Abstract
A controlled redox environment is essential for vascular cell maturation and function. During aging, an imbalance occurs, leading to endothelial dysfunction. We hypothesized that, according to the concept of hormesis, exposure to physiologic oxidative stress during the maturation phase of the endothelium will activate protective pathways involved in stress resistance. C57Bl/6 mice were treated with the polyphenol catechin for the last 3 (post-maturation) or 9 months prior study at 12 months of age. Endothelial dysfunction, assessed by acetylcholine-induced dilations of isolated renal arteries, was present at 12 months (P<0.05). Only the 3-month treatment with catechin fully prevented the decline in efficacy and sensitivity to acetylcholine (P<0.05). Splenocytes adhesion to the native endothelium, expression of CD18 and shedding of CD62L and PSGL-1 augmented in 12 months old mice (P<0.05): only 3-month catechin fully normalized adhesion and prevented the expression of adhesion molecules on splenocytes (P<0.05). Aging was associated with vascular gene alterations, which were prevented by 3-month catechin treatment (P<0.05). In contrast, 9-month catechin further increased COX-2, p22phox and reduced MnSOD (P<0.05). In conclusion, we demonstrate a pivotal role of cellular redox equilibrium: exposure to physiologic oxidative stress during the maturation phase of the endothelium is essential for its function.
Keywords: mouse arteries, endothelial dysfunction, adhesion, oxidative stress, hormesis
INTRODUCTION
Many cellular processes such as metabolism, proliferation, growth and inflammatory host defense involve oxidation/reduction reactions (Droge, 2002; Holliday, 2006). To function properly, the cell needs therefore to monitor and regulate tightly its redox environment (Gendron and Thorin, 2007; Szocs et al., 2002). Few data underscore the role of the redox poise on the cellular maturation and/or differentiation (Focardi et al., 2007). In particular, the role of the redox environment as a regulator of vascular endothelial cell function and maturation is not well defined.
Vascular aging leads to an unbalanced redox environment towards oxidation that is associated with impaired endothelium-dependent dilation, enhanced endothelial activation and inflammation (Csiszar et al., 2002; Donato et al., 2007). The mechanisms by which oxidative stress is responsible for endothelial cell dysfunction may include DNA, protein and lipid damages, alteration of gene expression and decreased NO availability (Kregel and Zhang, 2007; Rattan, 2008b). There is therefore a strong rationale for using antioxidants in preventing age-related cardiovascular decline (Baur and Sinclair, 2006). Antioxidant polyphenols, such as catechin, are abundant in fruits, vegetables, green tea and red wine; they increase the efficacy and/or production of endothelium-derived relaxing factors (Schini-Kerth et al., 2010; Stoclet et al., 2004), improve the efficacy of endogenous antioxidants and act as direct free radical scavengers (Nijveldt et al., 2001; Robak and Gryglewski, 1988). Polyphenols have been extensively studied regarding their effects on cardiovascular diseases (see for review (Arts and Hollman, 2005)). These studies have yielded mixed results, probably due to different experimental settings and conditions. Although no data are available in the literature, it is possible that if initiated too early in life, an antioxidant treatment may impair proper cell maturation and thus cell function. In accordance with the concept of hormesis where mild stress activates different endogenous mechanisms of repair and maintenance to protect cells against subsequent stresses (Gems and Partridge, 2008; Rattan, 2008a; Zhang et al., 2008), we hypothesized that exposure to physiologic oxidative stress during the maturation phase of the endothelium will activate protective pathways involved in stress resistance. In the present study, the antioxidant properties of the polyphenol catechin were used in order to indirectly demonstrate a pivotal role of cellular redox equilibrium in vascular cell development, maturation and function: the impact of catechin antioxidant treatment, initiated before and after maturation, on endothelial dysfunction and inflammation associated with aging of C57Bl/6 mice was investigated. Our data demonstrate that secondary catechin treatment is more effective than the treatment initiated before maturation at preserving endothelial function and avoiding inflammation, possibly by conserving the redox equilibrium essential for the proper maturation of the endothelium. The exposure of the endothelium to physiologic oxidative stress during its maturation phase is likely beneficial and may determine vascular longevity.
