Abstract
Background
GSK233705 is an inhaled, long-acting muscarinic antagonist in development for the treatment of chronic obstructive pulmonary disease (COPD). This study was performed to see if the addition of GSK233705 to salmeterol would provide greater bronchodilation than salmeterol or tiotropium alone in COPD.
Methods
In an incomplete-block, three-period, crossover design, dually responsive patients received three of the following five treatments: GSK233705 20 μg plus salmeterol 50 μg twice-daily; GSK233705 50 μg plus salmeterol 50 μg twice-daily; salmeterol 50 μg or placebo, each twice-daily; and tiotropium 18 μg or placebo once-daily for 7 days. Each treatment period was separated by a 14-day washout. The primary efficacy endpoint was morning (trough) forced expiratory volume in 1 second (FEV1) on Day 8, following 7 days of treatment. Secondary endpoints included pulmonary function, plethysmography, pharmacokinetics of GSK233705 and salmeterol, adverse events (AEs), electrocardiograms (ECGs), vital signs, and laboratory parameters.
Results
A total of 47 patients were randomized. The mean % predicted normal postbronchodilator FEV1 was 55% at screening. Compared with placebo (n = 24), the adjusted mean change from baseline in trough FEV1 on Day 8 was 215 mL higher with GSK233705 20 μg + salmeterol (n = 23) and 203 mL higher with GSK233705 50 μg + salmeterol (n = 27), whereas with salmeterol (n = 27) and tiotropium (n = 28) the changes were 101 mL and 118 mL higher, respectively. The primary efficacy results were supported by the results from the other secondary lung function assessments. AEs were reported by similar proportions of patients across the treatment groups, with headache the most frequently reported treatment-related AE reported by one subject receiving each of GSK233705 20 μg + salmeterol, tiotropium, and placebo. No significant differences were seen in vital signs, ECGs, or laboratory parameters between the groups.
Conclusion
The addition of GSK233705 to salmeterol in partially reversible COPD patients resulted in greater bronchodilation than salmeterol or tiotropium alone and was well tolerated.
Keywords: COPD, bronchodilation, dual therapy, LAMA, LABA
Background
Bronchodilators are the cornerstone of the symptomatic management of COPD and current Global initiative for chronic Obstructive Lung Disease guidelines recommend the use of long-acting inhaled bronchodilators in moderate to very severe COPD.1,2 The benefits of bronchodilators include not only the control of symptoms but also improvements in lung function, hyperinflation, exercise performance, exacerbation rate, and health status.
Long-acting inhaled bronchodilators include both long-acting β-agonists (LABA), such as formoterol or salmeterol, and long-acting muscarinic antagonists (LAMA), such as tiotropium bromide. For patients who are not sufficiently controlled on maintenance monotherapy, combining bronchodilators of different classes, in particular a LAMA and a LABA, has been advocated.3 LABA/LAMA combinations appear to play an important role in maximizing bronchodilation and are well tolerated by the majority of patients.4–8
The scientific rationale for combining a LABA and muscarinic antagonist in COPD has been reviewed by several authors.9,10 Complementary bronchodilation may be obtained either by directly relaxing the smooth muscle through stimulation of β2 adrenoceptors or by inhibiting the action of acetylcholine at muscarinic receptors with muscarinic antagonists, indirectly leading to smooth muscle relaxation. Most studies published to date have reported the use of a combination of a twice-daily LABA with tiotropium4–8,11,12 or twice-daily LABA with short-acting muscarinic antagonists.13,14
There are a number of LAMAs in clinical development as monotherapies and in fixed combinations with LABAs.9,10 GlaxoSmithKline (GSK) are also developing novel LAMAs and one such compound is twice-daily GSK233705, also known as darotropium, a high-affinity pan-active muscarinic receptor antagonist (data on file). Phase I and II studies have assessed the efficacy, safety, tolerability, pharmacodynamics, and pharmacokinetics of single and repeat doses of GSK233705 and shown it to be generally well tolerated and an effective bronchodilator.
This pilot study in COPD patients responsive to both β-agonists and antimuscarinics was performed to see if the addition of GSK233705 to salmeterol would provide greater bronchodilation than salmeterol or tiotropium alone.
Methods
Patients and methods
Patients were recruited between October 2006 and May 2007 at nine centers in Finland, Germany, the Netherlands, and the UK. All patients gave written informed consent and the protocol was approved by the appropriate institutional review boards and conducted in accordance with good clinical practice guidelines and the most recent version of the Declaration of Helsinki. Patients aged 40–75 years with a clinical history of COPD according to the American Thoracic Society/European Respiratory Society (ATS/ERS) definition,2 a smoking history of ≥10 pack years, a postbronchodilator forced expiratory volume in 1 second (FEV1) of ≥40% to ≤75% of predicted normal, and a postbronchodilator FEV1/forced vital capacity (FVC) ratio of ≤70% were recruited. In order to recruit the optimum patient population for the study, patients also had to demonstrate responsiveness/ reversibility to both 80 mcg ipratropium bromide and 400 mcg salbutamol. Responsiveness/Reversibility was defined as an increase in FEV1 of ≥12% and ≥150 mL following inhalation of each bronchodilator administered on different occasions. Subjects with very large improvements in FEV1 > 500 mL at testing were excluded as this suggested the possibility of an alternative diagnosis.
Patients were excluded from the study if they had suffered a COPD exacerbation, had changes in COPD medication, or taken antibiotics within 4 weeks prior to screening or during the run-in period. Patients were also excluded if they had: a current diagnosis of asthma, a history of asthma, known respiratory disorders (apart from COPD), previous lung surgery, or required long-term oxygen therapy or pulmonary rehabilitation at screening. Patients were excluded if they had significant unstable cardiovascular disease.
Study design
This was a multicenter, randomized, partially blinded (active versus placebo treatment), placebo-controlled, three-way crossover study. Each subject was randomized to receive three of a possible five treatments (incomplete block design). The five treatments were: GSK233705 20 μg plus salmeterol 50 μg twice-daily; GSK233705 50 μg plus salmeterol 50 μg twice-daily; salmeterol 50 μg twice-daily; tiotropium bromide 18 μg once daily; and placebo (GSK study number AC2106956; clinical trials.gov NCT00422604).15 After a 2-week run-in period to establish a stable baseline, subjects were randomized to treatment sequences which comprised three 7-day treatment periods, each separated by a 14-day wash-out period. Two types of inhaler were used for the study (Diskus/Accuhaler™ [GlaxoSmithKline, London, UK] for GSK233705, salmeterol, and placebo and HandiHaler™ [Boehringer Ingelheim, Ingelheim am Rhein, Germany] for tiotropium bromide and placebo).
