Abstract
A 9-year-old female Yorkshire terrier was presented for vomiting and diarrhea. Blood chemistry tests revealed hepatic dysfunction, cholestasis, and inflammation. Liver ultrasonography and liver biopsy were consistent with cholangiohepatitis. Fine-needle aspiration of the gallbladder revealed the presence of bacteria later identified as Clostridium spp. The cholangiohepatitis was successfully treated.
Résumé
Une chienne Yorkshire terrier de 9 ans est présentée pour vomissements et diahrrée. La biochimie sanguine révèle dysfonction hépatique, cholestase, et inflammation. L’échographie et la biopsie hépatique sont compatibles avec une cholangiohépatite. Une aspiration à l’aiguille fine de la vésicule billiaire révèle la presence de bactéries (Clostridium spp.).
(Traduit par les auteurs)
Cholangiohepatitis is a rare condition in dogs (1) with few cases reported (1–3), although there are occasional, limited, case descriptions in review articles on other topics (4). Clinical signs are typically non-specific (1). However, this case illustrates that early diagnosis can be made when appropriate complementary examinations, including imaging and clinical pathology, are used in synergy.
Case description
A 9-year-old, intact female, Yorkshire terrier, was presented with a 2-week history of anorexia, vomiting, and diarrhea. The report of the referring veterinarian revealed hyperthermia (39.8°C), weight-loss, and dullness at clinical examination. Complete blood work was performed and the data from hematology and biochemistry are presented in Tables 1 and 2, respectively. Remarkable results included hepatocellular injury (moderately increased alanine aminotransferase and moderate-to-marked increase in glutamate dehydrogenase activities), decreased hepatic function (moderately increased fasting bile acids, mild hypoalbuminemia, and slight hyperbilirubinemia) and cholestasis (markedly increased alkaline phosphatase and moderately-to-markedly increased gamma-glutamyl-transferase activities). There was also inflammation (slight mature neutrophilia, slight monocytosis, and moderate hyperglobulinemia). Slight azotemia, mild hypernatremia, and moderate hyperchloremia were attributed to dehydration, secondary to vomiting and diahrrhea. The results of routine urinalysis and coagulation tests were within reference ranges.
Table 1.
Hematology data for the Yorkshire terrier
| Parameter | Value on presentation | Value post-treatment | Reference range |
|---|---|---|---|
| Hematocrit (L/L) | 0.45 | 0.53 | 0.37–0.55 |
| Hemoglobin (g/L) | 157 | 185 | 120–180 |
| Red blood cells (×1012/L) | 6.4 | 7.46 | 5.5–8.5 |
| MCHC (g/L) | 348 | 349 | 310–362 |
| MCV (fL) | 71 | 71 | 60–77 |
| MCH (pg) | 25 | 24.8 | 19.5–25 |
| Platelets (×109/L) | 300 | 345 | 150–500 |
| MPV (fL) | 12 | 10.20 | 7–11 |
| WBC (×109/L) | 20 | 9.39 | 6–17 |
| Neutrophils (×109/L) | 13.5 | 6.87 | 3–11.5 |
| Band neutrophils (×109/L) | 0.59 | 0 | 0–0.3 |
| Lymphocytes (×109/L) | 2.9 | 1.67 | 1–3.6 |
| Monocytes (×109/L) | 1.55 | 0.5 | 0–1.35 |
| Eosinophils (×109/L) | 0.98 | 0.32 | 0–1.47 |
MCHC — mean corpuscular hemoglobin concentration, MCV — mean corpuscular volume, MCH — mean corpuscular hemoglobin, MPV — mean platelet volume, WBC — white blood cell count.
Table 2.