MATERIAL AND METHODS
Experimental groups
All experiments were performed using C57Bl/6 male mice. Mice were randomly assigned to the following 3 groups: treatment with the antioxidant polyphenol catechin (30 mg/kg/day; (Drouin et al., 2011; Gendron et al., 2010)) in the drinking water from 3 to 12 months (CAT 9; primary treatment), from 9 to 12 months (CAT 3; secondary treatment) or no treatment (control). The dose of catechin was chosen to provide an appropriate dietary intake of ≈ 0.75 mg/day and per mouse of catechin (Hishikawa et al., 2005; Loke et al. 2010; Miura et al., 2001; Waddington et al., 2004). Three months old C57Bl/6 mice were used as young reference mice. Twelve months old C57Bl/6 mice are considered in the present study as middle-aged mice, since 50 % of mortality occur around 25 months of age and the maximal life expectancy of C57Bl/6 mice is around 36 months of age (Forster et al., 2003; Wolf et al., 2000). At the end of the catechin treatment period, renal arteries were harvested for vascular reactivity studies (Gendron et al., 2010; Gendron and Thorin, 2007); the aorta was either snap frozen for total RNA extraction (Gendron and Thorin, 2007) or freshly prepared for the splenocyte adhesion onto the native endothelium (Gendron et al., 2010). All experiments have been approved by our ethical institutional committee and performed in accordance with the guidelines for animal experimentation of the Canadian Council on Animal care Protection (CCAP).
Vascular reactivity studies
Experiments were conducted in isolated and pressurized (100 mm Hg) mouse renal arteries (external diameter ≈ 400 μm) as previously described (Gendron et al., 2007). Arterial segments were pre-constricted with phenylephrine (PE; 30 μmol/L) and concentration-response curves to acetylcholine (ACh; 0.001 μmol/L - 30 μmol/L) were constructed. Half-maximum effective concentration (EC50) of ACh was measured from individual concentration-response curves; the pD2 value, the negative log of the EC50, was obtained. ACh-induced dilations are expressed as a percentage of the maximal diameter obtained in a calcium-free solution.
Splenocyte adhesion studies
The number of splenocytes adhering to the native endothelium was assessed as previously described (Gendron et al., 2010) and expressed per surface area of the aortic segment (splenocytes/mm2).
Flow cytometry studies
Splenocyte suspensions (5 × 106 cell/ml) were incubated with different monoclonal antibodies (CD18-, CD62L- or CD162 (PSGL-1)-phycoerythrein-conjugated, from Serotec) or their isotype-matched control IgGs, as previously described (Gendron et al., 2010). Antibody binding was determined as the percentage of positive splenocytes or the mean fluorescence intensity (MFI) over a fluorescence threshold gated over a splenocyte population stained with the proper isotype-matched control IgG (<2% of positive cells). The binding index (% of positive cells x MFI) was then calculated.
Quantification of gene expression by Real-Time polymerase chain reaction (qPCR)
Total RNA was extracted from aorta using an RNeasy mini-kit (Qiagen Inc). Efficient extraction was possible by performing additional steps of digestion with proteinase K (Qiagen Inc) and by eliminating DNA with a treatment with DNase I (Qiagen Inc). The reverse transcriptase reaction contained 5 ng/μL total RNA (each sample), M-MLV reverse transcriptase (200 U, Invitrogen), anti-sens primer (4 pM, Invitrogen), dNTPs (0.5 mmol/L, MBI Fermentas), and supplied optimal buffers. The reaction protocol consisted of 3 successive incubation steps: 1) 25°C for 10 minutes; 2) 37°C for 50 minutes; and 3) 70°C for 15 minutes.
qPCR was performed as previously (Gendron and Thorin, 2007) with 2 ng of cDNA template containing the appropriate primer concentration; Sirtuin-1 (100 nM); p22phox (300 nM); MnSOD (300 nM); COX-2 (300 nM); cyclophilin A (300 nM) and SYBR Green PCR master mix (Stratagene).