Concomitant medication
Inhaled salbutamol was provided as a relief medication. Other permitted medications included inhaled corticosteroids at a dose of up to 1000 μg/day of fluticasone propionate or equivalent (provided the dose had remained constant for 6 weeks prior to screening and would remain constant throughout the study). Patients who were taking LABA/ICS combination treatments had the LABA component stopped and replaced by the equivalent dose of inhaled corticosteroid (ICS) as monotherapy.
Outcome measurements
Lung function was determined by measuring the spirometry parameters FEV1 and FVC, and the plethysmography parameters airway conductance (sGaw), airways resistance (Raw), inspiratory capacity (IC), and residual volume (RV). Both trough (measured before the morning dose on Day 2 and Day 8) and postdose (measured on Day 1 and Day 7) spirometry and plethysmography values were recorded with plethysmography assessments conducted after spirometry. Serial spirometry was performed on Day 1 and Day 7 of each treatment period predose and at 1, 2, 4, 9, 12, and 24 hours following the morning dose of study medication. Plethysmography was performed predose and at 3, 11, and 25 hours postdose on Day 1 and Day 7. Subjects also recorded morning peak expiratory flow (PEF) prior to the morning dose of study medication and relief salbutamol rescue use on daily record cards during the run-in, wash-out, and treatment periods.
The primary efficacy endpoint was morning predose (trough) FEV1 on Day 8, following 7 days of treatment. Secondary lung function endpoints included trough FEV1 on Day 2; postdose FEV1, FVC, and plethysmography measurements on Day 1 and Day 7; trough FVC and trough plethysmography measurements on Day 2 and Day 8; and morning predose PEF and rescue medication recorded on the daily record card.
Safety endpoints included the incidence of adverse events (AEs), incidence of COPD exacerbations, vital signs (systolic and diastolic blood pressure and heart rate), and clinical laboratory tests (hematology, chemistry, and urinalysis). In addition, 12-lead electrocardiograms (ECGs) and 24-hour Holter monitoring were conducted during the study. Vital signs and 12-lead ECGs were measured at 15 and 45 minutes, 2, 4, 8, and 24 hours postdose on Day 1 and 7 of each treatment period. Lead II ECG monitoring was performed for 6 hours postdose at each treatment visit.
Pharmacokinetic assessments
Blood samples were collected to determine plasma concentrations of GSK233705 and salmeterol predose, 5, 15, and 30 minutes, and 1 hour and 2 hours postdose on Day 1. On Day 7, samples were collected as for Day 1 plus additional samples at 4 and 12 hours. Plasma samples were analyzed for GSK233705 and salmeterol using a validated analytical method based on protein precipitation, followed by high-performance liquid chromatography with mass spectrometry. The lower limit of quantification was 0.01 ng/mL for GSK233705 and 0.025 ng/mL for salmeterol. Urine samples were also analyzed for GSK233705 using a validated analytical method based on dilution followed by high-performance liquid chromatography with mass spectrometry analysis.
Statistical analysis
The sample size was based on estimates of precision of the anticipated treatment differences in FEV1, rather than formal powering, since this was a preliminary hypothesis-generating study. Three analysis populations were used. The modified per protocol (MPP) population, which comprised all subjects who completed at least one posttreatment lung-function assessment in at least two treatment periods and in which major deviations from the protocol did not occur, was used to analyze efficacy. The safety population included all subjects who were randomized and had received at least one dose of study medication and was used for all safety summaries and analyses, background, and demography summaries. Analyses of vital signs and 12-lead ECG data included all subjects in the safety population who recorded a posttreatment value for the relevant parameter in at least two treatment periods. The pharmacokinetic (PK) concentration population comprised all subjects in the safety population for whom a PK sample was obtained and analyzed.
The primary comparison for which treatment differences were estimated was of each dose of GSK233705 plus salmeterol versus placebo. Secondary comparisons for which treatment differences were estimated were for each dose of GSK233705 plus salmeterol versus salmeterol alone or tiotropium. Trough FEV1 values on Day 2 and Day 8 were analyzed using a mixed effects analysis of covariance with period, baseline FEV1 by day, treatment by day, and mean baseline FEV1 by day fitted as fixed effects and subject as a random coefficient; adjusted means and treatment differences were calculated along with corresponding 95% confidence intervals (CIs). Trough FEV1 on Day 2 and trough FVC endpoints were analyzed similarly. Prior to analysis, plethysmography parameters (sGaw, Raw, IC, and RV) were loge-transformed; the transformed parameters were analyzed in the same way as the primary efficacy parameter and back transformed prior to reporting. No formal statistical analysis was performed on safety data except for the maximum, minimum, and weighted mean parameters for 12-lead ECG QTc Fridericia’s formula (F) and QTc Bazzett’s formula (B) and supine vital signs data, which were analyzed in the same way as the primary efficacy parameter.
The PK parameters were calculated by standard noncompartmental analysis using WinNonlin® Pro (Pharsight, St Louis, MO). Plasma concentration data were listed and summarized (in tabular form as well as mean, median, and individual subject plots on the semilogarithmic and linear scales).
Results
Study population
A total of 47 dually responsive subjects were randomized, received treatment, and comprised the safety population. Four subjects (8.5%) were excluded from the MPP population due to failing to complete a baseline and posttreatment assessment for at least one efficacy parameter in at least two periods.
In the MPP population, 23 subjects received GSK233705 20 μg + salmeterol, 27 received GSK233705 50 μg + salmeterol, 27 received salmeterol alone, 28 received tiotropium alone, and 24 received placebo. The mean % predicted postbronchodilator FEV1 was 55% at screening and the mean percent reversibilities to ipratropium and salbutamol were 26% and 23%, respectively. The demographic and baseline characteristics of the safety and MPP study populations are shown in Table 1.
Table 1.
