016/0003-2697(69)90175-4. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer J., Finley F., Braude A. I. Release of 51Cr-endotoxin from bacteria as an assay of serum bactericidal activity. J Immunol. 1974 Jun;112(6):2184–2192. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Francoeur A. M., Peebles C. L., Gompper P. T., Tan E. M. Identification of Ki (Ku, p70/p80) autoantigens and analysis of anti-Ki autoantibody reactivity. J Immunol. 1986 Mar 1;136(5):1648–1653. [PubMed] [Google Scholar]
- Goldman R. C., Joiner K., Leive L. Serum-resistant mutants of Escherichia coli O111 contain increased lipopolysaccharide, lack an O antigen-containing capsule, and cover more of their lipid A core with O antigen. J Bacteriol. 1984 Sep;159(3):877–882. doi: 10.1128/jb.159.3.877-882.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Miller M. F. Complement attack of altered outer membrane areas synthesized after inhibition of the 3-deoxy-D-manno-octulosonate pathway leads to cell death. J Immunol. 1989 Jan 1;142(1):185–194. [PubMed] [Google Scholar]
- Guiney D. G., Hasegawa P., Davis C. E. Plasmid transfer from Escherichia coli to Bacteroides fragilis: differential expression of antibiotic resistance phenotypes. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7203–7206. doi: 10.1073/pnas.81.22.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A. Virulence plasmids of Salmonella typhimurium and other salmonellae. Microb Pathog. 1990 Jan;8(1):3–11. doi: 10.1016/0882-4010(90)90003-9. [DOI] [PubMed] [Google Scholar]
- Hackett J., Wyk P., Reeves P., Mathan V. Mediation of serum resistance in Salmonella typhimurium by an 11-kilodalton polypeptide encoded by the cryptic plasmid. J Infect Dis. 1987 Mar;155(3):540–549. doi: 10.1093/infdis/155.3.540. [DOI] [PubMed] [Google Scholar]
- Harriman G. R., Esser A. F., Podack E. R., Wunderlich A. C., Braude A. I., Lint T. F., Curd J. G. The role of C9 in complement-mediated killing of Neisseria. J Immunol. 1981 Dec;127(6):2386–2390. [PubMed] [Google Scholar]
- Harriman G. R., Podack E. R., Braude A. I., Corbeil L. C., Esser A. F., Curd J. G. Activation of complement by serum-resistant Neisseria gonorrhoeae. Assembly of the membrane attack complex without subsequent cell death. J Exp Med. 1982 Oct 1;156(4):1235–1249. doi: 10.1084/jem.156.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan E. J., Harwood J., Fierer J., Guiney D. The Salmonella typhimurium virulence plasmid complement resistance gene rck is homologous to a family of virulence-related outer membrane protein genes, including pagC and ail. J Bacteriol. 1992 Jan;174(1):84–91. doi: 10.1128/jb.174.1.84-91.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. II. C8 and C9 release C5b67 from the surface of Salmonella minnesota S218 because the terminal complex does not insert into the bacterial outer membrane. J Exp Med. 1982 Mar 1;155(3):809–819. doi: 10.1084/jem.155.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Schmetz M. A., Sanders M. E., Murray T. G., Hammer C. H., Dourmashkin R., Frank M. M. Multimeric complement component C9 is necessary for killing of Escherichia coli J5 by terminal attack complex C5b-9. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4808–4812. doi: 10.1073/pnas.82.14.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A. Studies on the mechanism of bacterial resistance to complement-mediated killing and on the mechanism of action of bactericidal antibody. Curr Top Microbiol Immunol. 1985;121:99–133. doi: 10.1007/978-3-642-45604-6_6. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Tartanian A. B., Hammer C. H., Schweinle J. E. Multimeric C9 within C5b-9 deposits in unique locations in the cell wall of Salmonella typhimurium. J Immunol. 1989 Jun 15;142(12):4450–4457. [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Brown E. J., Swanson J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. IV. C5b-9 forms high molecular weight complexes with bacterial outer membrane constituents on serum-resistant but not on serum-sensitive Neisseria gonorrhoeae. J Immunol. 1983 Sep;131(3):1443–1451. [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Hammer C., Frank M. M. Bactericidal but not nonbactericidal C5b-9 is associated with distinctive outer membrane proteins in Neisseria gonorrhoeae. J Immunol. 1985 Mar;134(3):1920–1925. [PubMed] [Google Scholar]
