Abstract
Long-acting bronchodilators have been shown to improve multiple clinical outcomes in chronic obstructive pulmonary disease (COPD) including lung function, symptoms, dyspnea, quality of life, and exacerbations. Indacaterol is a novel, inhaled, long-acting β2-agonist providing 24-hour bronchodilation with once-daily dosing. It is currently approved for the maintenance treatment of COPD to be administered as 150 or 300 μg once-daily doses as licensed in many countries and 75 μg as licensed in the US by means of a single-dose dry powder inhaler. The data from clinical development support a favorable safety and tolerability profile within the β2-agonist drug class, with no relevant issues identified. Current evidence indicates that indacaterol is suitable for use as first-line monotherapy in COPD patients with moderate disease (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage II) and beyond that do not require an inhaled corticosteroid (ICS) as per GOLD guidelines, or in combination with an ICS in severe or very severe patients with repeated exacerbations. Data from trials with the novel once-daily β2-agonist, indacaterol, indicate superior bronchodilation and clinical efficacy over twice-daily long-acting β2-agonists and at least equipotent bronchodilation as once-daily tiotropium. Bronchodilators are central in the symptomatic management of COPD. It is likely that once-daily dosing of a bronchodilator would be a significant convenience and probably a compliance-enhancing advantage, leading to improved overall clinical outcomes in patients with COPD.
Keywords: indacaterol, onset of action, chronic obstructive pulmonary disease, bronchodilators, once-daily, long-acting β2-agonists
Emerging treatment options in COPD
Chronic obstructive pulmonary disease (COPD) is a progressive obstructive airways disease with incomplete improvement in lung function in response to therapy. COPD is estimated to affect 10% of the world’s population aged ≥40 years and its prevalence is expected to continue to increase over the next decade or more.1 An accelerated decline in forced expiratory volume in 1 second (FEV1) occurs alongside a decline in ability to undertake physical activities and social functioning, reflected in the measurement of clinical outcomes including dyspnea and health status.2 Regular pharmacotherapy using long-acting inhaled bronchodilators has been shown to provide not only rapid improvement in lung function, but clinically important decreases in dyspnea and exacerbation rate with a corresponding improvement in health status.3 Bronchodilators have therefore become the cornerstone of treatment for COPD of all severity stages.
Current guidelines recommend that patients with moderate to severe COPD initiate therapy with long-acting bronchodilators if short-acting bronchodilators are not providing sufficient symptom relief.4 Currently available agents include the twice-daily long-acting β2-agonists (LABAs) formoterol and salmeterol and the once-daily anticholinergic tiotropium. Although long-acting bronchodilators are considered more effective and convenient than short-acting agents,5 symptoms remain limiting for many subjects.6
The shift in treatment preference from short-acting bronchodilators with multiple dosing per day to long-acting bronchodilators with once-daily or twice-daily dosing and prolonged duration of bronchodilation has resulted in improved clinical outcomes for COPD patients.7 The consequent reduction in dosing frequency not only simplifies disease management, but has the potential to improve patient adherence and compliance.5
Until recently, the only available LABAs were salmeterol and formoterol, both of which have an approximate 12-hour duration of bronchodilator action, and hence are used twice daily for maintenance therapy in COPD. Indacaterol is the first once-daily long-acting β2-selective agent, sometimes referred to as an ultra-LABA, indicated for maintenance treatment in patients with moderate to severe COPD, has been approved in more than 40 countries (including throughout the European Union) at a recommended dose of 150 μg once daily and a maximum dose of 300 μg once daily,8 and has been shown to be effective and well tolerated by subjects with COPD during 12 weeks of treatment.9 With any new treatment intended for long-term use, it is important to evaluate safety and to determine if efficacy remains unblunted with regular use. A thorough characterization of safety is particularly relevant for a long-acting bronchodilator, given the recent interest in the safety of these agents in the treatment of COPD.10
The 75 μg dose of indacaterol as licensed in the US was evaluated only in studies of 12 weeks’ duration.11 Overall, the results with indacaterol 150 μg and 300 μg demonstrate a good profile of safety and tolerability that is comparable with the other evaluated long-acting bronchodilators. Compared with these two doses, results with the 75 μg dose of indacaterol were occasionally anomalous but must be considered in the context that these data are less robust in terms of shorter length of treatment (maximum 12 weeks) and fewer patients exposed to this dose.12 However, in the two studies evaluating the 75 μg dose, the safety and tolerability profiles were similar to placebo.11
Few deaths occurred in any of the active treatment groups, and the number of deaths adjusted per patient-year was not increased with any of the LABA treatments relative to placebo. The finding of no deaths among the indacaterol 75 μg group should be interpreted with caution, given that most of the data came from two 12-week studies.12
Pharmacology, mode of action, and pharmacokinetics
Sequential modifications of the chemical structure of catecholamines13 allowed synthesis of agonists with improved selectivity for the β2-adrenoreceptor and led to the subsequent development of short-acting β2-bronchodilators such as fenoterol, salbutamol (albuterol), and terbutaline, and the long-acting β2-agonists, salmeterol and formoterol.