Primers for each gene were obtained from distinct exons that spanned an intron by using the Ensembl genome browser (http://www.ensembl.org). The sequence specificity of each primer was verified with the Blast program derived from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov). The primers used were as follows: for mouse Sirtuin-1: forward 5′-GAGCAGGTTGCA GGAATCCA-3′ and reverse 5′-CCTGATTAAAAATGTCTCCACGAA-3′; for mouse p22phox: forward 5′-GGCTGCCCTCCACTTCCT-3′ and reverse 5′-CTCCTTGGGTTTAGGCTCAATG-3′; for mouse MnSOD: foward 5′-GGCCAAGGGAGATGTTACAA-3′ and reverse 5′-GCTTGATAGCCTC CAGCAAC-3′; for mouse COX-2: forward 5′-GAACATGGACTCACT CAGTTTGTTG-3′ and reverse 5′-CAAAGATAGCATCTGGACGAGGT-3′; for mouse cyclophilin A: forward 5′-CCGATGACGAGCCCTTGG-3′ and reverse 5′-GCCGCCAGTGCCATTATG-3′.
PCR products were purified, sequenced and confirmed to be the genes of interest.
Statistical Analysis
In every case, n refers to the number of animals used in each protocol. Continuous variables are expressed as means ± standard error of the mean (SEM). ANOVA studies followed by a Scheffé’s F test were performed to compare Emax and pD2 of dose-response curves as well for adhesion and qPCR studies. Unpaired t-tests were performed for flow cytometry studies. Differences were considered to be statistically significant for a P value <0.05.
RESULTS
Secondary catechin treatment fully prevents age-dependent endothelial dysfunction
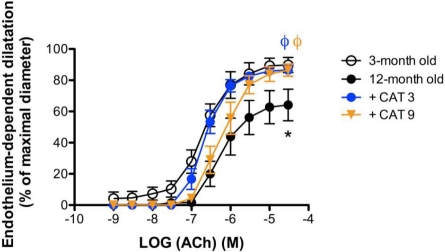
Vascular sensitivity (pD2: 6.7±0.1 versus 6.3±0.1, 3- versus 12-month old) and maximal dilation (Emax: 90±5 versus 64±10 %, 3- versus 12-month old) to ACh were lower (P<0.05) at 12-month old than at 3-month old (Fig. 1). Both secondary (3 months) and primary (9 months) treatments with catechin normalized Emax (87±3 versus 86±4 %, CAT-3- versus CAT-9, P<0.05). Only the secondary treatment, however, normalized (P<0.05) the vascular sensitivity to ACh comparable to that measured at the age of 3 months (6.6±0.1 versus 6.2±0.1, CAT-3- versus CAT-9, P<0.05).
FIGURE 1.
Secondary catechin treatment fully prevents age-dependent endothelial dysfunction. Dose-response curves to acetylcholine of renal arteries isolated from 3-month old mice, 12-month old untreated mice and 12-month old mice treated for the last 3 months (CAT 3) or the last 9 months (CAT 9) with catechin. Data are mean±SEM, n = 7. *: P<0.05 compared to endothelium-dependent dilations observed in 3-month old mice; ϕ: P<0.05 compared to endothelium-dependent dilations observed in 12-month old mice.
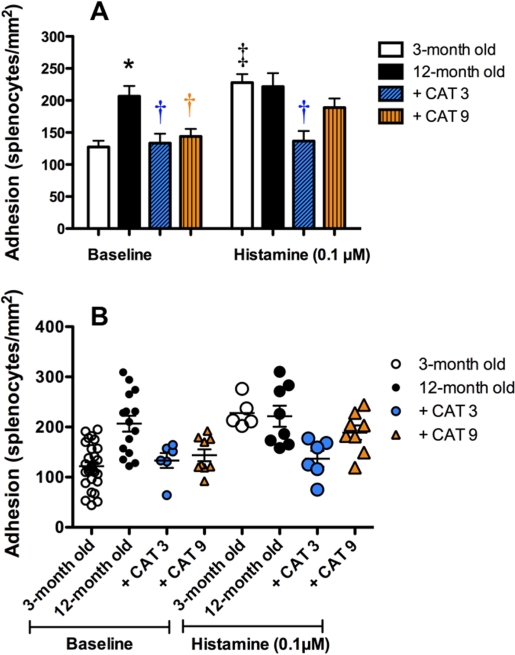
Secondary catechin treatment fully prevents age-dependent splenocyte adhesion onto the endothelium
Basal splenocyte adhesion onto the endothelium increased significantly in 12-month old mice (207±16 splenocytes/mm2) compared to 3-month old mice (127±10 splenocytes/mm2, P<0.05) (Fig. 2). To determine whether the endothelium and/or the splenocytes were dysfunctional, we first performed a crossover study in which the endothelium of 3-month old mice was exposed to splenocytes of 12-month old mice and vice-versa: in both cases adhesiveness was increased (P<0.05; data not shown). Catechin prevented (P<0.05) the rise of basal splenocyte adhesion irrespective of the duration of the treatment (Fig. 2). This rise of splenocyte adhesion with aging and its prevention by catechin was also observed by confocal microscopy using CD45 and VE-cadherin to visualize splenocytes and endothelial cells, respectively (data not shown).