- Kawahara K., Hamaoka T., Suzuki S., Nakamura M., Murayama S. Y., Arai T., Terakado N., Danbara H. Lipopolysaccharide alteration mediated by the virulence plasmid of Salmonella. Microb Pathog. 1989 Sep;7(3):195–202. doi: 10.1016/0882-4010(89)90055-7. [DOI] [PubMed] [Google Scholar]
- Kitamura F., Shimada K., Suzuki T., Nishioka K. A simplified method for the preparation of EAC14 intermediate cells using human serum treated with methylprednisolone. J Immunol Methods. 1985 Dec 27;85(2):363–370. doi: 10.1016/0022-1759(85)90145-0. [DOI] [PubMed] [Google Scholar]
- Krause M., Roudier C., Fierer J., Harwood J., Guiney D. Molecular analysis of the virulence locus of the Salmonella dublin plasmid pSDL2. Mol Microbiol. 1991 Feb;5(2):307–316. doi: 10.1111/j.1365-2958.1991.tb02111.x. [DOI] [PubMed] [Google Scholar]
- Kroll H. P., Voigt W. H., Taylor P. W. Stable insertion of C5b-9 complement complexes into the outer membrane of serum treated, susceptible Escherichia coli cells as a prerequisite for killing. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Dec;258(2-3):316–326. doi: 10.1016/s0176-6724(84)80050-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Risse H. J., Ruschmann E., Schlecht S., Schmidt G., Schulte-Holthausen H., Wheat R., Westphal O., Schlosshardt J. Structural relationship of Salmonella O and R antigens. Ann N Y Acad Sci. 1966 Jun 30;133(2):349–374. doi: 10.1111/j.1749-6632.1966.tb52376.x. [DOI] [PubMed] [Google Scholar]
- MacKay S. L., Dankert J. R. Bacterial killing and inhibition of inner membrane activity by C5b-9 complexes as a function of the sequential addition of C9 to C5b-8 sites. J Immunol. 1990 Nov 15;145(10):3367–3371. [PubMed] [Google Scholar]
- McCutchan J. A., Levine S., Braude A. I. Influence of colony type on susceptibility of gonococci to killing by human serum. J Immunol. 1976 Jun;116(6):1652–1655. [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Bliska J. B., Falkow S. Nucleotide sequence of the Yersinia enterocolitica ail gene and characterization of the Ail protein product. J Bacteriol. 1990 Feb;172(2):1062–1069. doi: 10.1128/jb.172.2.1062-1069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Farmer J. J., 3rd, Hill W. E., Falkow S. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect Immun. 1989 Jan;57(1):121–131. doi: 10.1128/iai.57.1.121-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Mayden J., Achtman M., Levine R. P. Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing. Infect Immun. 1983 Dec;42(3):907–913. doi: 10.1128/iai.42.3.907-913.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Biesecker G., Kolb W. P., Müller-Eberhard H. J. The C5b-6 complex: reaction with C7, C8, C9. J Immunol. 1978 Aug;121(2):484–490. [PubMed] [Google Scholar]
- Podack E. R., Biesecker G., Müller-Eberhard H. J. Membrane attack complex of complement: generation of high-affinity phospholipid binding sites by fusion of five hydrophilic plasma proteins. Proc Natl Acad Sci U S A. 1979 Feb;76(2):897–901. doi: 10.1073/pnas.76.2.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Circular polymerization of the ninth component of complement. Ring closure of the tubular complex confers resistance to detergent dissociation and to proteolytic degradation. J Biol Chem. 1982 Dec 25;257(24):15204–15212. [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. Enharncement of the hemolytic activity of the second component of human complement by oxidation. J Exp Med. 1967 Dec 1;126(6):1013–1025. doi: 10.1084/jem.126.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puentes S. M., Sacks D. L., da Silva R. P., Joiner K. A. Complement binding by two developmental stages of Leishmania major promastigotes varying in expression of a surface lipophosphoglycan. J Exp Med. 1988 Mar 1;167(3):887–902. doi: 10.1084/jem.167.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen W. S., Miller S. I. A Salmonella typhimurium virulence protein is similar to a Yersinia enterocolitica invasion protein and a bacteriophage lambda outer membrane protein. J Bacteriol. 1991 Jan;173(1):86–93. doi: 10.1128/jb.173.1.86-93.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen M., Sukupolvi S. The role of the traT gene of the Salmonella typhimurium virulence plasmid for serum resistance and growth within liver macrophages. Microb Pathog. 1988 Oct;5(4):275–285. doi: 10.1016/0882-4010(88)90100-3. [DOI] [PubMed] [Google Scholar]
