Abstract
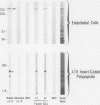
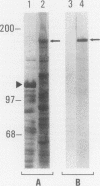
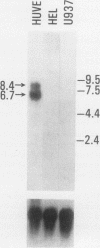
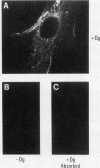
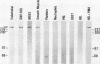
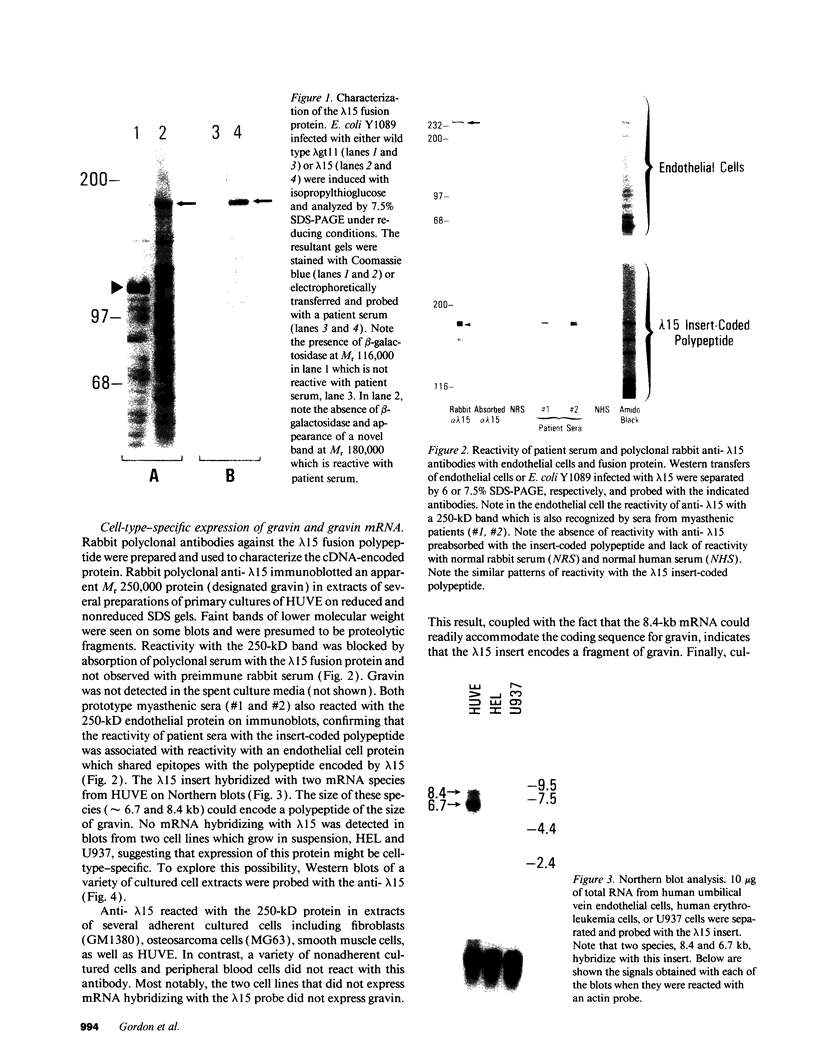
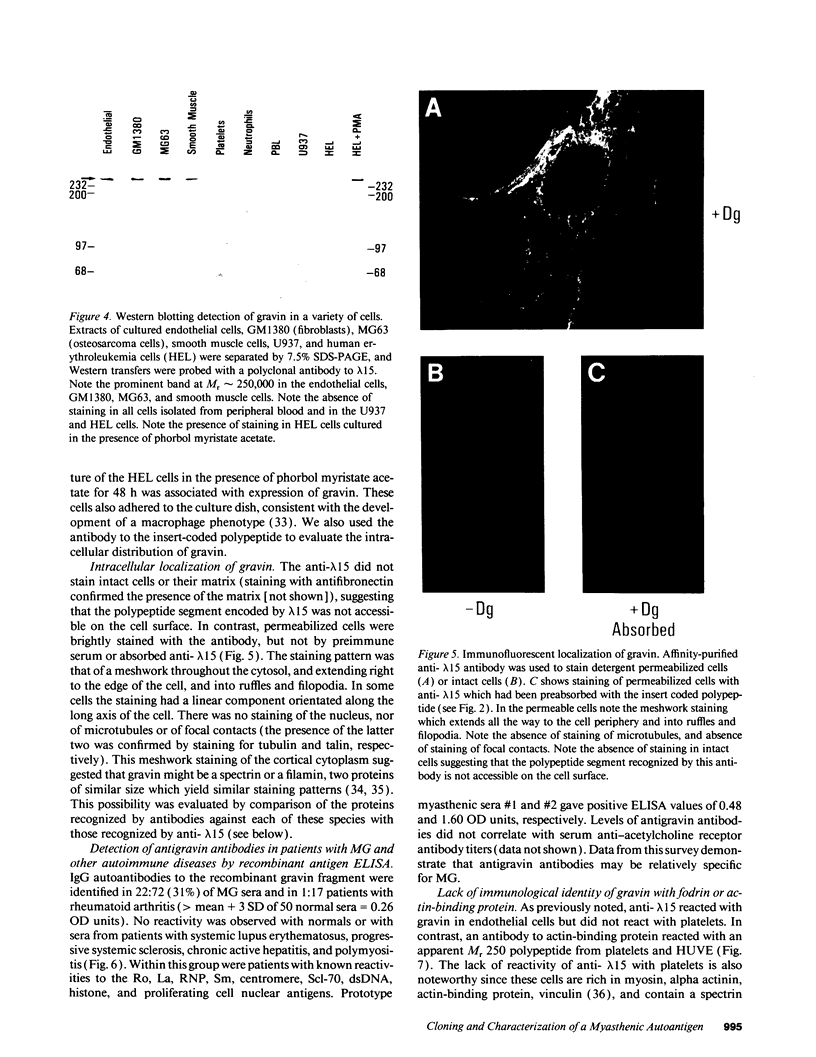
A cDNA clone was isolated by screening of a lambda gt11 endothelial expression library with serum from a patient with myasthenia gravis (MG). Rabbit antisera raised against the recombinant protein and human MG sera reactive with the clone immunoblotted an M(r) integral of 250,000 polypeptide (gravin) present in endothelial cells and several adherent cells. Gravin was not detected in platelets, leukocytes, U937, or human erythroleukemic (HEL) cell lines, but was expressed in HEL cells after induction with phorbol myristate acetate. Northern blot analysis showed two transcripts of approximately 6.7 and 8.4 kb in endothelial cells but not U937 or HEL cells. Indirect immunofluorescence of permeabilized cells revealed a trabecular network of gravin staining with a distinct linear component. Antibodies to gravin, were present in sera from 22:72 (31%) of MG patients. In contrast 0:50 normal sera and 1:72 sera from patients with other autoimmune diseases contained antigravin antibodies. Gravin is not likely to be a nonerythroid spectrin, talin, myosin, or actin-binding protein based on the lack of reactivity of antigravin with these polypeptides in immunoblots. The nucleotide sequence of the immunoreactive clone indicated that it encodes a highly acidic polypeptide fragment that contains the carboxyl terminus of the protein. Neither amino acid nor nucleotide sequences were present in Genbank, EMBL, or Swissprot databases as of March, 1992. These data indicate that gravin is an inducible, cell type-specific cytoplasmic protein and that auto-antibodies to gravin may be highly specific for MG.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Woods V. L., Jr, Tani P., Lindstrom J. M., Schmidt D., McMillan R. Autoantibodies to platelet glycoprotein IIb/IIIa and to the acetylcholine receptor in a patient with chronic idiopathic thrombocytopenic purpura and myasthenia gravis. Ann Intern Med. 1984 Jun;100(6):829–831. doi: 10.7326/0003-4819-100-6-829. [DOI] [PubMed] [Google Scholar]
- Baron M. D., Davison M. D., Jones P., Critchley D. R. The sequence of chick alpha-actinin reveals homologies to spectrin and calmodulin. J Biol Chem. 1987 Dec 25;262(36):17623–17629. [PubMed] [Google Scholar]