Baseline demographics and characteristics of the safety and modified per protocol (MPP) populations
| Safety population (N = 47) | MPP population (N = 43) | |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 62.6 (6.9) | 62.3 (7.1) |
| Range | 47–75 | 47–75 |
| Gender, n (%) | ||
| Female | 18 (38%) | 18 (42%) |
| Male | 29 (62%) | 25 (58%) |
| Ethnicity, n (%) | ||
| White | 47 (100%) | 43 (100%) |
| Body mass index (kg/m2) | ||
| Mean (SD) | 25.3 (2.9) | 25.3 (3.0) |
| Range | 20.2–30.9 | 20.2–30.9 |
| Smoking history | ||
| Current/former n (%) | 23 (49%)/24 (51%) | 21 (49%)/22 (51%) |
| Years smoked | ||
| Mean (SD) | 38.8 (8.9) | 38.6 (9.0) |
| Range | 20–60 | 20–60 |
| Number of cigarettes/day | ||
| Mean (SD) | 20.5 (7.8) | 20.7 (7.8) |
| Range | 5–40 | 5–40 |
| Smoking pack years | ||
| Mean (SD) | 40.0 (19.2) | 40.3 (19.4) |
| Range | 11.5–90.0 | 11.5–90.0 |
| Postbronchodilator | ||
| FEV1 at screening % predicted normal FEV1 | ||
| Mean (SD) | 55.3 (10.5) | 54.8 (9.5) |
| Range | 39.8–84.9 | 39.8–72.1 |
| % reversibility in baseline FEV1 ipratropium | ||
| Mean (SD) | 26.3 (11.3) | 25.7 (10.7) |
| Range | 9.3–52.4 | 9.3–52.4 |
| Salbutamol | ||
| Mean (SD) | 24.6 (11.5) | 23.3 (9.4) |
| Range | 10.6–66.7 | 10.6–52.7 |
| COPD medication during treatment | ||
| N (%) | 37 (79%) | 34 (79%) |
| Inhaled corticosteroids during treatment | ||
| N (%) | 25 (53%) | 23 (53%) |
Abbreviations: FEV1, forced expiratory volume in 1 second; SD, standard deviation.
Efficacy
Primary endpoint
Trough FEV1 on Day 8
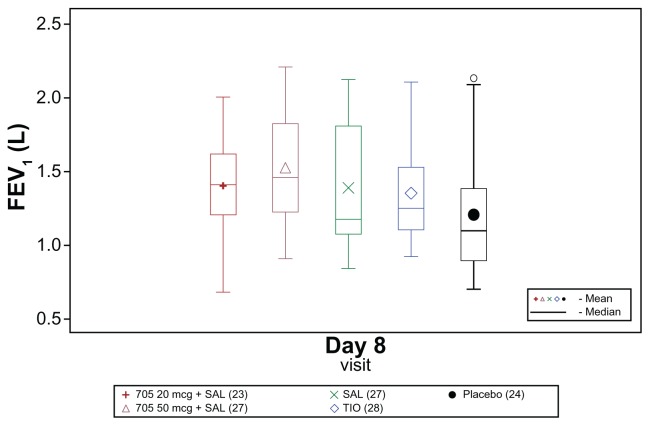
Raw mean trough FEV1 on Day 8 was higher for all active treatments than for placebo (Figure 1). All active treatments increased trough FEV1 on Day 8 by more than 120 mL compared with baseline, whereas the increase after placebo treatment was only 20 mL (adjusted mean differences).
Figure 1.
Box plot of raw mean trough (morning, predose) forced expiratory volume in 1 second (FEV1) on Day 8 (modified per protocol population).
Abbreviations: SAL, salmeterol; TIO, tiotropium.
Compared with placebo, the increase in trough FEV1 was 215 mL higher with GSK233705 20 μg + salmeterol and 203 mL higher with GSK233705 50 μg + salmeterol, whereas with salmeterol and tiotropium alone the increases were 101 mL and 118 mL higher, respectively. Larger increases from baseline in trough FEV1 on Day 8 were also seen with the combination treatments compared with the individual treatments (Table 2). For all treatment comparisons performed, the lower 95% CI for the treatment difference was >0, giving strong statistical evidence of a benefit of the combination treatments over placebo, salmeterol, and tiotropium, and of salmeterol and tiotropium over placebo (Table 2).
Table 2.
Analysis of trough (morning, predose) forced expiratory volume in 1 second (L) on Day 8 (modified per protocol population)
| GSK233705 20 μg + salmeterol | GSK233705 50 μg + salmeterol | Salmeterol | Tiotropium | Placebo | |
|---|---|---|---|---|---|
| n | 22 | 26 | 27 | 28 | 24 |
| Baseline raw mean (SD) | 1.16 (0.31) | 1.29 (0.43) | 1.29 (0.42) | 1.20 (0.34) | 1.19 (0.36) |
| Endpoint adjusted mean (SE) | 1.46 (0.03) | 1.45 (0.03) | 1.35 (0.03) | 1.37 (0.03) | 1.25 (0.03) |
| Adjusted mean change from baseline (SE) | 0.24 (0.03) | 0.22 (0.03) | 0.12 (0.03) | 0.14 (0.03) | 0.02 (0.03) |
| Difference from placebo (SE) 95% CI | 0.21 (0.04) (0.14, 0.29) | 0.20 (0.03) (0.13, 0.27) | 0.10 (0.03) (0.03, 0.17) | 0.12 (0.03) (0.05, 0.18) | |
| Difference from salmeterol (SE) 95% CI | 0.11 (0.03) (0.04, 0.18) | 0.10 (0.03) (0.04, 0.17) | |||
| Difference from tiotropium (SE) 95% CI | 0.10 (0.03) (0.03, 0.17) | 0.08 (0.03) (0.02, 0.15) |
Abbreviations: CI, confidence interval; SD, standard deviation; SE, standard error of the mean.
Secondary endpoints
Trough FEV1 on Day 2
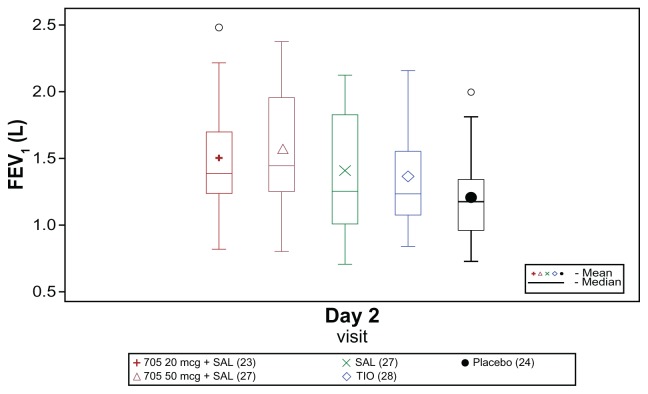
Raw mean trough FEV1 on Day 2 was higher for all active treatments than for placebo (Figure 2), and all treatments increased trough FEV1 on Day 2 compared with baseline. Compared with placebo, the increase from baseline in trough FEV1 was 306 mL higher with GSK233705 20 μg + salmeterol and 251 mL higher with GSK233705 50 μg + salmeterol, whereas with salmeterol and tiotropium the increases were 105 mL and 136 mL higher, respectively. Larger increases from baseline in trough FEV1 on Day 2 were also seen with the combination treatments compared with the individual treatments: 201 mL versus salmeterol and 170 mL versus tiotropium for GSK233705 20 μg + salmeterol and 145 mL versus salmeterol and 115 mL versus tiotropium for GSK233705 50 μg + salmeterol.