In vitro studies during the preclinical development of indacaterol13 characterized this new agent as having high agonist efficacy at the human β2-adrenoreceptor, with a binding affinity similar to formoterol, an intrinsic activity higher than salmeterol, and a functional selectivity similar to formoterol over the β1-adrenoreceptor, and similar to formoterol and salbutamol over the β3-adrenoreceptor. In vivo, indacaterol produced a prolonged bronchoprotective effect against pharmacologically induced bronchoconstriction, and showed an improved cardiovascular safety profile.14
Reports on pharmacokinetics15 showed that indacaterol is rapidly absorbed and distributed after inhalation, with a median time to reach peak serum concentrations of approximately 15 minutes after single or repeated inhaled doses, although systemic exposure results from a composite of pulmonary and intestinal absorption. The faster onset and longer duration of action of indacaterol compared with some other β2-adrenoceptor agonists may be related to lipid membrane interactions. The sum of these small differences, including higher partitioning of indacaterol into the microenvironment of the receptor and its faster membrane permeation, is likely to contribute to its faster onset and longer duration of therapeutic action.16
In the toxicological assessments,17 indacaterol was considered negative in the standard battery of in vitro and in vivo genotoxicity tests, and did not raise concerns regarding potential carcinogenicity. There was also no evidence of teratogenicity in the embryo-fetal development studies. An analysis of the effect of age, gender, and weight on systemic exposure after inhalation indicated that indacaterol can be used safely in all age and weight groups within the COPD population, and regardless of gender. No difference was suggested either between ethnic subgroups in the population analyzed.
Pharmacokinetic data taken during multiple-dose studies of indacaterol 400 μg or 800 μg once daily for 14 days demonstrated rapid absorption and a mean elimination half- life >30 hours, whereas in a single-dose study, doses between 600 μg and 2000 μg were rapidly absorbed, with maximum serum concentrations reached within 15 minutes.18
Efficacy of indacaterol compared with established therapies
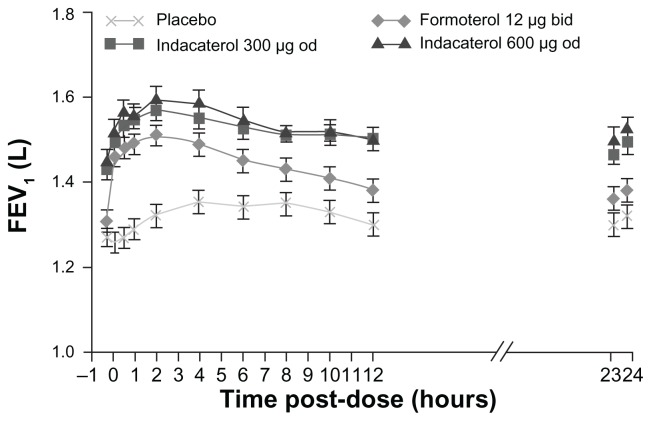
In large-scale registration studies, indacaterol provided 24-hour bronchodilation on once-daily dosing with an effect that was sustained during treatment for up to 1 year.19 The relative effect of indacaterol on trough FEV1 (measured 24 hours following dosing) after 12 weeks of treatment was reported to be 40–50 mL greater than the once-daily anticholinergic bronchodilator, tiotropium, and 60–100 mL greater than the trough FEV1 measured 12 hours after dosing with the twice-daily β2-agonists, salmeterol and formoterol20 (Figure 1).
Figure 1.
Serial measurements of FEV1 from 15 minutes to 24 hours postdose measured in a subset with serial spirometry measurements (12-hour serial spirometry subset) at week 12.