Biochemistry data for the Yorkshire terrier
| Parameter | Value on presentation | Value post-treatment | Reference range |
|---|---|---|---|
| Total protein (g/L) | 76 | 70.9 | 54–71 |
| Albumin (g/L) | 30 | 36.4 | 31–40 |
| Globulin (g/L) | 46 | 34.5 | 28–42 |
| ALT (U/L) | 429 | 27 | 0–50 |
| GLDH (U/L) | 94 | ND | 0–10 |
| ALP (U/L) | 4336 | 49 | 0–140 |
| GGT (U/L) | 27 | 0 | 0–8 |
| Bile acids (μmol/L) | 37 | ND | 0–15 |
| Post prandial bile acids (μmol/L) | 13.7 | ND | |
| Total bilirubin (μmol/L) | 10.6 | ND | 0.9–10 |
| Amylase (U/L) | 876 | 731 | 0–730 |
| Lipase (U/L) | 51 | 161 | 0–130 |
| Creatine kinase (U/L) | 47 | 110 | 0–50 |
| Cholesterol (mmol/L) | 5.8 | 5.65 | 3.2–6.5 |
| Glucose (mmol/L) | 3.8 | 5.7 | 3–6.5 |
| Urea (mmol/L) | 10 | 4.3 | 3.6–8.6 |
| Creatinine (μmol/L) | 68 | 79 | 20–120 |
| Sodium (mmol/L) | 152 | 148 | 137–151 |
| Chloride (mmol/L) | 113 | 108.5 | 99–110 |
| Calcium (mmol/L) | 2.7 | 2.59 | 2.3–3 |
| Phosphorus (mmol/L) | 1.8 | 0.9 | 0.8–1.8 |
| Potassium (mmol/L) | 3.9 | 4.05 | 3.7–5.8 |
ALT — alanine aminotransferase, GLDH — glutamate dehydrogenase, ALP — alkaline phosphatase, GGT — gamma-glutamyl transferase.
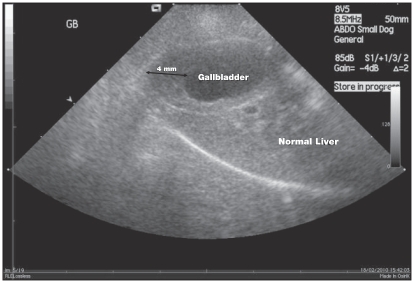
In order to investigate suspected liver damage, abdominal ultrasound was performed that showed gallbladder wall thickening, up to 4 mm (reference range: 2 to 3 mm) (5) (Figure 1). The common bile duct was marginally distended, up to 3 mm near the duodenal papilla. The liver was otherwise normal. In addition, there was a focal area of gastric wall thickening at the level of the lesser curvature of the pyloric canal measuring up to 1 cm. In the mucosal surface of this area there was a persistent gas accumulation generating multiple comet tail artefacts. These findings were suggestive of distal gastric ulceration. Endoscopy was not performed.
Figure 1.
Ultrasonography of the liver and the gallbladder showing thickening of the gallbladder wall.
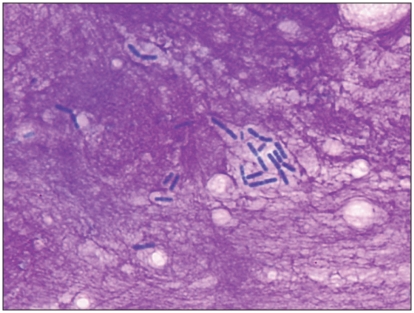
An ultrasound-guided, fine-needle aspiration of the gallbladder was performed. A direct smear of the bile was prepared for microscopic examination and a sample was sent for culture. On direct smears, uniform, large bacterial rods in pairs and small chains typical of clostridia (> 25/100× objective field) were seen in an abundant bright-pink, fibrillar background (Figure 2). No intact cells were seen. Bacterial culture resulted in a pure culture of Clostridium sp. At this stage, clostridial bactibilia was diagnosed.
Figure 2.
Microscopic appearance of the bile aspirated from the gallbladder showing clostridial bactibilia (Wright Giemsa, ×1000 magnification).
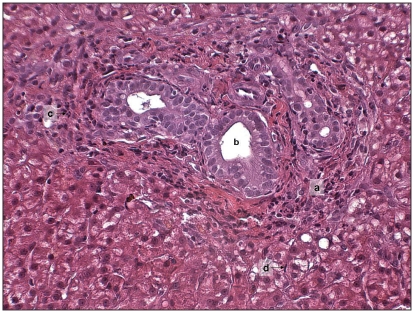
The possibility of a concurrent cholangiohepatitis was investigated by 2.5 cm length core liver biopsy, using a 16-gauge tissue-core biopsy needle under ultrasonographic control. Microscopy of the biopsy revealed periportal expansion by macrophages, plasma cells, neutrophils, and occasional lymphocytes. Bile ducts were prominent with low grade hyperplasia (Figure 3). Hepatocytes were mildly swollen and occasionally contained dark yellow bile granules. Kupffer cells were prominent and sinusoids contained moderate numbers of neutrophils. Mild, multifocal, hepatocyte necrosis with moderate neutrophilic and histiocytic infiltration was present. The diagnosis of cholangitis with mild-to-moderate, subacute, hepato-pericholangitis was therefore made.