Figure 2.
Box plot of raw mean trough (morning, predose) forced expiratory volume in 1 second (FEV1) on Day 2 (modified per protocol population).
Abbreviations: SAL, salmeterol; TIO, tiotropium.
For all treatment comparisons performed, the lower 95% CI for the treatment difference was >0, giving strong statistical evidence of a benefit of the combination treatments over placebo, salmeterol, and tiotropium, and of salmeterol and tiotropium over placebo (Table 3).
Table 3.
Analysis of trough (morning, predose) forced expiratory volume in 1 second (L) on Day 2 (modified per protocol population)
| GSK233705 20 μg + salmeterol | GSK233705 50 μg + salmeterol | Salmeterol | Tiotropium | Placebo | |
|---|---|---|---|---|---|
| n | 23 | 27 | 27 | 28 | 24 |
| Baseline raw mean (SD) | 1.18 (0.32) | 1.29 (0.42) | 1.29 (0.42) | 1.20 (0.34) | 1.19 (0.36) |
| Endpoint adjusted mean (SE) | 1.55 (0.03) | 1.49 (0.03) | 1.34 (0.03) | 1.37 (0.03) | 1.24 (0.03) |
| Adjusted mean change from baseline (SE) | 0.32 (0.03) | 0.23 (0.028) | 0.12 (0.03) | 0.15 (0.03) | 0.01 (0.03) |
| Difference from placebo (SE) 95% CI | 0.31 (0.04) (0.23, 0.38) | 0.25 (0.04) (0.17, 0.33) | 0.10 (0.04) (0.03, 0.18) | 0.14 (0.037) (0.06, 0.21) | |
| Difference from salmeterol (SE) 95% CI | 0.20 (0.04) (0.12, 0.28) | 0.14 (0.04) (0.07, 0.22) | |||
| Difference from tiotropium (SE) 95% CI | 0.17 (0.04) (0.09, 0.25) | 0.11 (0.04) (0.04, 0.19) |
Abbreviations: CI, confidence interval; SD, standard deviation; SE, standard error of the mean.
Trough FVC and postdose FVC
Trough FVC and postdose FVC values followed a similar pattern to that observed for the FEV1 endpoints. Compared with placebo, the change from baseline in trough FVC on Day 8 was 411 mL higher with GSK233705 20 μg + salmeterol and 355 mL higher with GSK233705 50 μg + salmeterol, whereas with salmeterol and tiotropium the changes were 144 mL and 257 mL higher, respectively. Trough FVC values and treatment differences on Day 2 were generally higher than those recorded on Day 8. For all comparisons of combination treatments and tiotropium with placebo, the lower 95% CI for the treatment difference was >0, giving strong statistical evidence of a benefit. The evidence was less strong for the benefit of salmeterol over placebo. Correspondingly there was statistical evidence (lower 95% CI > 0) of a benefit of combination treatment over salmeterol, but no consistent statistical evidence of a benefit over tiotropium.
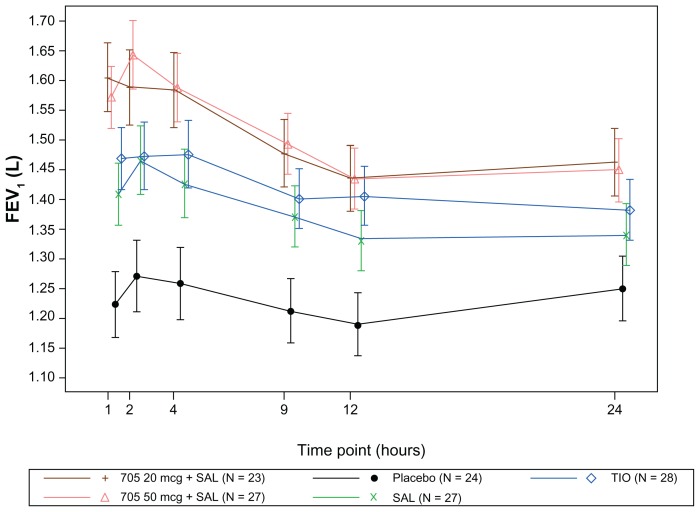
All active treatments increased postdose serial FEV1, and postdose FVC relative to placebo at all time points on both days, with the combination treatments again showing an improvement compared with salmeterol alone and tiotropium alone (Figure 3). For all comparisons of combination treatments with placebo, the lower 95% CI for the ratio between treatments was >0, giving strong statistical evidence of a benefit.
Figure 3.
Adjusted mean (95% confidence interval) forced expiratory volume in 1 second (FEV1) over time on Day 7 (modified per protocol population).
Abbreviations: SAL, salmeterol; TIO, tiotropium.
Plethysmography
All active treatments increased trough sGaw on Day 8 by more than 22% compared with baseline, whereas placebo treatment showed a 3% decrease from baseline. The ratio to baseline compared with placebo was 49.8% higher with GSK233705 20 μg + salmeterol and 65.9% higher with GSK233705 50 μg + salmeterol, whereas with salmeterol and tiotropium bromide the ratios were 26.6% and 29.8% higher, respectively.
Postdose sGaw, trough Raw, and postdose Raw also reflected the spirometric results (as did IC and RV to a lesser extent) (Table 4).
Table 4.