Notes: Data are least squares means 6 SE. Treatment differences: P < 0.001 for indacaterol (both doses) versus placebo at each time point; P < 0.05 for formoterol versus placebo at time points from 5 minutes to 11 hours and 45 minutes postdose; P < 0.05 for indacaterol 300 mg versus formoterol at 15 minutes, 2 hours, and from 6 hours to 23 hours 45 minutes; P < 0.05 for indacaterol 600 mg versus formoterol at all time points apart from 5 minutes postdose.20
Abbreviations: FEV1, forced expiratory volume in 1 second; bd, twice daily; od, once daily.
The efficacy and safety of indacaterol was evaluated in an extensive Phase III clinical program in which patients received doses of up to 600 μg once daily for up to 52 weeks.20 In an analysis of data from 801 patients with moderate to severe COPD after 2 weeks of treatment, indacaterol 150 μg once daily was identified as the lowest dose that was numerically superior to the active comparators (formoterol twice daily and open-label tiotropium once daily) and, along with the next highest dose (300 μg), was selected for further evaluation.21
Chapman et al22 recently reported on the safety of indacaterol 150 μg and 300 μg once daily versus placebo during the 26-week extension phase of the 26-week open-label tiotropium comparison study, thereby providing data over a total duration of 52 weeks. In their analysis, adverse events occurred in 76%, 77%, and 68% of subjects receiving indacaterol 150 μg, 300 μg, and placebo, respectively, whereas serious adverse events occurred in 10.4%, 12.3%, and 10.5%, respectively. There were no clinically significant effects with indacaterol at either dose on QTc interval or potassium or glucose levels.
This additional evaluation showed that indacaterol 150 μg and 300 μg provided statistically significant and clinically relevant improvements in trough FEV1 versus placebo up to 26 weeks.19 Although indacaterol 150 μg and 300 μg had similar effects on trough FEV1, the higher dose was associated with incremental benefits in terms of symptomatic relief, such as dyspnea, particularly for patients with more severe COPD. Further, the overall clinical trial program indicated that indacaterol has a similar safety and tolerability profile across all of the doses evaluated.22,23
Results of a recent 14-day crossover study24 that compared indacaterol 300 μg with open-label salmeterol showed that indacaterol dosed once daily has a bronchodilator profile that is consistently numerically superior to salmeterol dosed twice daily. A larger 26-week comparison with blinded salmeterol suggested that in addition to improved bronchodilator efficacy, once-daily dosing with indacaterol is generally more effective than twice-daily salmeterol.23
COPD exacerbations were significantly reduced versus placebo for indacaterol 150 μg or 300 μg once daily. In a 52-week study,20 once-daily treatment with indacaterol prolonged the time to first COPD exacerbation and was effective in reducing the incidence and frequency of COPD exacerbations, with no significant difference between indacaterol and formoterol. Patients treated with indacaterol had a significantly higher percentage of days with no use of as-needed rescue salbutamol than placebo recipients in all large studies.
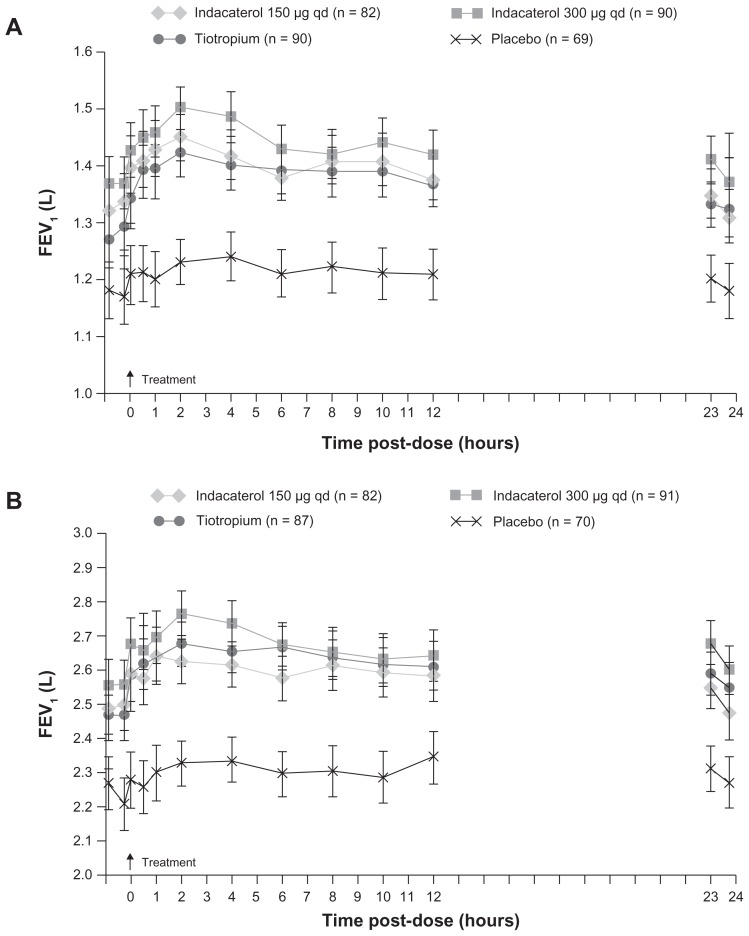
Only the once-daily bronchodilators (indacaterol and tiotropium) provided more than the 120 mL improvement in trough FEV1, prespecified as the threshold of clinical interest in several studies studies.9,19,20,23 For trough FEV1 at week 12, indacaterol 150 μg and 300 μg were statistically superior to open-label tiotropium, indacaterol 150 μg was statistically superior to salmeterol, and indacaterol 300 μg was statistically superior to formoterol. The efficacy of indacaterol was maintained over the study durations (ie, 6 months19,23 and 1 year20). Indacaterol has a fast onset of bronchodilator effect following the first dose. The mean FEV1 measured at 5 minutes after the first dose with indacaterol was 110–130 mL greater than after placebo (P < 0.001), approximately double the corresponding values with tiotropium (70 mL; P < 0.001 versus tiotropium) and salmeterol (60 mL; P < 0.001 versus salmeterol), and similar to formoterol (140 mL; not significantly different, Figure 2).