- Roantree R. J., Rantz L. A. A STUDY OF THE RELATIONSHIP OF THE NORMAL BACTERICIDAL ACTIVITY OF HUMAN SERUM TO BACTERIAL INFECTION. J Clin Invest. 1960 Jan;39(1):72–81. doi: 10.1172/JCI104029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudier C., Krause M., Fierer J., Guiney D. G. Correlation between the presence of sequences homologous to the vir region of Salmonella dublin plasmid pSDL2 and the virulence of twenty-two Salmonella serotypes in mice. Infect Immun. 1990 May;58(5):1180–1185. doi: 10.1128/iai.58.5.1180-1185.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder G., Brandenburg K., Brade L., Seydel U. Pore formation by complement in the outer membrane of gram-negative bacteria studied with asymmetric planar lipopolysaccharide/phospholipid bilayers. J Membr Biol. 1990 Nov;118(2):161–170. doi: 10.1007/BF01868473. [DOI] [PubMed] [Google Scholar]
- Stoorvogel J., van Bussel M. J., Tommassen J., van de Klundert J. A. Molecular characterization of an Enterobacter cloacae outer membrane protein (OmpX). J Bacteriol. 1991 Jan;173(1):156–160. doi: 10.1128/jb.173.1.156-160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terakado N., Hamaoka T., Danbara H. Plasmid-mediated serum resistance and alterations in the composition of lipopolysaccharides in Salmonella dublin. J Gen Microbiol. 1988 Jul;134(7):2089–2093. doi: 10.1099/00221287-134-7-2089. [DOI] [PubMed] [Google Scholar]
- Terakado N., Ushijima T., Samejima T., Ito H., Hamaoka T., Murayama S., Kawahara K., Danbara H. Transposon insertion mutagenesis of a genetic region encoding serum resistance in an 80 kb plasmid of Salmonella dublin. J Gen Microbiol. 1990 Sep;136(9):1833–1838. doi: 10.1099/00221287-136-9-1833. [DOI] [PubMed] [Google Scholar]
- Tinge S. A., Curtiss R., 3rd Isolation of the replication and partitioning regions of the Salmonella typhimurium virulence plasmid and stabilization of heterologous replicons. J Bacteriol. 1990 Sep;172(9):5266–5277. doi: 10.1128/jb.172.9.5266-5277.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson S., Taylor P. W., Morgan B. P., Luzio J. P. Killing of gram-negative bacteria by complement. Fractionation of cell membranes after complement C5b-9 deposition on to the surface of Salmonella minnesota Re595. Biochem J. 1989 Oct 15;263(2):505–511. doi: 10.1042/bj2630505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Podack E. R., Müller-Eberhard H. J. The membrane attack complex of complement: C5b-8 complex as accelerator of C9 polymerization. J Immunol. 1985 Jan;134(1):495–499. [PubMed] [Google Scholar]
- Vandenbosch J. L., Kurlandsky D. R., Urdangaray R., Jones G. W. Evidence of coordinate regulation of virulence in Salmonella typhimurium involving the rsk element of the 95-kilobase plasmid. Infect Immun. 1989 Aug;57(8):2566–2568. doi: 10.1128/iai.57.8.2566-2568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbosch J. L., Rabert D. K., Jones G. W. Plasmid-associated resistance of Salmonella typhimurium to complement activated by the classical pathway. Infect Immun. 1987 Nov;55(11):2645–2652. doi: 10.1128/iai.55.11.2645-2652.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbosch J. L., Rabert D. K., Kurlandsky D. R., Jones G. W. Sequence analysis of rsk, a portion of the 95-kilobase plasmid of Salmonella typhimurium associated with resistance to the bactericidal activity of serum. Infect Immun. 1989 Mar;57(3):850–857. doi: 10.1128/iai.57.3.850-857.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Levine R. P. How complement kills E. coli. I. Location of the lethal lesion. J Immunol. 1981 Sep;127(3):1146–1151. [PubMed] [Google Scholar]