Trough sGaw, Raw, IC, and RV on Day 2 and Day 8 (modified per protocol population)
| GSK233705 20 μg + salmeterol | GSK233705 50 μg + salmeterol | Salmeterol | Tiotropium | Placebo | |
|---|---|---|---|---|---|
| Day 2 | |||||
| n | 23 | 27 | 27 | 27 | 24 |
| Trough sGaw | |||||
| Endpoint adjusted geometric mean | 0.79 | 0.84 | 0.56 | 0.59 | 0.47 |
| Ratio of individual treatments to placebo (95% CI) | 1.68 (1.48, 1.91) | 1.77 (1.57, 2.00) | 1.19 (1.05, 1.35) | 1.26 (1.11, 1.42) | |
| Trough Raw | |||||
| Endpoint adjusted geometric mean | 0.26 | 0.25 | 0.36 | 0.33 | 0.42 |
| Ratio of individual treatments to placebo (95% CI) | 0.62 (0.54, 0.70) | 0.60 (0.53, 0.67) | 0.86 (0.76, 0.97) | 0.80 (0.71, 0.90) | |
| Trough IC | |||||
| Endpoint adjusted geometric mean | 2.25 | 2.17 | 2.01 | 2.09 | 1.98 |
| Ratio of individual treatments to placebo (95% CI) | 1.13 (1.06, 1.21) | 1.09 (1.03, 1.16) | 1.02 (0.96, 1.08) | 1.06 (0.99, 1.12) | |
| Trough RV | |||||
| Endpoint adjusted geometric mean | 3.02 | 3.06 | 3.31 | 3.32 | 3.57 |
| Ratio of individual treatments to placebo (95% CI) | 0.85 (0.78, 0.92) | 0.857 (0.79, 0.93) | 0.93 (0.85, 1.01) | 0.932 (0.86, 1.01) | |
| Day 8 | |||||
| n | 21 | 26 | 27 | 28 | 24 |
| Trough sGaw | |||||
| Endpoint adjusted geometric mean | 0.66 | 0.73 | 0.56 | 0.57 | 0.44 |
| Ratio of individual treatments to placebo (95% CI) | 1.50 (1.31, 1.70) | 1.66 (1.47, 1.87) | 1.27 (1.12, 1.43) | 1.30 (1.15, 1.47) | |
| Trough Raw | |||||
| Endpoint adjusted geometric mean | 0.31 | 0.29 | 0.36 | 0.35 | 0.44 |
| Ratio of individual treatments to placebo (95% CI) | 0.71 (0.62, 0.81) | 0.65 (0.58, 0.74) | 0.81 (0.72, 0.92) | 0.80 (0.71, 0.91) | |
| Trough IC | |||||
| Endpoint adjusted geometric mean | 2.16 | 2.17 | 2.03 | 2.07 | 1.93 |
| Ratio of individual treatments to placebo (95% CI) | 1.12 (1.04, 1.20) | 1.12 (1.05, 1.20) | 1.05 (0.98, 1.12) | 1.07 (1.00, 1.14) | |
| Trough RV | |||||
| Endpoint adjusted geometric mean | 3.03 | 3.03 | 3.33 | 3.31 | 3.59 |
| Ratio of individual treatments to placebo (95% CI) | 0.84 (0.79, 0.90) | 0.84 (0.79, 0.90) | 0.93 (0.87, 0.98) | 0.92 (0.87, 0.98) | |
Abbreviations: CI, confidence interval; IC, inspiratory capacity; Raw, airways resistance; RV, residual volume; sGaw, plethysmography parameters airway conductance.
Morning PEF
The combination treatments both increased PEF by approximately 35 L/min compared with placebo whereas salmeterol and tiotropium increased PEF by 20 L/min and 15 L/min, respectively. For all treatment comparisons performed, the lower 95% CI for the treatment difference was >0, indicating strong statistical evidence of a benefit of the combination treatments over placebo, salmeterol and tiotropium, and of salmeterol and tiotropium over placebo.
Rescue use
For subjects receiving GSK233705 20 μg + salmeterol and GSK233705 50 μg + salmeterol, 52.4%, and 46.2% never needed to use their salbutamol rescue medication during the treatment period, compared with 44.4% of subjects receiving salmeterol, 32.1% of subjects receiving tiotropium, and 16.7% of subjects receiving placebo.
Safety
AEs
The safety profiles were similar across all treatments. The most common AEs with onset while on-treatment were headache and ventricular extrasystoles (Table 5). Headache was the most frequently reported treatment-related AE and was reported by one subject on each of GSK233705 20 μg + salmeterol, tiotropium, and placebo. No serious AEs were reported in treated subjects. Four subjects were withdrawn; three due to an exacerbation (two following treatment with placebo and one after salmeterol) and one subject on salmeterol treatment had an AE of nonsustained ventricular tachycardia (<30 beats), which led to withdrawal from the study.
Table 5.
Adverse events (AEs) with onset on-treatment experienced by more than one subject on any treatment (safety population)
| AE n (%) | GSK233705 20 μg + salmeterol (N = 23) | GSK233705 50 μg + salmeterol (N = 27) | Salmeterol (N = 29) | Tiotropium (N = 28) | Placebo (N = 26) |
|---|---|---|---|---|---|
| Subjects with any AE | 14 (61%) | 11 (41%) | 16 (55%) | 16 (57%) | 12 (46%) |
| Nasopharyngitis | 1 (4%) | 0 | 1 (3%) | 1 (4%) | 2 (8%) |
| Urinary tract infection | 1 (4%) | 0 | 1 (3%) | 1 (4%) | 2 (8%) |
| Influenza | 2 (9%) | 0 | 0 | 2 (7%) | 0 |
| Headache | 4 (17%) | 2 (7%) | 2 (7%) | 5 (18%) | 3 (11%) |
| Dizziness | 1 (4%) | 2 (7%) | 0 | 1 (4%) | 0 |
| Ventricular extrasystoles | 1 (4%) | 1 (4%) | 3 (10%) | 1 (4%) | 0 |
| Ventricular tachycardia | 1 (4%) | 0 | 2 (7%) | 1 (4%) | 0 |
| Supraventricular tachycardia | 1 (4%) | 0 | 0 | 2 (7%) | 0 |
| Supraventricular extrasystoles | 0 | 0 | 2 (7%) | 0 | 0 |
| Back pain | 0 | 0 | 2 (7%) | 0 | 3 (11%) |
| Leukocyturia | 0 | 2 (7%) | 0 | 1 (4%) | 0 |
| Fatigue | 0 | 0 | 0 | 1 (4%) | 2 (8%) |
Vital signs
The changes in heart rate from baseline on Day 1 and Day 7 were similar for all treatments including placebo. There was some evidence of a small increase in heart rate on salmeterol compared with placebo on Day 1, with lower CIs above zero for the comparisons of both weighted mean and maximum heart rate over 0–24 hours. Similar evidence was seen for a decrease in heart rate on the combination treatments compared with salmeterol, with upper CIs < 0 for the comparison of each combination treatment with salmeterol for weighted mean and minimum heart rate, and for the comparison of GSK233705 50 μg + salmeterol with salmeterol for maximum heart rate. These differences were small, with maximum and minimum 95% CIs less than ±6.4 bpm. However, similar evidence was not seen on Day 7.
The changes in systolic and diastolic blood pressure from baseline on Day 1 and Day 7 were similar between all treatments including placebo. Treatment differences for weighted mean, maximum and minimum systolic and diastolic blood pressure over 0–4 hours and 0–24 hours were small, and in all cases except one the CIs for all treatment comparisons contained zero.