Figure 3.
Microscopic appearance of the liver periportal area showing periportal expansion (a), prominent bile ducts (b), neutrophilic infiltration (c), and prominent Kupffer cells (d). (Hematoxylin & Eosin, ×200 magnification).
The 3.7-kg dog was successfully treated for 6 wk with clindamycin (Antirobe; Pfizer, Dublin, Ireland) 40 mg and amoxicillin clavulanate (Synulox; Pfizer), 50 mg, PO, twice daily. The dog also received sucralfate (Antepsin; Chugai Pharma UK, London, UK), 0.5 g PO, TID, ursodeoxycholic acid (Destolit, Norgine, Harefield, UK), 50 mg PO, SID, and lansoprazole (Destolit; Norgine), 3.75 mg PO, SID. Hematologic and serum biochemical results obtained 2 mo after diagnosis had no abnormalities (Tables 1 and 2).
Discussion
In the dog, gallbladder wall thickening has been identified in cases of acute or chronic hepatitis, cholecystitis, or cholangio-hepatitis (6,5). It can also be seen in association with other conditions such as sepsis and neoplasia. Ultrasonographic-guided, percutaneous liver cholecystocentesis for cytological examination and culture of bile can help to determine the cause of gallbladder wall thickening (4). However, this procedure has long been perceived as carrying an unacceptable risk of bile peritonitis. To the authors’ knowledge, there are no data on side-effects of cholecystocentesis in dogs suffering from bacterial cholangitis. However, in healthy dogs, it has been shown to be safe and easy to perform (7).
The bactibilia, together with the gallbladder thickening, was suggestive of cholecystitis. The absence of inflammatory cells on bile microscopic examination has been previously reported in humans with clinically significant cholecystitis (3). This may be due to bile cytolytic activity and the bright-pink fibrillar background seen in our case may be chromatin. Cholecystitis is frequently associated with cholangiohepatitis. Microscopic examination of the biopsy confirmed a mild-to-moderate, subacute, hepato-pericholangitis. The clinicopathologic and liver biopsy findings could have been a consequence of acute pancreatitis. However, this possibility was not likely as both lipase and amylase activities were not significantly increased and as pancreatic ultrasonography was normal. Canine pancreatic lipase immunoreactivity (cPLI) could have helped exclude pancreatitis but was not performed. Clindamycin and amoxicillin clavulanate were chosen 1) for their activity against gram-positive anaerobic bacteria, and 2) because they reach their highest concentrations in liver-tissue and bile, respectively. Ursodeoxycholic acid was used as a choleretic and as a broad-spectrum antibiotic. Sucralfate and lansoprazole were administered in order to treat the suspected gastric ulcers.
Cholangitis is the inflammation of the intrahepatic biliary ducts and leads to cholangiohepatitis when it is associated with secondary inflammation of the surrounding hepatic parenchyma (1). It is a rare disorder in dogs (8), cattle (7), and humans (9) but is commonly seen in cats (10). There are 4 case reports in the literature on cholangitis in dogs (1–4), and there are occasional, limited, case descriptions in review articles on other topics (11,12). In the previous case reports, 3 dogs responded to medical treatment alone (1,2), 2 dogs didn’t respond to initial medical treatment but recovered after cholecystotomy (1), and 1 dog had to be euthanized despite emergency gallbladder resection and supportive care (3). Higher incidence in cats is attributed to the common bile duct and the major pancreatic duct being closer together than in the dog.
Clinical manifestations of cholangiohepatitis are variable, but commonly include hyperthermia, anorexia, vomiting, weight loss, dullness, and icterus. As in the present case, clinical pathology usually indicates abnormal hepatocellular, hepatobiliary, and functional liver biomarkers, with mild-to-moderate systemic inflammation (1). Marginal hyperbilirubinemia was in agreement with previous reports stating that bilirubin increase is an inconstant finding (6). A ratio of ALP/GGT greater than 100 has also been previously reported in hepatobilliary disorders (13). Pre- and post-prandial bile acids discordance has been reported in approximately 20% of dogs with suspected liver disease (14) and confounding factors to consider in this case are lack of cholecystokinin-stimulated contraction of the diseased gallbladder, spontaneous gallbladder contraction, and bacterial overgrowth (14). There is only one more cholangiohepatitis case report for which bile acids were measured. They were increased at both time-points, although lower post-prandially (3).