- Bloch R. J., Hall Z. W. Cytoskeletal components of the vertebrate neuromuscular junction: vinculin, alpha-actinin, and filamin. J Cell Biol. 1983 Jul;97(1):217–223. doi: 10.1083/jcb.97.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Cohen C. M. Platelets contain proteins immunologically related to red cell spectrin and protein 4.1. Blood. 1985 Jan;65(1):52–59. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dropcho E. J., Chen Y. T., Posner J. B., Old L. J. Cloning of a brain protein identified by autoantibodies from a patient with paraneoplastic cerebellar degeneration. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4552–4556. doi: 10.1073/pnas.84.13.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Sullivan K. F., Machlin P. S., Cooke C. A., Kaiser D. A., Pollard T. D., Rothfield N. F., Cleveland D. W. Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J Cell Biol. 1987 Apr;104(4):817–829. doi: 10.1083/jcb.104.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel E. K., Trotter J. L., McFarlin D. E., McIntosh C. L. Thymic epithelial cell contains acetylcholine receptor. Lancet. 1977 Jun 18;1(8025):1310–1311. doi: 10.1016/s0140-6736(77)91343-5. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985 Nov 29;230(4729):1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Gabbiani F., Lombardi D., Schwartz S. M. Organization of actin cytoskeleton in normal and regenerating arterial endothelial cells. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2361–2364. doi: 10.1073/pnas.80.8.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Plessmann U., Weber K. The complete amino acid sequence of the major mammalian neurofilament protein (NF-L). FEBS Lett. 1985 Mar 25;182(2):475–478. doi: 10.1016/0014-5793(85)80357-4. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Taylor L., Painter R. G. The mechanism of thrombin-induced platelet factor 4 secretion. Blood. 1980 Apr;55(4):661–668. [PubMed] [Google Scholar]
- Habets W. J., Sillekens P. T., Hoet M. H., Schalken J. A., Roebroek A. J., Leunissen J. A., van de Ven W. J., van Venrooij W. J. Analysis of a cDNA clone expressing a human autoimmune antigen: full-length sequence of the U2 small nuclear RNA-associated B" antigen. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2421–2425. doi: 10.1073/pnas.84.8.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. Secretion of granule constituents and increased oxidation of glucose. J Clin Invest. 1975 Oct;56(4):1053–1061. doi: 10.1172/JCI108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin E. G. Latent tissue plasminogen activator produced by human endothelial cells in culture: evidence for an enzyme-inhibitor complex. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6804–6808. doi: 10.1073/pnas.80.22.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J. Immunobiology of myasthenia gravis, experimental autoimmune myasthenia gravis, and Lambert-Eaton syndrome. Annu Rev Immunol. 1985;3:109–131. doi: 10.1146/annurev.iy.03.040185.000545. [DOI] [PubMed] [Google Scholar]
- Loftus J. C., Plow E. F., Frelinger A. L., 3rd, D'Souza S. E., Dixon D., Lacy J., Sorge J., Ginsberg M. H. Molecular cloning and chemical synthesis of a region of platelet glycoprotein IIb involved in adhesive function. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7114–7118. doi: 10.1073/pnas.84.20.7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran T., Beckerle M. C., Burridge K. Identification of talin as a major cytoplasmic protein implicated in platelet activation. Nature. 1985 Oct 3;317(6036):449–451. doi: 10.1038/317449a0. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Nakamoto B., Yokochi T., Chait A., Kannagi R. Human erythroleukemia cell line (HEL) undergoes a drastic macrophage-like shift with TPA. Blood. 1983 Oct;62(4):832–845. [PubMed] [Google Scholar]
- Peers J., McDonald B. L., Dawkins R. L. The reactivity of the antistriational antibodies associated with thymoma and myasthenia gravis. Clin Exp Immunol. 1977 Jan;27(1):66–73. [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Loftus J. C., Levin E. G., Fair D. S., Dixon D., Forsyth J., Ginsberg M. H. Immunologic relationship between platelet membrane glycoprotein GPIIb/IIIa and cell surface molecules expressed by a variety of cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6002–6006. doi: 10.1073/pnas.83.16.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D. Actin-binding protein evolution. 1984 Nov 29-Dec 5Nature. 312(5993):403–403. doi: 10.1038/312403a0. [DOI] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt B. M., Harris A. S., Morrow J. S., Madri J. A. Mechanisms of cytoskeletal regulation. Modulation of aortic endothelial cell spectrin by the extracellular matrix. Am J Pathol. 1984 Dec;117(3):349–354. [PMC free article] [PubMed] [Google Scholar]
- Sadler J. E., Shelton-Inloes B. B., Sorace J. M., Harlan J. M., Titani K., Davie E. W. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6394–6398. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillekens P. T., Habets W. J., Beijer R. P., van Venrooij W. J. cDNA cloning of the human U1 snRNA-associated A protein: extensive homology between U1 and U2 snRNP-specific proteins. EMBO J. 1987 Dec 1;6(12):3841–3848. doi: 10.1002/j.1460-2075.1987.tb02721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O. I., Hartwig J. H., Brotschi E. A., Stossel T. P. Distribution of actin-binding protein and myosin in macrophages during spreading and phagocytosis. J Cell Biol. 1980 Feb;84(2):215–224. doi: 10.1083/jcb.84.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolpen A. H., Guinan E. C., Fiers W., Pober J. S. Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am J Pathol. 1986 Apr;123(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- Strauss A. J., Kemp P. G., Jr Serum autoantibodies in myasthenia gravis and thymoma: selective affinity for I-bands of striated muscle as a guide to identification of antigen(s). J Immunol. 1967 Nov;99(5):945–953. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen H., Etzerodt M., Reuter R., Schneider C., Lottspeich F., Argos P., Lührmann R., Philipson L. Cloning of the human cDNA for the U1 RNA-associated 70K protein. EMBO J. 1986 Dec 1;5(12):3209–3217. doi: 10.1002/j.1460-2075.1986.tb04631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetters J. M. Immunofluorescence staining patterns in skeletal muscle using serum of myasthenic patients and normal controls. Immunology. 1965 Jul;9(1):93–95. [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Hollenberg S. M., Ong E. S., Harmon J. M., Brower S. T., Cidlowski J., Thompson E. B., Rosenfeld M. G., Evans R. M. Identification of human glucocorticoid receptor complementary DNA clones by epitope selection. Science. 1985 May 10;228(4700):740–742. doi: 10.1126/science.2581314. [DOI] [PubMed] [Google Scholar]
- White G. E., Fujiwara K. Expression and intracellular distribution of stress fibers in aortic endothelium. J Cell Biol. 1986 Jul;103(1):63–70. doi: 10.1083/jcb.103.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. L., Lennon V. A. Thymic B lymphocyte clones from patients with myasthenia gravis secrete monoclonal striational autoantibodies reacting with myosin, alpha actinin, or actin. J Exp Med. 1986 Oct 1;164(4):1043–1059. doi: 10.1084/jem.164.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A. J., Pollard T. D., Herman I. M. Actin filament stress fibers in vascular endothelial cells in vivo. Science. 1983 Feb 18;219(4586):867–869. doi: 10.1126/science.6681677. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Sato T., Sugita H. Antifilamin, antivinculin, and antitropomyosin antibodies in myasthenia gravis. Neurology. 1987 Aug;37(8):1329–1333. doi: 10.1212/wnl.37.8.1329. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]