ECGs
No clinically significant changes in ECG traces during any visit postbaseline were reported for any treatment. Summary statistics for Holter ECG parameters (maximum, mean and minimum heart rate, maximum and mean uncorrected QT, maximum RR, mean QTc(B) and QTc(F), number of RR intervals >2 msec, and number of ventricular couplets, triplets and nonsustained ventricular tachycardias, and supraventricular tachycardias) were similar for all treatments at screening and on Day 1 and Day 7.
Pharmacokinetics
The bioanalytical method (LLQ of 0.01 ng/mL) for GSK233705 was not sensitive enough to fully characterize the PK profile of subjects due to the low level of GSK233705 detected in plasma. Overall, 52% of data were nonquantifiable. Following single and repeat inhaled dose administration, GSK233705 was rapidly absorbed. The median tmax was 5 minutes, irrespective of dose levels (20 μg and 50 μg) and sampling days (Day 1 and Day 7). Plasma concentrations declined rapidly following the occurrence of Cmax. Plasma concentrations following repeat inhaled administration were on average higher than those after single dosing for both dose levels suggestive of accumulation. Moderate to high between-subject variability in Cmax was observed with values of the coefficient of variation ranging from 42% to 60% on Day 1, and from 42% to 52% on Day 7 across dose levels. Moderate to high between-subject variability in AUC(0–t) was also observed with values of the coefficient of variation ranging from 29% to 67% on Day 1, and from 98% to 130% on Day 7 across dose levels (Table 6).
Table 6.
Summary pharmacokinetic statistics on Day 1 and Day 7
| Parameter | Treatment | Analyte: GSK233705 | Analyte: Salmeterol | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Day 1 | Day 7 | Day 1 | Day 7 | ||||||
|
|
|
|
|
||||||
| N/n | Geometric mean 95% CI/CV (%) | N/n | Geometric mean 95% CI/CV (%) | N/n | Geometric mean 95% CI/CV (%) | N/n | Geometric mean 95% CI/CV (%) | ||
| AUC(0–t) (h*ng/mL) | 705 20 μg + SAL | 23/4 | 0.020 (0.013, 0.032)/29 | 23/12 | 0.022 (0.013, 0.037)/98 | 23/10 | 0.055 (0.039, 0.077)/49 | 23/16 | 0.116 (0.084, 0.162)/68 |
| 705 50 μg + SAL | 27/14 | 0.018 (0.013, 0.026)/67 | 27/26 | 0.088 (0.059, 0.131)/130 | 27/11 | 0.059 (0.042, 0.083)/54 | 27/20 | 0.071 (0.045, 0.112)/124 | |
| SAL | N/A | N/A | N/A | N/A | 29/14 | 0.059 (0.049, 0.072)/35 | 29/24 | 0.091 (0.064, 0.129)/99 | |
| Cmax_high (ng/mL) | 705 20 μg + SAL | 23/23 | 0.013 (0.011, 0.015)/42 | 23/22 | 0.019 (0.016, 0.023)/42 | 23/23 | 0.036 (0.032, 0.041)/28 | 23/22 | 0.052 (0.042, 0.064)/50 |
| 705 50 μg + SAL | 27/27 | 0.023 (0.019, 0.029)/60 | 27/27 | 0.048 (0.040, 0.058)/52 | 27/27 | 0.036 (0.031, 0.041)/38 | 27/27 | 0.052 (0.042, 0.064)/53 | |
| SAL | N/A | N/A | N/A | N/A | 29/29 | 0.037 (0.032, 0.042) | 29/29 | 0.049 (0.042, 0.056)/41 | |
| tmax (h)a | 705 20 μg + SAL | 23/10 | 0.080 (0.05, 0.27) | 23/20 | 0.080 (0.08, 0.50) | 23/20 | 0.520 (0.05, 2.08) | 23/20 | 0.425 (0.08, 1.98) |
| 705 50 μg + SAL | 27/25 | 0.080 (0.03, 2.08) | 27/27 | 0.080 (0.05, 0.52) | 27/19 | 0.400 (0.08, 1.07) | 27/24 | 0.500 (0.05, 11.92) | |
| SAL | N/A | N/A | N/A | N/A | 29/23 | 0.480 (0.05, 2.00) | 29/26 | 0.250 (0.08, 4.00) | |
| tlast (h)a | 705 20 μg + SAL | 23/10 | 0.230 (0.08, 2.10) | 23/20 | 0.495 (0.08, 4.17) | 23/20 | 1.000 (0.05, 2.10) | 23/20 | 2.010 (0.08, 11.85) |
| 705 50 μg + SAL | 27/25 | 0.500 (0.03, 2.08) | 27/27 | 4.020 (0.25, 11.95) | 27/19 | 1.000 (0.08, 2.10) | 27/24 | 2.000 (0.50, 11.93) | |
| SAL | N/A | N/A | N/A | N/A | 29/23 | 1.900 (0.12, 2.25) | 29/16 | 2.050 (0.32, 12.00) | |
Note:
Presented as median and range.
Abbreviations: AUC(0–t), area under concentration–time curve from time 0 to time of last quantifiable concentration; CI, confidence interval; Cmax, maximum observed plasma concentration; CV, coefficient of variation; N/A, not applicable; SAL, salmeterol; tmax, time of maximum observed plasma concentration; tlast, last time point where the concentration is above the limit of quantification.
The bioanalytical method (LLQ of 0.025 ng/mL) for salmeterol was not sensitive enough to fully characterize the PK profile of subjects due to the low level of salmeterol detected in plasma. For salmeterol, overall 55% (604/1092) of data was nonquantifiable. The median tmax was 30 minutes or less irrespective of sampling day or whether salmeterol was administered alone or in combination with GSK233705. Moderate between-subject variability in Cmax was observed with values of the coefficient of variation ranging from 29% to 38% on Day 1, and from 41% to 53% on Day 7 across treatment regimens. Moderate to high between-subject variability in AUC(0–t) was also observed with values of the coefficient of variation ranging from 35% to 54% on Day 1, and from 68% to 124% on Day 7 across treatment regimens.
Discussion
The objectives of this pilot study were to investigate the bronchodilatory efficacy, safety, and tolerability of twice-daily therapy with two different long-acting bronchodilators in a COPD population optimized to demonstrate a response. These were administered concurrently (GSK233705 + salmeterol) and compared primarily with placebo but also compared with twice-daily salmeterol monotherapy or once-daily tiotropium monotherapy in subjects with moderate to severe COPD. The primary lung function measurements were made using spirometry, and this was supported by plethysmography, which provides a further sensitive assessment of lung function16 and also takes into account effects on both the large and small airways.