FIGURE 2.
Secondary catechin treatment fully prevents age-dependent splenocyte adhesion onto the endothelium. Splenocyte adhesion onto the native endothelium of aortic segments isolated from 3-month old mice, 12-month old untreated mice and 12-month old mice treated for the last 3 months (+ CAT 3) or the last 9 months (+ CAT 9) with catechin. Responses were obtained either in basal conditions (baseline) or following stimulation of the native endothelium with histamine (0.1 μM).
Data are mean±SEM (A) and the corresponding individual data (B) of n = 5–29.
*: P<0.05 compared to 3-month old mice; †: P<0.05 compared to 12-month old mice; ‡: P<0.05 compared to baseline.
Stimulation of the endothelium with histamine increased significantly adhesion in 3-month old mice from 127±10 to 228±13 splenocyte/mm2, a level similar to that measured in 12-month old mice in basal conditions, confirming the activated state of the endothelium (Fig. 2). Histamine, however, did not further increase splenocyte adhesion at 12 months when compared to baseline. Only the secondary catechin treatment fully prevented histamine-induced adhesion of the splenocytes to the endothelium (Fig. 2).
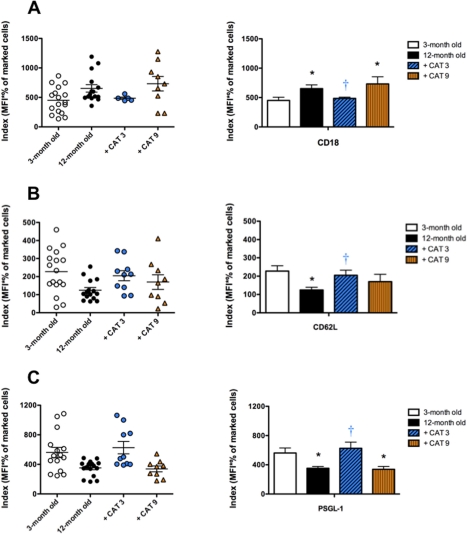
Secondary catechin treatment fully prevents age-dependent increase in the expression of splenocyte cell adhesion molecules
Aging was associated with a significant up-regulation of splenocyte CD18 expression and shedding of CD62L and PSGL-1 (Fig. 3). Only the secondary catechin regimen completely prevented these changes (Fig. 3).
FIGURE 3.
Secondary catechin treatment fully prevents age-dependent increase in the expression of splenocyte cell adhesion molecules. Expression of cell adhesion molecules measured by flow cytometry on the surface of splenocytes isolated from 3-month old mice, 12-month old untreated mice and 12-month old mice treated for the last 3 months (+ CAT 3) or the last 9 months (+ CAT 9) with catechin.
Individual data and the corresponding mean±SEM, n = 9–18 are illustrated for (A) CD18, (B) CD62L and (C) PSGL-1. *: P<0.05 compared to 3-month old mice; †: P<0.05 compared to 12-month old mice.
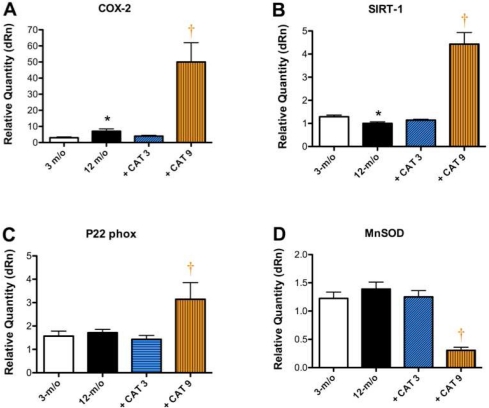
Secondary catechin treatment prevents age-dependent changes in gene expression
Expression of SIRT-1 was decreased (P<0.05) in 12-month old when compared to 3-month old mice (Fig. 4B). In contrast, aging was associated with a 2-fold increase in COX-2 expression (P<0.05; Fig. 4A) without any changes in p22phox and MnSOD expression (Fig. 4). The secondary catechin treatment prevented the age-dependent rise in COX-2 expression and the decline in SIRT-1 expression. In contrast, primary treatment increased by 50 times COX-2 expression, enhanced significantly mRNA expression of the free radical producing enzyme p22phox, while it decreased significantly the expression of the free radical inactivating enzyme MnSOD (Fig. 4). Finally, the expression of the polyphenol-sensitive longevity gene SIRT-1 was 4-fold increased by the primary treatment with catechin (Fig. 4B).
