Abstract
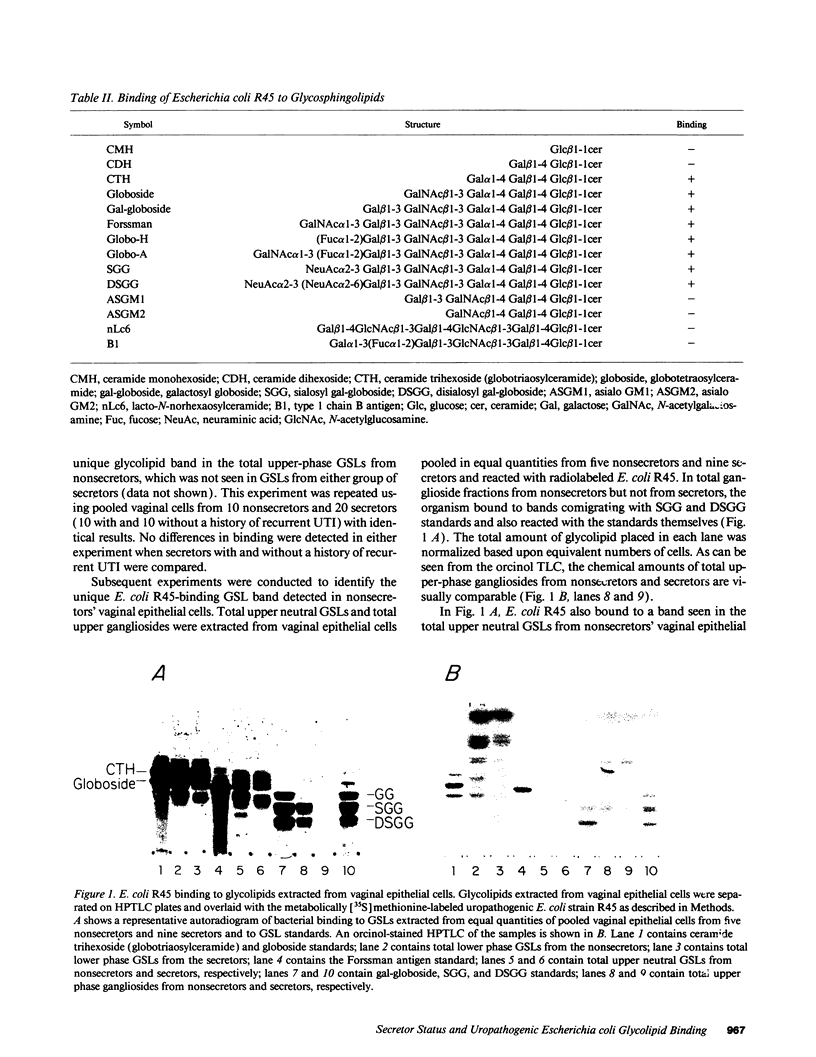
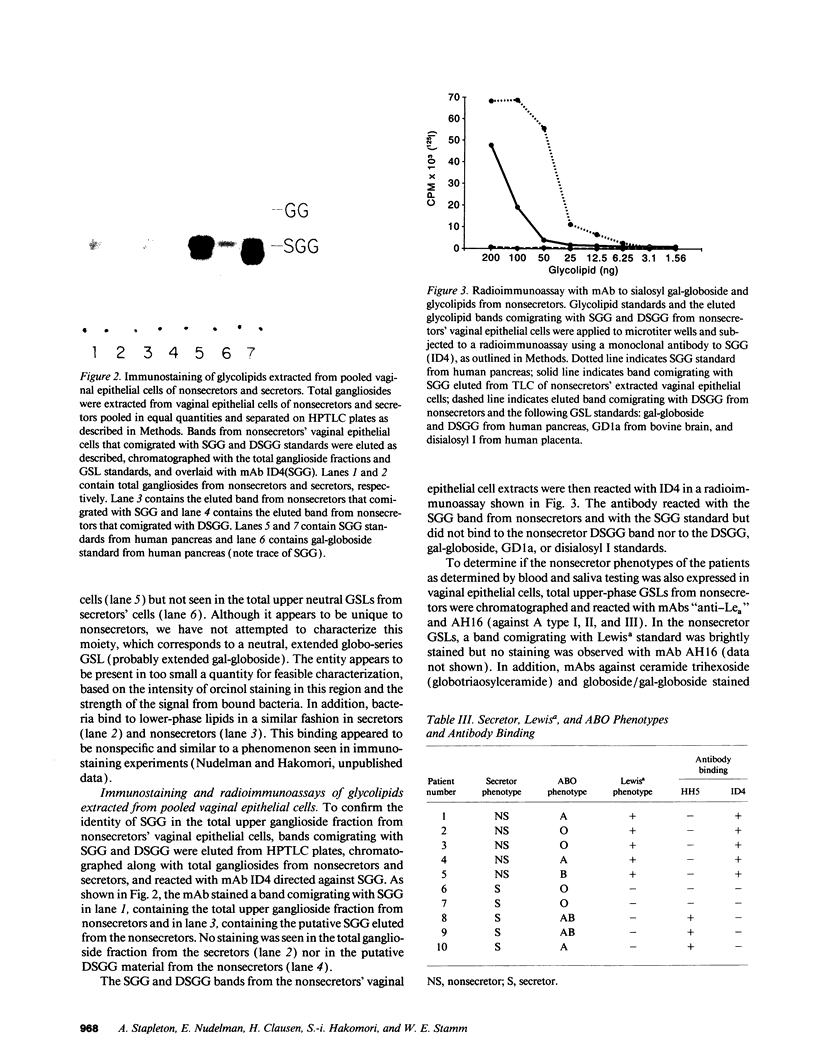
Women with a history of recurrent Escherichia coli urinary tract infections (UTIs) are two to three times more likely to be nonsecretors of histo-blood group antigens than are women without such a history. Further, uroepithelial cells from women who are nonsecretors show enhanced adherence of uropathogenic E. coli compared with cells from secretors. To investigate the hypothesis that nonsecretors express unique receptors for uropathogenic E. coli related to their genetic background, we extracted glycosphingolipids (GSLs) from vaginal epithelial cells collected from nonsecretors and secretors and used an assay in which radiolabeled uropathogenic E. coli were bound to these GSLs separated on TLC plates. An E. coli strain (R45) expressing both P and F adhesins, which was isolated from one of these patients' UTIs, was metabolically labeled with 35S for the TLC binding assay. The radiolabeled E. coli R45 bound to two extended globo-series GSLs, sialosyl gal-globoside (SGG) and disialosyl gal-globoside (DSGG), found in the GSL extracts from nonsecretors but not from secretors. The identity of SGG in the nonsecretor GSL extracts was confirmed in radioimmunoassays using an mAb to SGG and in immunofluorescence assays with this mAb and native vaginal epithelial cells. We show that SGG and DSGG are selectively expressed by epithelial cells of nonsecretors, presumably as a result of sialylation of the gal-globoside precursor glycolipid, which in secretors is fucosylated and processed to ABH antigens. The presence of SGG and DSGG may account for the increased binding of E. coli to uroepithelial cells from nonsecretors and for their increased susceptibility to recurrent UTI.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Levery S. B., Hakomori S. The antibody specific to type 1 chain blood group A determinant. J Immunol. 1984 Apr;132(4):1951–1954. [PubMed] [Google Scholar]
- Arthur M., Johnson C. E., Rubin R. H., Arbeit R. D., Campanelli C., Kim C., Steinbach S., Agarwal M., Wilkinson R., Goldstein R. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect Immun. 1989 Feb;57(2):303–313. doi: 10.1128/iai.57.2.303-313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock K., Breimer M. E., Brignole A., Hansson G. C., Karlsson K. A., Larson G., Leffler H., Samuelsson B. E., Strömberg N., Edén C. S. Specificity of binding of a strain of uropathogenic Escherichia coli to Gal alpha 1----4Gal-containing glycosphingolipids. J Biol Chem. 1985 Jul 15;260(14):8545–8551. [PubMed] [Google Scholar]
- Breimer M. E., Hansson G. C., Leffler H. The specific glycosphingolipid composition of human ureteral epithelial cells. J Biochem. 1985 Nov;98(5):1169–1180. doi: 10.1093/oxfordjournals.jbchem.a135383. [DOI] [PubMed] [Google Scholar]
- Breimer M. E., Jovall P. A. Structural characterization of a blood group A heptaglycosylceramide with globo-series structure. The major glycolipid based blood group A antigen of human kidney. FEBS Lett. 1985 Jan 1;179(1):165–172. doi: 10.1016/0014-5793(85)80213-1. [DOI] [PubMed] [Google Scholar]