A crossover design was chosen because the within-patient variability in FEV1 was expected to be less than between-patient variability, with each patient acting as their own control. An incomplete-block, rather than a complete-block, crossover design was adopted (with three periods) to reduce the study duration and overall burden on patients. To fully blind the study, each subject would have needed to use both inhalers on a double-dummy basis and to take multiple inhalations morning and evening. In order to reduce the number of inhalers subjects needed to take daily, the study was blinded for active versus placebo treatments but not with respect to inhaler type. The duration of 2 weeks for washout was also sufficient to minimize the possibility of carry-over effects of both salmeterol and tiotropium. It is recognized however that a limitation of the study was that there was no GSK233705 monotherapy arm.
Concurrent use of GSK233705 at doses of 20 μg or 50 μg twice-daily plus salmeterol 50 μg twice-daily for 7 days resulted in clinically important improvements in lung function which were greater than those seen with placebo, salmeterol 50 μg twice-daily alone, or tiotropium 18 μg once-daily alone. The improvement versus placebo in bronchodilation with both doses of GSK233705 plus salmeterol was not only higher than the 100 mL criterion described by Donohue17 as a difference that COPD patients can perceive but also was well above the range (100–140 mL) proposed as a minimal clinically important difference.18
The clinical improvements seen in lung function were also supported by the results of daily diary card assessments, morning PEF measurements, and rescue medication use, all of which suggested that dual bronchodilator treatment has the potential to produce a clinically important additional benefit on symptoms compared with a single agent. It has previously been shown that these two classes of bronchodilator added together result in a significant reduction in salbutamol use as rescue therapy and symptoms of dyspnoea.5,19 This study provides further support for these observations. Although this study did not include symptom assessments, the need for rescue medication is a useful surrogate of symptomatic benefit and reductions in the use of rescue medication were seen with the combination treatments compared with each of the individual treatments.
The positive primary efficacy results were supported by the results from all of the remaining lung function assessments.
Although this was a small study, and the endpoints not formally powered, the results were consistent across virtually every endpoint studied and the sizes of the treatment differences were compelling. No obvious dose response was observed between the two doses of GSK233705 in combination with salmeterol for any of the endpoints, but the study was not designed to differentiate between doses.
The safety findings from this study demonstrated that the safety profiles were similar across all treatments. No clinically significant effects on heart rate were observed with concurrent administration of GSK233705 and salmeterol and, importantly, analysis of 24-hour Holter ECG measurements did not identify any change in the incidence of the minor rhythm abnormalities frequently observed in COPD patients.
The pharmacokinetic information obtained in this study was limited due to a large amount of nonquantifiable data. However, the results showed that, following single and repeat inhaled doses, there was rapid absorption of both GSK233705 and salmeterol with a median time to Cmax (tmax) of 5 minutes for GSK233705 and 30 minutes for salmeterol. The PK parameters for salmeterol were similar in the presence and absence of GSK233705, demonstrating an absence of any PK interaction between the two drugs.
Results from this study support previous findings which have demonstrated that a long-acting β-agonist added to a long-acting anticholinergic (tiotropium bromide) produce incremental effects on lung function. Data from both short-term4–6,16 and long-term studies provide additional evidence that combining LAMAs and LABAs helps to maximize bronchodilation and improve symptoms and quality of life in patients with moderate to severe disease.7–8,20 Importantly, in this study no increase in the risk of side effects was observed with the combinations compared with monotherapies.
Most subjects recruited for this study had moderate disease and were documented to demonstrate significant bronchodilator responses to both anticholinergics and β2-agonists. This further supports the use of LABA/LAMA combination therapy in moderate COPD if maintenance treatment with a single long-acting bronchodilator does not suffice.1–3 Published evidence to date also supports the use of a combination therapy with tiotropium plus a LABA even in those COPD patients with more severe symptoms.8,21,22 However, limited analyses have been performed to date to assess the differential effects of LAMA therapy by severity of COPD. Clinical studies have included patients with disease severity ranging from moderate to very severe disease. Tashkin et al6 found that while improvements in lung function were greater in more severe COPD, dyspnea scores improved irrespective of severity.
It has been hypothesized that activity of the sympathetic system is more prominent during the day, whereas increased parasympathetic system activity is found during the night.21–25 It therefore seems appropriate to target bronchoconstriction through two distinct mechanisms (anticholinergic and sympathomimetic) to maximize the bronchodilator response over 24 hours and help to overcome inter- and intra-patient variability in bronchomotor tone associated with COPD.26
A range of once and twice daily LAMAs are currently in clinical development as monotherapies in COPD.9,10 A number of novel once-daily fixed-dose combination bronchodilators are also in phase III development, including QVA149 (a combination of indacaterol and NVA237)27 and vilanterol plus GSK573719.28
Conclusion
This study helps to demonstrate that combinations of these two classes of medications can achieve significant bronchodilation compared to monotherapy. The addition of GSK233705 to salmeterol resulted in greater bronchodilatation than salmeterol or tiotropium alone and was well tolerated. This particular bronchodilator combination is at present not being progressed further because once-daily alternatives are favored and being developed.
Acknowledgments
The authors thank the patients who took part and the staff at the participating clinical centers. The authors would also like to thank Diana Jones of Cambrian Clinical Associates Limited, a professional medical writer sponsored by GSK, for her assistance in the preparation of this manuscript.
Footnotes
Disclosure
This study was funded by GSK, Stockley Park, UK. Amanda Deans, Jean Brooks, Claire Maden, Suus Baggen, Rashmi Mehta, and Anthony Cahn are employees of GSK. Jutta Beier has given presentations at symposia sponsored by Almirall, Novartis, and Revotar and has received fees for consulting from Almirall, Cytos, Novartis, Pfizer, and Revotar. Jan van Noord has given presentations at symposia sponsored by AstraZeneca, Boehringer Ingelheim, and GSK, and has received fees for contract research from AstraZeneca, Boehringer Ingelheim, and GSK.
Authors’ contributions
Amanda Deans, Jean Brooks, Claire Maden, Suus Baggen, Rashmi Mehta, and Anthony Cahn (as employees of the study sponsor, GSK) contributed to the design, analysis, and interpretation of the study, and oversaw its conduct. All authors contributed equally to the development of the manuscript, and approved the final version for submission.
References
- 1.Global initiative for chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [updated 2008] [Accessed December 8, 2011]. Available from: http://www.goldcopd.com.