FIGURE 4.
Secondary catechin treatment prevents age-dependent changes in gene expression. Gene expression from total RNA isolated from the aorta of 3-month old mice, 12-month old untreated mice and 12-month old mice treated for the last 3 months (+ CAT 3) or the last 9 months (+ CAT 9) with catechin. Gene expression of (A) COX-2, (B) SIRT-1, (C) p22phox and (D) MnSOD was measured by qPCR.
Data are mean±SEM, n = 3–5. *: P<0.05 compared to 3- month old mice; †: P<0.05 compared to 12-month old mice.
DISCUSSION
In the present study, we show that the endothelial function is dependent on a tightly regulated redox environment. We demonstrate that a secondary antioxidant treatment (from 9- to 12-month of age) with the polyphenol catechin is more efficient than a primary treatment (from 3- to 12-month of age) in preventing endothelial dysfunction associated with normal aging in mice. This is highlighted by a better endothelial dilatory sensitivity to ACh, a reduced adhesion of splenocytes onto the endothelium, an improved expression profile of splenocytes adhesion molecules, and finally by the maintenance of a favorable vascular-related gene expression. Thus, a physiological redox-sensitive maturation process likely occurs in the endothelium, suggesting that the exposure of the endothelium to physiologic oxidative stress during its maturation phase is beneficial. This is in accordance with the concept of hormesis, where exposure to a mild stress (oxidative stress in this case) promotes protection against further stress.
In 12-month old mice, we observed that vascular sensitivity and maximal dilation to ACh are reduced when compared to 3-month old mice. This age-dependent decline in the endothelial function is in accordance with our previous work (Gendron et al., 2007; Krummen et al., 2006) and the literature (Brandes et al., 2005). The endothelial capacity to limit leukocyte adhesion is also weakened with aging. Our data show that both the endothelium and the splenocytes are prone to adhesion at 12 months of age. The shortest and latest catechin treatment paradigm proved to be the most efficient at preventing endothelium dysfunction and the increase in splenocyte/endothelium interactions with aging. This secondary treatment also prevented the age-dependent increase in CD18 expression and shedding of CD62L and PSGL-1, suggesting a normalization of splenocyte function. The primary treatment with catechin neither fully prevented endothelial dysfunction nor splenocyte adhesion molecule expression. It is well established that the expression of cell adhesion molecules increases with raised free radical production and is sensitive to an antioxidant treatment (Chen et al., 2004; Ludwig et al., 2004), but the duration of such therapy may considerably influence the outcome. Our data suggest that catechin treatment initiated during the maturation of the endothelium (from 3 to 9 months of age) could prevent adaptive pathways responsible for cellular and physiological homeostasis, such as antioxidant enzymes to maintain an appropriate redox environment (Zhang et al., 2008). Cellular maintenance processes are necessary to protect against the molecular damages that cause aging (Gems and Partridge, 2008), and we propose that an early and long exposure to catechin prevents the normal establishment of these protective processes. Exposure to oxidative stress during the maturation of the endothelium likely enables it to more successfully cope with the rise in oxidative stress associated with aging. This is the concept of hormesis, adapted to the context of aging (Rattan, 2008a; Rattan, 2008b), where the “hormetin”, i.e. the stress inducing resistance against subsequent stress, is oxidative stress.