- Bremer E. G., Levery S. B., Sonnino S., Ghidoni R., Canevari S., Kannagi R., Hakomori S. Characterization of a glycosphingolipid antigen defined by the monoclonal antibody MBr1 expressed in normal and neoplastic epithelial cells of human mammary gland. J Biol Chem. 1984 Dec 10;259(23):14773–14777. [PubMed] [Google Scholar]
- Clausen H., Hakomori S. ABH and related histo-blood group antigens; immunochemical differences in carrier isotypes and their distribution. Vox Sang. 1989;56(1):1–20. doi: 10.1111/j.1423-0410.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Clausen H., Stroud M., Parker J., Springer G., Hakomori S. Monoclonal antibodies directed to the blood group A associated structure, galactosyl-A: specificity and relation to the Thomsen-Friedenreich antigen. Mol Immunol. 1988 Feb;25(2):199–204. doi: 10.1016/0161-5890(88)90068-5. [DOI] [PubMed] [Google Scholar]
- Clausen H., Watanabe K., Kannagi R., Levery S. B., Nudelman E., Arao-Tomono Y., Hakomori S. Blood group A glycolipid (Ax) with globo-series structure which is specific for blood group A1 erythrocytes: one of the chemical bases for A1 and A2 distinction. Biochem Biophys Res Commun. 1984 Oct 30;124(2):523–529. doi: 10.1016/0006-291x(84)91585-7. [DOI] [PubMed] [Google Scholar]
- Daifuku R., Stamm W. E. Bacterial adherence to bladder uroepithelial cells in catheter-associated urinary tract infection. N Engl J Med. 1986 May 8;314(19):1208–1213. doi: 10.1056/NEJM198605083141902. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Leffler H. Glycosphingolipids of human urinary tract epithelial cells as possible receptors for adhering Escherichia coli bacteria. Scand J Infect Dis Suppl. 1980;Suppl 24:144–147. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hakomori S., Wang S. M., Young W. W., Jr Isoantigenic expression of Forssman glycolipid in human gastric and colonic mucosa: its possible identity with "A-like antigen" in human cancer. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3023–3027. doi: 10.1073/pnas.74.7.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Larson G., Strömberg N., Thurin J. Carbohydrate-specific adhesion of bacteria to thin-layer chromatograms: a rationalized approach to the study of host cell glycolipid receptors. Anal Biochem. 1985 Apr;146(1):158–163. doi: 10.1016/0003-2697(85)90410-5. [DOI] [PubMed] [Google Scholar]
- Jacobsen S. H., Lins L. E., Svenson S. B., Källenius G. P fimbriated Escherichia coli in adults with acute pyelonephritis. J Infect Dis. 1985 Aug;152(2):426–427. doi: 10.1093/infdis/152.2.426. [DOI] [PubMed] [Google Scholar]
- Johnson J. R., Roberts P. L., Stamm W. E. P fimbriae and other virulence factors in Escherichia coli urosepsis: association with patients' characteristics. J Infect Dis. 1987 Jul;156(1):225–229. doi: 10.1093/infdis/156.1.225. [DOI] [PubMed] [Google Scholar]
- Kannagi R., Levery S. B., Hakomori S. Blood group H antigen with globo-series structure. Isolation and characterization from human blood group O erythrocytes. FEBS Lett. 1984 Oct 1;175(2):397–401. doi: 10.1016/0014-5793(84)80776-0. [DOI] [PubMed] [Google Scholar]