Figure 2.
Serial measurements of (A) FEV1 and (B) FVC from 250 minutes to 23 hours 45 minutes postdose measured in subsets with 12-hour serial spirometry at week 26.
Notes: Data are least squares means 6 SE. Treatment differences for FEV1: P = 0.05 for indacaterol (both doses) and tiotropium versus placebo at all time points; P = 0.05 for indacaterol 300 mg versus tiotropium at 250, 215, and 5 minutes, 2 hours, 4 hours, and 23 hours 10 minutes. Treatment differences for FVC: P = 0.05 for indacaterol (both doses) and tiotropium versus placebo at all time points; P = 0.05 for indacaterol 300 mg versus 150 mg at 2 hours, 4 hours, 23 hours 10 minutes, and 24 hours 45 minutes.19
Abbreviations: FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; qd, once daily.
Indacaterol improved breathlessness assessed using the transition dyspnea index, with an effect close to or greater than the threshold for clinical relevance. Indacaterol had a similar (150 μg dose) or greater (300 μg dose) effect than open-label tiotropium, and a greater effect than the twice-daily bronchodilators formoterol and salmeterol. These differences are evident both in terms of the effect on mean transition dyspnea index scores and the percentage of patients who achieved at least a clinically relevant improvement in breathlessness. Indacaterol, along with all the other bronchodilators, showed a statistically significant effect compared with placebo at later time points during the 6-month and 1-year studies.9,19,20,23
Patients with COPD treated with indacaterol used less as-needed salbutamol, with a decrease of approximately 1.5 puffs per day from baseline. The decrease was greater than that seen for patients receiving either tiotropium or formoterol. Patients receiving indacaterol also recorded more days when they took no salbutamol (55%–60%), which is more than was recorded with tiotropium (46%) and formoterol (52%).9,19,20,23
Exacerbation rates were numerically reduced compared with placebo with all the bronchodilators, but in most cases the effect was not statistically significant. These studies were not designed to measure exacerbations as a primary outcome, so only stable patients were recruited (annual exacerbation rates with placebo treatment were in the range 0.7–1.0).9,19,20,23 Health status, assessed using the St George’s Respiratory Questionnaire, was improved relative to placebo with indacaterol and with the two twice-daily bronchodilators, but not with tiotropium, although it should be noted that tiotropium was given “open-label”. As seen in previous studies with bronchodilators, most treatments did not produce a change that exceeded the minimal clinically important difference when compared with placebo, but this was achieved with indacaterol 150 μg9,23 and with salmeterol.23 A similar pattern of results was seen for patients with severe COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage III). This is appropriate for primary care, given that a recent large European study in primary care found that nearly 40% of patients had GOLD stage III or IV disease.25
Safety and tolerability compared with established therapies
Systemic bioavailability of β2-agonist bronchodilators can elicit known β2-adrenoceptor-mediated cardiovascular effects,26 such as palpitations, tachycardia, changes in blood pressure, and electrocardiographic abnormalities such as a prolonged QT interval, as well as hypokalemia, hyperglycemia, headache, and skeletal muscle tremor. The available data for indacaterol overall support an excellent safety profile, with no relevance of its long duration of action in terms of side effects.