The effectiveness of secondary versus primary catechin treatment is further demonstrated by the expression profile of various genes involved in the pathways that regulate vascular tone and the redox environment. We previously reported that aging was associated with a reduction in eNOS and an augmentation in COX-2 expression (Gendron et al., 2007). The secondary treatment that fully preserved endothelial function also prevented the rise in COX-2. In contrast, primary treatment with catechin drastically increased COX-2 gene expression. Our data therefore suggest that during the course of maturation, expression of key endothelial players occurs and is altered by the primary catechin treatment. We propose that during this phase, the reducing environment created by catechin, as demonstrated by the decrease in MnSOD expression (Zhang et al., 2002), is deleterious. This hypothesis is supported by the up-regulation of the p22phox, a subunit of the NADPH oxidase (Bedard and Krause, 2007) observed only in the group of mice treated with catechin for 9 months. Primary catechin treatment induces a rupture in the normal redox environment and likely alters the multiple protective and adaptive cellular pathways responsible for stress resistance.
We demonstrate that chronic exposure to catechin during the maturation period of the mouse increases SIRT-1 gene expression. Although the increase in SIRT-1 expression had to be expected since it is a marker of the polyphenol response (Kaeberlein et al., 2005), it is striking that SIRT-1 expression increases only if the treatment is initiated at 3 months of age. Absence of impact of the secondary treatment with catechin on SIRT-1 gene expression does not, however, utterly mean that the polyphenol did not have any impact. It will be important to study the longevity of mice treated with catechin from the age of 3 months compared to that of animals treated from the age of 9 months. One should be interested to see if preserving a seemingly “young” vascular phenotype, as observed with the secondary treatment, is as effective as increasing SIRT-1 gene expression in prolonging lifespan. This study would also be informative considering that resveratrol has been shown to protect mice on a high fat diet starting at 12 months of age (Baur et al., 2006).
To conclude, our results reveal that the age-dependent changes in endothelial function were best prevented by a secondary, post-maturation, catechin treatment. This was associated with maintenance of endothelial dilatory function, a reduced adhesiveness of splenocytes onto the endothelium, an improved expression profile of both splenocytes and endothelial cell adhesion molecules, and maintenance of most vascular-related gene expression tested. This highlights the importance of a tightly regulated redox environment to provide an adequate development/maturation and function. This essential maturation process likely includes protective and adaptive cellular pathways responsible for stress resistance, according to the hormetic concept in aging (Le Bourg and Rattan, 2009): mild oxidative stress during maturation promotes stress resistance later in life. Hormesis could contribute to the beneficial effects of the late-catechin regimen, by promoting resistance to endothelial dysfunction associated with healthy aging.
Acknowledgments
This work has been supported in part by the Foundation of the Montreal Heart Institute, the Heart and Stroke Foundation of Quebec, and the Canadian Institute for Health Research (MOP 14496). ME Gendron was supported by the Frederick Banting and Charles Best Canada Graduate Scholarships - Doctoral Award, in association with the Canadian Institute for Health Research.
REFERENCES
- Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–94. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Chen WC, Hayakawa S, Shimizu K, Chien CT, Lai MK. Catechin prevents substance P-induced hyperactive bladder in rats via the downregulation of ICAM and ROS. Neurosci Lett. 2004;367:213–217. doi: 10.1016/j.neulet.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Drouin A, Bolduc V, Thorin-Trescases N, Belanger E, Fernandes P, Baraghis E, Lesage F, Gillis MA, Villeneuve L, Hamel E, Ferland G, Thorin E. Catechin treatment improves cerebrovascular flow-mediated dilation and learning abilities in atherosclerotic mice. Am J Physiol Heart Circ Physiol. 2011;300:H1032–H1043. doi: 10.1152/ajpheart.00410.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focardi M, Dick GM, Picchi A, Zhang C, Chilian WM. Restoration of coronary endothelial function in obese Zucker rats by a low-carbohydrate diet. Am J Physiol Heart Circ Physiol. 2007;292:H2093–H2099. doi: 10.1152/ajpheart.01202.2006. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17:690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell Metab. 2008;7:200–203. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Gendron ME, Theoret JF, Mamarbachi AM, Drouin A, Nguyen A, Bolduc V, Thorin-Trescases N, Merhi Y, Thorin E. Late chronic catechin antioxidant treatment is deleterious to the endothelial function in aging mice with established atherosclerosis. Am J Physiol Heart Circ Physiol. 2010;298:H2062–H2070. doi: 10.1152/ajpheart.00532.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron ME, Thorin E. A change in the redox environment and thromboxane A2 production precede endothelial dysfunction in mice. Am J Physiol Heart Circ Physiol. 2007;293:H2508–H2515. doi: 10.1152/ajpheart.00352.2007. [DOI] [PubMed] [Google Scholar]
- Gendron ME, Thorin-Trescases N, Villeneuve L, Thorin E. Aging associated with mild dyslipidemia reveals that COX-2 preserves dilation despite endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H451–H458. doi: 10.1152/ajpheart.00551.2006. [DOI] [PubMed] [Google Scholar]
- Hishikawa K, Nakaki T, Fujita T. Oral flavonoid supplementation attenuates atherosclerosis development in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:442–446. doi: 10.1161/01.ATV.0000148404.24271.fc. [DOI] [PubMed] [Google Scholar]
- Holliday R. Aging is no longer an unsolved problem in biology. Ann N Y Acad Sci. 2006;1067:1–9. doi: 10.1196/annals.1354.002. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- Krummen S, Drouin A, Gendron ME, Falck JR, Thorin E. ROS-sensitive cytochrome P450 activity maintains endothelial dilatation in ageing but is transitory in dyslipidaemic mice. Br J Pharmacol. 2006;147:897–904. doi: 10.1038/sj.bjp.0706679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourg E, Rattan SI. “Is hormesis applicable as a pro-healthy aging intervention in mammals and human beings, and how?” Introduction to a special issue of Dose-Response. Dose Response. 2009;8:1–3. doi: 10.2203/dose-response.09-052.LeBourg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke WM, Proudfoot JM, Hodgson JM, McKinley AJ, Hime N, Magat M, Stocker R, Croft KD. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2010;30:749–757. doi: 10.1161/ATVBAHA.109.199687. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Lorenz M, Grimbo N, Steinle F, Meiners S, Bartsch C, Stangl K, Baumann G, Stangl V. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochem Biophys Res Commun. 2004;316:659–665. doi: 10.1016/j.bbrc.2004.02.099. [DOI] [PubMed] [Google Scholar]
- Miura Y, Chiba T, Tomita I, Koizumi H, Miura S, Umegaki K, Hara Y, Ikeda M, Tomita T. Tea catechins prevent the development of atherosclerosis in apoprotein E-deficient mice. J Nutr. 2001;131:27–32. doi: 10.1093/jn/131.1.27. [DOI] [PubMed] [Google Scholar]
- Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Hormesis in aging. Ageing Res Rev. 2008a;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Increased molecular damage and heterogeneity as the basis of aging. Biol Chem. 2008b;389:267–272. doi: 10.1515/BC.2008.030. [DOI] [PubMed] [Google Scholar]
- Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988;37:837–841. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- Schini-Kerth VB, Auger C, Kim JH, Etienne-Selloum N, Chataigneau T. Nutritional improvement of the endothelial control of vascular tone by polyphenols: role of NO and EDHF. Pflugers Arch. 2010;459:853–862. doi: 10.1007/s00424-010-0806-4. [DOI] [PubMed] [Google Scholar]
- Stoclet JC, Chataigneau T, Ndiaye M, Oak MH, El Bedoui J, Chataigneau M, Schini-Kerth VB. Vascular protection by dietary polyphenols. Eur J Pharmacol. 2004;500:299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Szocs K, Lassegue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- Waddington E, Puddey IB, Croft KD. Red wine polyphenolic compounds inhibit atherosclerosis in apolipoprotein E-deficient mice independently of effects on lipid peroxidation. Am J Clin Nutr. 2004;79:54–61. doi: 10.1093/ajcn/79.1.54. [DOI] [PubMed] [Google Scholar]
- Wolf NS, Li Y, Pendergrass W, Schmeider C, Turturro A. Normal mouse and rat strains as models for age-related cataract and the effect of caloric restriction on its development. Exp Eye Res. 2000;70:683–692. doi: 10.1006/exer.2000.0835. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Pi J, Woods CG, Jarabek AM, Clewell HJ, 3rd, Andersen ME. Hormesis and adaptive cellular control systems. Dose Response. 2008;6:196–208. doi: 10.2203/dose-response.07-028.Zhang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Hashimoto K, Yu GY, Sakagami H. Decline of superoxide dismutase activity during antioxidant-induced apoptosis in HL-60 cells. Anticancer Res. 2002;22:219–224. [PubMed] [Google Scholar]