- Kannagi R., Levery S. B., Ishigami F., Hakomori S., Shevinsky L. H., Knowles B. B., Solter D. New globoseries glycosphingolipids in human teratocarcinoma reactive with the monoclonal antibody directed to a developmentally regulated antigen, stage-specific embryonic antigen 3. J Biol Chem. 1983 Jul 25;258(14):8934–8942. [PubMed] [Google Scholar]
- Kannagi R., Nudelman E., Levery S. B., Hakomori S. A series of human erythrocyte glycosphingolipids reacting to the monoclonal antibody directed to a developmentally regulated antigen SSEA-1. J Biol Chem. 1982 Dec 25;257(24):14865–14874. [PubMed] [Google Scholar]
- Karlsson K. A., Strömberg N. Overlay and solid-phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987;138:220–232. doi: 10.1016/0076-6879(87)38019-x. [DOI] [PubMed] [Google Scholar]
- Karr J. F., Nowicki B. J., Truong L. D., Hull R. A., Moulds J. J., Hull S. I. pap-2-encoded fimbriae adhere to the P blood group-related glycosphingolipid stage-specific embryonic antigen 4 in the human kidney. Infect Immun. 1990 Dec;58(12):4055–4062. doi: 10.1128/iai.58.12.4055-4062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane D. F., Blackwell C. C., Brettle R. P., Weir D. M., Winstanley F. P., Elton R. A. ABO blood group, secretor state, and susceptibility to recurrent urinary tract infection in women. Br Med J (Clin Res Ed) 1982 Jul 3;285(6334):7–9. doi: 10.1136/bmj.285.6334.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Möllby R., Svenson S. B., Helin I., Hultberg H., Cedergren B., Winberg J. Occurrence of P-fimbriated Escherichia coli in urinary tract infections. Lancet. 1981 Dec 19;2(8260-61):1369–1372. doi: 10.1016/s0140-6736(81)92797-5. [DOI] [PubMed] [Google Scholar]
- Källenius G., Svenson S. B., Möllby R., Korhonen T., Winberg J., Cedergren B., Helin I., Hultberg H. Carbohydrate receptor structures recognized by uropathogenic E. coli. Scand J Infect Dis Suppl. 1982;33:52–60. [PubMed] [Google Scholar]
- Källenius G., Svenson S., Möllby R., Cedergren B., Hultberg H., Winberg J. Structure of carbohydrate part of receptor on human uroepithelial cells for pyelonephritogenic Escherichia coli. Lancet. 1981 Sep 19;2(8247):604–606. doi: 10.1016/s0140-6736(81)92743-4. [DOI] [PubMed] [Google Scholar]
- Leffler H., Svanborg-Edén C. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect Immun. 1981 Dec;34(3):920–929. doi: 10.1128/iai.34.3.920-929.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidefelt K. J., Bollgren I., Källenius G., Svenson S. B. P-fimbriated Escherichia coli in children with acute cystitis. Acta Paediatr Scand. 1987 Sep;76(5):775–780. doi: 10.1111/j.1651-2227.1987.tb10564.x. [DOI] [PubMed] [Google Scholar]