The bronchodilator efficacy of indacaterol increased from the first dose to the measurements performed at the end of 12 weeks of treatment. In a longer-term study, the efficacy of indacaterol 150 μg once daily was not reduced with repeated dosing for 6 or 12 months, showing persistence of bronchodilator response without tachyphylaxis.22
In COPD patients, a large data set was generated from various studies primarily aimed at dose ranging and safety analysis.9,27 Safety variables were also secondary endpoints in all studies primarily aimed at efficacy endpoints. Overall, the rate of adverse events was low and similar across treatment groups in the different trials. There was no evidence of drug-related or dose-related changes in hematological parameters, and no clinically relevant differences were observed between treatment groups in any of the biochemical variables measured.
The incidence of other β2-agonist-related effects, including muscle spasm, headache, and tremor, was comparable between indacaterol and placebo groups. Data generated across a wide dose range support a good cardiovascular safety, with no clinically significant differences in mean pulse rate, no drug-related trends in systolic or diastolic blood pressure, no statistically significant differences in mean QTc interval, and no differences in the numbers of subjects with notable QTc interval increases. Serious adverse events in subjects receiving indacaterol occurred with frequencies similar or inferior to placebo groups, and none were classified as suspected to be related to the study drug in COPD patients in the trials reported.
In various trials, cough upon drug inhalation was reported with a mean frequency ranging from 2.9% to 17.8% in indacaterol groups and 0.9% to 7.3% in placebo groups. The cough was described as occurring within 15 seconds following drug inhalation, with a median duration of 6 seconds, and having no association with bronchospasm or increased study discontinuation rates.9
More than 4000 patients have completed treatment with indacaterol so far in controlled clinical studies of at least 12 weeks’ duration, and no significant safety concerns have arisen. Safety has been evaluated during up to 1 year’s treatment with the approved daily doses of 150 μg and 300 μg, and with a higher (unlicensed) dose of 600 μg once daily. Adverse events generally occurred with a similar incidence in the indacaterol and placebo groups. The most common adverse events reflected the symptoms and manifestations of COPD, such as worsening of COPD and respiratory tract infections. As with the other long-acting bronchodilators,28 indacaterol has a good profile of cardiovascular safety in patients with COPD, and has little or no effect on vital signs and QTc interval, the derived electrocardiographic measurement used to indicate risk of arrhythmias.29 Approximately 20% of patients experience a mild transient cough in the first few minutes after inhalation of indacaterol. This typically lasts for several seconds and is not associated with loss of efficacy, increased dropout rates, or any safety concerns.
Patient considerations
Indacaterol may offer small but clinically important advantages in the treatment of COPD. The dosing regimen requiring less frequent dosing was shown to be associated with improved patient compliance with therapy.30 Therefore, an effective once-daily β2-agonist bronchodilator would add a significant advance to the therapeutic toolbox for COPD. Long-term safety of a new treatment is important for subjects with COPD, who are often elderly, frequently have significant comorbidity, and tend to be receiving multiple medications.31 In all studies designed to investigate whether indacaterol has the same tolerability as that of the LABAs already on the market, indacaterol was well tolerated at all doses and with a good overall safety profile.32
The inhalation device used for indacaterol, ie, the Breezhaler, is a simple dry powder inhaler in which capsules of powdered medication are loaded, pierced, and inhaled. While similar in function to the Handihaler used for tiotropium, the Breezhaler is a lower resistance device. When compared with the Handihaler in a 2-week patient handling and preference study, patients handled both devices well and without error, but preferred the smaller size and lower resistance characteristics of the Breezhaler.33
Conclusion, place in therapy
Indacaterol is a new once-daily LABA with a rapid onset of action (within 5 minutes), a peak effect at approximately 3 hours, and a duration of bronchodilation lasting at least 24 hours. Indacaterol has become the first effective once-daily LABA that is widely approved for use in COPD. Indacaterol, now approved in the European Union and US for COPD, provides effective 24-hour bronchodilation and a fast onset of action, with an efficacy at least comparable or superior to current bronchodilator therapy standards. We restrict our discussion only to the European product because in the US there were only two studies that evaluated 12 weeks’ duration. The data from clinical development support a favorable safety and tolerability profile within the β2-agonist drug class, with no relevant safety issues identified. The recommended dose is 150 μg once daily, delivered using a single-dose dry powder inhaler device. A 300 μg per capsule presentation for once-daily dosing is also approved and may provide additional clinical benefit for patients with severe COPD. The maximum dose is 300 μg once daily. As expected, improvements in lung function have translated into correspondingly beneficial effects on patient-reported outcomes, including significant reductions in exacerbations, dyspnea, and days with poor control, and clinically meaningful improvements in health-related quality of life, particularly when comparisons are made with placebo.