It is suspected that, in infectious cholangiohepatitis, intestinal bacteria ascend via the bile duct due to some predisposing pathology, such as inflammatory bowel disease, cholestasis, gallbladder stones, chronic pancreatitis, immunosuppression, or altered gut motility (15). Consistently, bacteria involved in previous cholangiohepatitis case reports were Escherichia coli (3,1), Klebsiella sp. (2) and, as in the present case, Clostridium sp. (1,4); all being normal constituents of the intestinal flora.
This case report illustrates that, although cholangiohepatitis is rare in dogs, clinical pathology, imaging, and anatomopathology used in synergy allow early detection of this condition and successful medical treatment. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.O’Neill EJ, Day MJ, Hall EJ, et al. Bacterial cholangitis/cholangiohepatitis with or without concurrent cholecystitis in four dogs. J Small Anim Pract. 2006;47:325–335. doi: 10.1111/j.1748-5827.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 2.Forrester SD, Rogers KS, Relford RL. Cholangiohepatitis in a dog. J Am Vet Med Assoc. 1992;200:1704–1706. [PubMed] [Google Scholar]
- 3.Neel JA, Tarigo J, Grindem CB. Gallbladder aspirate from a dog. Vet Clin Pathol. 2006;35:467–470. doi: 10.1111/j.1939-165x.2006.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 4.Rivers BJ, Walters PA, Johnston GR, Merkel LK, Hardy RM. Acalculous cholecystitis in four canine cases: Ultrasonographic findings and use of ultrasonographic-guided, percutaneous cholecystocentesis in diagnosis. J Am Anim Hosp Assoc. 1997;33:207–214. doi: 10.5326/15473317-33-3-207. [DOI] [PubMed] [Google Scholar]
- 5.Spaulding KA. Gall bladder wall thickness. Veterinary Radiology & Ultrasound. 1993;34:270–272. [Google Scholar]
- 6.Center SA. Diseases of the gallbladder and biliary tree. Vet Clin North Am Small Anim Pract. 2009;39:543–598. doi: 10.1016/j.cvsm.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Coombs DK, MacWilliams PS, Phillips LA, Nelson KM, Darien BJ. Cholangiohepatitis in a calf. Vet Rec. 2002;150:551–552. doi: 10.1136/vr.150.17.551. [DOI] [PubMed] [Google Scholar]
- 8.Voros K, Sterczer A, Manczur F, Gaal T. Percutaneous ultrasound-guided cholecystocentesis in dogs. Acta Vet Hung. 2002;50:385–393. doi: 10.1556/AVet.50.2002.4.2. [DOI] [PubMed] [Google Scholar]
- 9.Helmberger H, Hellerhoff K, Rüll T, Rösch T. Chronic infections of the biliary system. Radiologe. 2000;40:530–536. doi: 10.1007/s001170050750. [DOI] [PubMed] [Google Scholar]
- 10.Day DG. Feline cholangiohepatitis complex. Vet Clin North Am Small Anim Pract. 1995;25:375–385. doi: 10.1016/s0195-5616(95)50032-4. [DOI] [PubMed] [Google Scholar]
- 11.Morrison S, Prostredny J, Roa D. Retrospective study of 28 cases of cholecystoduodenostomy performed using endoscopic gastrointestinal anastomosis stapling equipment. J Am Anim Hosp Assoc. 2008;44:10–18. doi: 10.5326/0440010. [DOI] [PubMed] [Google Scholar]
- 12.Uno T, Okamoto K, Onaka T, Fujita K, Yamamura H, Sakai T. Correlation between ultrasonographic imaging of the gallbladder and gallbladder content in eleven cholecystectomised dogs and their prognoses. J Vet Med Sci. 2009;71:1295–1300. doi: 10.1292/jvms.001295. [DOI] [PubMed] [Google Scholar]
- 13.Center SA. Interpretation of liver enzymes. Vet Clin North Am Small Anim Pract. 2007;37:297–333. doi: 10.1016/j.cvsm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Center SA, ManWarren T, Slater MR, Wilentz E. Evaluation of twelve-hour preprandial and two-hour postprandial serum bile acids concentrations for diagnosis of hepatobiliary disease in dogs. J Am Vet Med Assoc. 1991;199:217–226. [PubMed] [Google Scholar]
- 15.Kearns S. Infectious hepatopathies in dogs and cats. Top Companion Anim Med. 2009;24:189–198. doi: 10.1053/j.tcam.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]