- Lindstedt R., Baker N., Falk P., Hull R., Hull S., Karr J., Leffler H., Svanborg Edén C., Larson G. Binding specificities of wild-type and cloned Escherichia coli strains that recognize globo-A. Infect Immun. 1989 Nov;57(11):3389–3394. doi: 10.1128/iai.57.11.3389-3394.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt R., Larson G., Falk P., Jodal U., Leffler H., Svanborg C. The receptor repertoire defines the host range for attaching Escherichia coli strains that recognize globo-A. Infect Immun. 1991 Mar;59(3):1086–1092. doi: 10.1128/iai.59.3.1086-1092.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomberg H., Cedergren B., Leffler H., Nilsson B., Carlström A. S., Svanborg-Edén C. Influence of blood group on the availability of receptors for attachment of uropathogenic Escherichia coli. Infect Immun. 1986 Mar;51(3):919–926. doi: 10.1128/iai.51.3.919-926.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Marklund B. I., Strömberg N., Lindberg F., Karlsson K. A., Normark S. Uropathogenic Escherichia coli can express serologically identical pili of different receptor binding specificities. Mol Microbiol. 1988 Mar;2(2):255–263. doi: 10.1111/j.1365-2958.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Mabeck C. E. Treatment of uncomplicated urinary tract infection in non-pregnant women. Postgrad Med J. 1972 Feb;48(556):69–75. [PMC free article] [PubMed] [Google Scholar]
- Magnani J. L., Smith D. F., Ginsburg V. Detection of gangliosides that bind cholera toxin: direct binding of 125I-labeled toxin to thin-layer chromatograms. Anal Biochem. 1980 Dec;109(2):399–402. doi: 10.1016/0003-2697(80)90667-3. [DOI] [PubMed] [Google Scholar]
- Nudelman E. D., Mandel U., Levery S. B., Kaizu T., Hakomori S. A series of disialogangliosides with binary 2----3 sialosyllactosamine structure, defined by monoclonal antibody NUH2, are oncodevelopmentally regulated antigens. J Biol Chem. 1989 Nov 5;264(31):18719–18725. [PubMed] [Google Scholar]
- Nudelman E., Fukushi Y., Levery S. B., Higuchi T., Hakomori S. Novel fucolipids of human adenocarcinoma: disialosyl Lea antigen (III4FucIII6NeuAcIV3NeuAcLc4) of human colonic adenocarcinoma and the monoclonal antibody (FH7) defining this structure. J Biol Chem. 1986 Apr 25;261(12):5487–5495. [PubMed] [Google Scholar]
- O'Hanley P., Low D., Romero I., Lark D., Vosti K., Falkow S., Schoolnik G. Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. N Engl J Med. 1985 Aug 15;313(7):414–420. doi: 10.1056/NEJM198508153130704. [DOI] [PubMed] [Google Scholar]
- Orntoft T. F., Wolf H., Clausen H., Hakomori S., Dabelsteen E. Blood group ABO-related antigens in fetal and normal adult bladder urothelium. Immunohistochemical study of type 2 chain structures with a panel of mouse monoclonal antibodies. Lab Invest. 1988 May;58(5):576–583. [PubMed] [Google Scholar]
- Sandberg T., Kaijser B., Lidin-Janson G., Lincoln K., Orskov F., Orskov I., Stokland E., Svanborg-Edén C. Virulence of Escherichia coli in relation to host factors in women with symptomatic urinary tract infection. J Clin Microbiol. 1988 Aug;26(8):1471–1476. doi: 10.1128/jcm.26.8.1471-1476.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer A. J., Jones J. M., Dunn J. K. Association of vitro Escherichia coli adherence to vaginal and buccal epithelial cells with susceptibility of women to recurrent urinary-tract infections. N Engl J Med. 1981 Apr 30;304(18):1062–1066. doi: 10.1056/NEJM198104303041802. [DOI] [PubMed] [Google Scholar]