Some evidence shows that indacaterol is suitable for use as first-line monotherapy in COPD patients with moderate disease (GOLD stage II) and beyond that do not require inhaled corticosteroids as per GOLD guidelines, or in combination with an inhaled corticosteroid in severe or very severe patients with repeated exacerbations. As with long-acting bronchodilators, a short-acting bronchodilator such as salbutamol can be employed as on-demand rescue medication if needed. Further research should provide evidence on the benefits and safety profile of combining indacaterol with a long-acting anticholinergic bronchodilator.
Current COPD guidelines recommend inhaled bronchodilators as the mainstay of COPD pharmacotherapy and at least one long-acting inhaled bronchodilator as first-line maintenance therapy for patients with moderate symptomatic COPD, without signifying a preference for either a LABA or a once-daily long-acting antimuscarinic (LAMA). However, in actual practice, when a single long-acting inhaled bronchodilator is prescribed, it is most often a LAMA (tiotropium), rather than a twice-daily LABA (salmeterol or formoterol). The reason for this real world preference is unclear, but may be related to the greater convenience of the once-daily LAMA than the twice-daily LABA, a distinction that will be eliminated when indacaterol becomes widely available.
The GOLD guidelines also recommend combining a LAMA and a LABA in COPD patients not responding satisfactorily to monotherapy with either class of long-acting inhaled bronchodilator, a strategy that has been found to result in additive improvements in lung function in short-term clinical trials. However, this recommendation is infrequently followed; instead, in patients not responding to tiotropium alone, a LABA-inhaled corticosteroid combination is generally added as the next step. The availability of a once-daily LABA, such as indacaterol, would provide a convenient alternative to adding a twice-daily LABA-inhaled corticosteroid to a once-daily LAMA because the combined use of tiotropium and indacaterol in separate devices would entail only once-daily dosing.
Indacaterol provides a level of bronchodilation that is similar to tiotropium and greater than the twice-daily agents, formoterol and salmeterol. The measures of breathlessness and health status used in the indacaterol studies encompass components reflecting symptoms and the ability to undertake activities of daily living. Indacaterol was effective at reducing breathlessness, the most troublesome COPD symptom, and the 300 μg dose was significantly more effective in this regard than tiotropium and the twice-daily agents.
The beneficial effects of indacaterol on breathlessness and health status in GOLD stage II patients suggest that the overall study results provide a useful guide to the level of efficacy that may be expected in milder patients who may be seen predominantly in a primary care patient population. Indacaterol can also be combined with a once-daily inhaled corticosteroid, thus providing a more convenient once-daily alternative to existing twice-daily LABA-inhaled corticosteroid combinations (fluticasone-salmeterol and budesonide-formoterol) for the treatment of severe COPD.
A noticeable side effect has been cough, which has been mild and with attenuation over time and without pre-mature withdrawal from the trials. It has occurred within seconds of inhalation with rapid resolution, and has not been associated with bronchospasm. The mechanism of the cough remains unclear but presumably is related to a drug-specific stimulant effect on cough receptors in the upper/central airways; it seems that tachyphylaxis to this effect develops over time. No clinically meaningful effects have been noted on cardiovascular parameters, including heart rate, blood pressure, or QTc interval, or on serum potassium or blood glucose.