- 2.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ /ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 3.Tashkin DP, Cooper CB. The role of long-acting bronchodilators in the management of stable COPD. Chest. 2004;125(1):249–259. doi: 10.1378/chest.125.1.249. [DOI] [PubMed] [Google Scholar]
- 4.van Noord JA, Aumann JL, Janssens E, et al. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J. 2005;26(2):214–222. doi: 10.1183/09031936.05.00140404. [DOI] [PubMed] [Google Scholar]
- 5.van Noord JA, Aumann JL, Janssens E, et al. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest. 2006;129(3):509–517. doi: 10.1378/chest.129.3.509. [DOI] [PubMed] [Google Scholar]
- 6.Tashkin DP, Donohue JF, Mahler DA, et al. Effects of arformoterol twice daily, tiotropium once daily, and their combination in patients with COPD. Respir Med. 2009;103(4):516–524. doi: 10.1016/j.rmed.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Vogelmeier C, Kardos P, Harari S, Gans SJ, Stenglein S, Thirlwell J. Formoterol mono- and combination therapy with tiotropium in patients with COPD: a 6-month study. Respir Med. 2008;102(11):1511–1520. doi: 10.1016/j.rmed.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146(8):545–555. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- 9.Cazzola M, Molimard M. The scientific rationale for combining long-acting b2-agonistsandmuscarinic antagonists in COPD. Pulm Pharmacol Ther. 2010;23(4):257–267. doi: 10.1016/j.pupt.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Laine DI. Long-acting muscarinic antagonists for the treatment of chronic obstructive pulmonary disease. Expert Rev Clin Pharmacol. 2010;3(1):43–53. doi: 10.1586/ecp.09.48. [DOI] [PubMed] [Google Scholar]
- 11.Rabe KF, Timmer W, Sagkriotis A, Viel K. Comparison of a combination of tiotropium plus formoterol to salmeterol plus fluticasone in moderate COPD. Chest. 2008;134(2):255–262. doi: 10.1378/chest.07-2138. [DOI] [PubMed] [Google Scholar]
- 12.Cazzola M, Santus P, Verga M, et al. The functional impact of adding salmeterol to tiotropium in patients with stable COPD. Respir Med. 2004;98(12):1214–1221. doi: 10.1016/j.rmed.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 13.van Noord JA, de Munck DR, Bantje TA, et al. Long-term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. Eur Respir J. 2000;15(5):878–885. doi: 10.1034/j.1399-3003.2000.15e11.x. [DOI] [PubMed] [Google Scholar]
- 14.D’Urzo AD, De Salvo MC, Ramirez-Rivera A, et al. In patients with COPD, treatment with a combination of formoterol and ipratropium is more effective than a combination of salbutamol and ipratropium: a 3-week, randomized, double-blind, within-patient, multicenter study. Chest. 2001;119(5):1347–1356. doi: 10.1378/chest.119.5.1347. [DOI] [PubMed] [Google Scholar]
- 15.GlaxoSmithKline. ClinicalTrials.gov [website on the Internet] Bethseda, MD: US National Library of Medicine; 2011. [Accessed December 15, 2011]. Safety and efficacy of GSK233705 plus salmeterol compared with 2 active comparators and placebo in subjects with chronic obstructive pulmonary disease (COPD) [updated March 17, 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT00422604. NLM identifier: NCT00422604. [Google Scholar]
- 16.Singh D, Tal-Singer R, Faiferman I, et al. Plethysmography and impulse oscillometry assessment of tiotropium and ipratropium bromide; a randomized, double-blind, placebo-controlled, cross-over study in healthy subjects. Br J Clin Pharmacol. 2006;61(4):398–404. doi: 10.1111/j.1365-2125.2006.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2(1):111–124. doi: 10.1081/copd-200053377. [DOI] [PubMed] [Google Scholar]
- 18.Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416–469. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- 19.Van Noord J, Aumann L, Janssens E, Smeets J, Mueller A, Cornelissen P. Combination therapy of tiotropium plus salmeterol superior to single agent therapy in terms of dyspnea improvement in COPD [abstract] Chest. 2005;(128):177S. [Google Scholar]
- 20.Tashkin D, Pearle J, Iezzoni D, Varghese ST. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD. 2009;6(1):17–25. doi: 10.1080/15412550902724073. [DOI] [PubMed] [Google Scholar]
- 21.Singh D, Brooks J, Hagan G, Cahn A, O’Connor BJ. Superiority of “triple” therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax. 2008;63(7):592–598. doi: 10.1136/thx.2007.087213. [DOI] [PubMed] [Google Scholar]
- 22.Cazzola M, Andò F, Santus P, et al. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe-to-very severe COPD. Pulm Pharmacol Ther. 2007;20(5):556–561. doi: 10.1016/j.pupt.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Postma DS, Keyzer JJ, Koeter GH, Sluiter HJ, DeVries K. Influence of the parasympathetic and sympathetic nervous system on nocturnal bronchial obstruction. Clin Sci (Lond) 1985;69(3):251–258. doi: 10.1042/cs0690251. [DOI] [PubMed] [Google Scholar]
- 24.Gaultier C, Reinberg A, Girard E. Circadian rhythms in lung resistance and dynamic lung compliance of healthy children. Effects of two bronchodilators. Respir Physiol. 1977;31(2):169–182. doi: 10.1016/0034-5687(77)90100-1. [DOI] [PubMed] [Google Scholar]
- 25.Furlan R, Guzzetti S, Crivellaro W, et al. Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and RR variabilities in ambulant subjects. Circulation. 1990;81(2):537–547. doi: 10.1161/01.cir.81.2.537. [DOI] [PubMed] [Google Scholar]
- 26.Cazzola M, Tashkin DP. Combination of formoterol and tiotropium in the treatment of COPD: effects on lung function. COPD. 2009;6(5):404–415. doi: 10.1080/15412550903156333. [DOI] [PubMed] [Google Scholar]
- 27.van Noord JA, Buhl R, LaForce C, et al. QVA149 demonstrates superior bronchodilation compared with indacaterol or placebo in patients with chronic obstructive pulmonary disease. Thorax. 2010;65(12):1086–1091. doi: 10.1136/thx.2010.139113. [DOI] [PubMed] [Google Scholar]
- 28.GlaxoSmithKline. ClinicalTrialsgov [Internet] Bethesda, MD: National Library of Medicine (US); December 23. 2009. Safety, tolerability, pharmacokinetics and pharmacodynamics of the combination of GSK573719 and GW642444 in subjects with COPD. [last updated on May 13, 2010]. Available from: http://clinicaltrials.gov/ct2/show/NCT01039675. NLM identifier: NCT01039675. [Google Scholar]