- Schoolrik G. K. How Escherichia coli infects the urinary tract. N Engl J Med. 1989 Mar 23;320(12):804–805. doi: 10.1056/NEJM198903233201211. [DOI] [PubMed] [Google Scholar]
- Sheinfeld J., Schaeffer A. J., Cordon-Cardo C., Rogatko A., Fair W. R. Association of the Lewis blood-group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989 Mar 23;320(12):773–777. doi: 10.1056/NEJM198903233201205. [DOI] [PubMed] [Google Scholar]
- Skipski V. P. Thin-layer chromatography of neutral glycosphingolipids. Methods Enzymol. 1975;35:396–425. doi: 10.1016/0076-6879(75)35178-1. [DOI] [PubMed] [Google Scholar]
- Stamey T. A., Sexton C. C. The role of vaginal colonization with enterobacteriaceae in recurrent urinary infections. J Urol. 1975 Feb;113(2):214–217. doi: 10.1016/s0022-5347(17)59447-1. [DOI] [PubMed] [Google Scholar]
- Stapleton A., Moseley S., Stamm W. E. Urovirulence determinants in Escherichia coli isolates causing first-episode and recurrent cystitis in women. J Infect Dis. 1991 Apr;163(4):773–779. doi: 10.1093/infdis/163.4.773. [DOI] [PubMed] [Google Scholar]
- Strömberg N., Marklund B. I., Lund B., Ilver D., Hamers A., Gaastra W., Karlsson K. A., Normark S. Host-specificity of uropathogenic Escherichia coli depends on differences in binding specificity to Gal alpha 1-4Gal-containing isoreceptors. EMBO J. 1990 Jun;9(6):2001–2010. doi: 10.1002/j.1460-2075.1990.tb08328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg-Edén C., Jodal U. Attachment of Escherichia coli to urinary sediment epithelial cells from urinary tract infection-prone and healthy children. Infect Immun. 1979 Dec;26(3):837–840. doi: 10.1128/iai.26.3.837-840.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Hultberg H., Källenius G., Korhonen T. K., Möllby R., Winberg J. P-fimbriae of pyelonephritogenic Escherichia coli: identification and chemical characterization of receptors. Infection. 1983 Jan-Feb;11(1):61–67. doi: 10.1007/BF01651362. [DOI] [PubMed] [Google Scholar]
- Väisänen-Rhen V., Elo J., Väisänen E., Siitonen A., Orskov I., Orskov F., Svenson S. B., Mäkelä P. H., Korhonen T. K. P-fimbriated clones among uropathogenic Escherichia coli strains. Infect Immun. 1984 Jan;43(1):149–155. doi: 10.1128/iai.43.1.149-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen V., Elo J., Tallgren L. G., Siitonen A., Mäkelä P. H., Svanborg-Edén C., Källenius G., Svenson S. B., Hultberg H., Korhonen T. Mannose-resistant haemagglutination and P antigen recognition are characteristic of Escherichia coli causing primary pyelonephritis. Lancet. 1981 Dec 19;2(8260-61):1366–1369. doi: 10.1016/s0140-6736(81)92796-3. [DOI] [PubMed] [Google Scholar]
- Westerlund B., Siitonen A., Elo J., Williams P. H., Korhonen T. K., Mäkelä P. H. Properties of Escherichia coli isolates from urinary tract infections in boys. J Infect Dis. 1988 Nov;158(5):996–1002. doi: 10.1093/infdis/158.5.996. [DOI] [PubMed] [Google Scholar]