A patient with confirmed COPD who remains troubled by symptoms limiting daily activities despite a short-acting bronchodilator is a candidate for long-acting bronchodilator treatment. Factors to consider are the patient’s symptomatic response and preference, as well as drug side effects and cost. Indacaterol is attractive as a once-daily bronchodilator because it has slightly greater efficacy than the twice-daily bronchodilators. On the basis of the evidence reviewed, we conclude that once-daily indacaterol is an effective and beneficial maintenance bronchodilator treatment for patients with moderate to severe COPD, including patients with GOLD stage II disease.
Bronchodilators are central in the symptomatic management of COPD. It is likely that once-daily dosing of a bronchodilator would be a significant convenience and probably a compliance-enhancing advantage, leading to improved overall clinical outcomes in patients with COPD.
Footnotes
Disclosure
KRC holds the GSK-CIHR Research Chair in Respiratory Healthcare Delivery at the University Health Network, has served as a consultant to CSL Behring, GlaxoSmithKline, Novartis, Nycomed (Takeda), and Talecris (Grifols), and has received payment for lectures or service on speakers bureaus from Boehringer-Ingelheim, GlaxoSmithKline, Grifols, Nycomed (Takeda), Family Physicians Airways Group of Canada, Canadian Network for Respiratory Care, and Talecris.
References
- 1.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 2.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28:523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 3.Barr RG, Bourbeau J, Camargo CA, Ram FS. Tiotropium for stable chronic obstructive pulmonary disease: a meta-analysis. Thorax. 2006;61:854–862. doi: 10.1136/thx.2006.063271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GOLD Guidelines. COPD Diagnosis and Management. At-A-Glance Desk Reference. Global Initiative for Chronic Obstructive Pulmonary Disease. [Accessed December 29, 2011]. Available from: http://www.goldcopd.com.
- 5.Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63:831–838. doi: 10.1136/thx.2007.086041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an Internet survey. Curr Med Res Opin. 2009;25:2043–2048. doi: 10.1185/03007990903103006. [DOI] [PubMed] [Google Scholar]
- 7.Tashkin DP, Cooper CB. The role of long-acting bronchodilators in the management of stable COPD. Chest. 2004;125:249–259. doi: 10.1378/chest.125.1.249. [DOI] [PubMed] [Google Scholar]
- 8.Cazzola M, Matera MG, Lotvall J. Ultra long-acting beta 2-agonists in development for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs. 2005;14:775–783. doi: 10.1517/13543784.14.7.775. [DOI] [PubMed] [Google Scholar]
- 9.Feldman G, Siler T, Prasad N, et al. INLIGHT 1study group Efficacy and safety of indacaterol 150 microg once-daily in COPD: a double-blind, randomised, 12-week study. BMC Pulm Med. 2010;(10):11. doi: 10.1186/1471-2466-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calverley PM, Anderson JA, Celli B, et al. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010;65:719–725. doi: 10.1136/thx.2010.136077. [DOI] [PubMed] [Google Scholar]
- 11.Kerwin EM, Meli J, Henley M, Lassen C, Kramer B. Efficacy and safety of indacaterol 75 μg once daily in patients with moderate to-severe COPD. Am J Respir Crit Care Med. 2011;(183):A1595. [Google Scholar]
- 12.Donohue JF, Singh D, Kornmann O, Lawrence D, Lassen C, Kramer B. Safety of indacaterol in the treatment of patients with COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:477–492. doi: 10.2147/COPD.S23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aranson R, Rau JL., Jr The evolution of beta-agonists. Respir Care Clin N Am. 1999;5:479–519. [PubMed] [Google Scholar]
- 14.Battram C, Charlton SJ, Cuenoud B, et al. In vitro and in vivo pharmacological characterization of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1--hydroxy-ethyl]-8-hydroxy-1H-quino lin-2-one (indacaterol), a novel inhaled beta(2) adrenoceptor agonist with a 24-h duration of action. J Pharmacol Exp Ther. 2006;317:762–770. doi: 10.1124/jpet.105.098251. [DOI] [PubMed] [Google Scholar]
- 15.Pearlman DS, Greos L, LaForce C, Orevillo CJ, Owen R, Higgins M. Bronchodilator efficacy of indacaterol, a novel once-daily beta2-agonist, in patients with persistent asthma. Ann Allergy Asthma Immunol. 2008;101:90–95. doi: 10.1016/S1081-1206(10)60840-X. [DOI] [PubMed] [Google Scholar]
- 16.Lombardi D, Cuenoud B, Krämer SD. Lipid membrane interactions of indacaterol and salmeterol: do they influence their pharmacological properties. Eur J Pharm Sci. 2009;38:533–547. doi: 10.1016/j.ejps.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 17.EMEA European Medicines Agency. Evaluation of Medicines for Human Use. Assessment Report EMA/659981/2009. [Accessed January 5, 2012]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-Public_assessment_report/human/001114/WC500053735.pdf.
- 18.Duvauchelle T, Elharrar B, Knight H, et al. Single-dose indacaterol, a novel 24-hour. 2-agonist, is well tolerated in patients with mild asthma. Eur Respir J. 2005;26(Suppl 49):253s. [Google Scholar]
- 19.Donohue JF, Fogarty C, Lotvall J, et al. Am J Respir Crit Care Med. Vol. 182. Once; 2010. -daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium; pp. 155–162. [DOI] [PubMed] [Google Scholar]
- 20.Dahl R, Chung KF, Buhl R, et al. Efficacy of a new once-daily LABA, indacaterol, versus the twice-daily LABA, formoterol, in COPD. Thorax. 2010;65:473–479. doi: 10.1136/thx.2009.125435. [DOI] [PubMed] [Google Scholar]
- 21.Barnes PJ, Pocock SJ, Magnussen H, et al. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. Pulm Pharmacol Ther. 2010;23:165–171. doi: 10.1016/j.pupt.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Chapman KR, Rennard SI, Dogra A, Owen R, Lassen C, Kramer B. Long-term safety and efficacy of indacaterol, a long-acting β2-agonist, in subjects with COPD: a randomized, placebo-controlled study. Chest. 2011;140:68–75. doi: 10.1378/chest.10-1830. [DOI] [PubMed] [Google Scholar]
- 23.Kornmann O, Dahl R, Centanni S, et al. Once-daily indacaterol vs twice-daily salmeterol for COPD: a placebo controlled comparison. Eur Respir J. 2011;37:273–279. doi: 10.1183/09031936.00045810. [DOI] [PubMed] [Google Scholar]
- 24.Laforce C, Aumann J, de Teresa Parreno L, et al. Sustained 24-hour efficacy of once-daily indacaterol (300 mg) in patients with chronic obstructive pulmonary disease: a randomized, crossover study. Pulmon Pharmacol Ther. 2011;24:162–168. doi: 10.1016/j.pupt.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Jones PW, Brusselle G, Dal Negro RW, et al. Health-related quality of life in patients by COPD severity within primary care in Europe. Respir Med. 2011;105:57–66. doi: 10.1016/j.rmed.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Sears MR. Adverse effects of beta-agonists. J Allergy Clin Immunol. 2002;110:S322–328. doi: 10.1067/mai.2002.129966. [DOI] [PubMed] [Google Scholar]
- 27.Beier J, Beeh KM, Brookman L, Peachey G, Hmissi A, Pascoe S. Bronchodilator effects of indacaterol and formoterol in patients with COPD. Pulm Pharmacol Ther. 2009;22:492–496. doi: 10.1016/j.pupt.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigo GJ, Castro-Rodriguez JA, Nannini LJ, Plaza Moral V, Schiavi EA. Tiotropium and risk for fatal and nonfatal cardiovascular events in patients with chronic obstructive pulmonary disease: systematic review with meta-analysis. Respir Med. 2009;103:1421–1429. doi: 10.1016/j.rmed.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Worth H, Chung KF, Felser JM, Hu H, Rueegg P. Cardio- and cerebrovascular safety of indacaterol vs formoterol, salmeterol, tiotropium and placebo in COPD. Respir Med. 2011;105:571–579. doi: 10.1016/j.rmed.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 30.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 31.Barr RG, Celli BR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med. 2009;122:348–355. doi: 10.1016/j.amjmed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cazzola M, Proietto A, Matera MG. Indacaterol for chronic obstructive pulmonary disease (COPD) Drugs Today (Barc) 2010;46:139–150. doi: 10.1358/dot.2010.46.3.1450070. [DOI] [PubMed] [Google Scholar]
- 33.Chapman KR, Fogarty CM, Peckitt C, et al. Delivery characteristics and patients’ handling of two single-dose dry-powder inhalers used in COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:353–363. doi: 10.2147/COPD.S18529. [DOI] [PMC free article] [PubMed] [Google Scholar]