Abstract
JAM-C is an adhesion molecule that is expressed on cells within the vascular compartment and epithelial cells and, to date, has been largely studied in the context of inflammatory events. Using immunolabeling procedures in conjunction with confocal and electron microscopy, we show here that JAM-C is also expressed in peripheral nerves and that this expression is localized to Schwann cells at junctions between adjoining myelin end loops. Sciatic nerves from JAM-C–deficient [having the JAM-C gene knocked out (KO)] mice exhibited loss of integrity of the myelin sheath and defective nerve conduction as indicated by morphological and electrophysiological studies, respectively. In addition, behavioral tests showed motor abnormalities in the KO animals. JAM-C was also expressed in human sural nerves with an expression profile similar to that seen in mice. These results demonstrate that JAM-C is a component of the autotypic junctional attachments of Schwann cells and plays an important role in maintaining the integrity and function of myelinated peripheral nerves.
JAM-C is a member of an immunoglobulin subfamily of junctional adhesion molecules, composed (as far as is known) of JAM-A, -B, -C, JAM4, ESAM, and CAR, which are specifically enriched at tight junctions of cell-cell contacts (1-3). To date, human JAM-C has been reported to be expressed on the cell surface of platelets and certain leukocyte subtypes, as well as at junctions between endothelial cells (ECs) and intestinal epithelial cells, and has largely been investigated in the context of inflammatory and vascular events (1-8). In addition, JAM-C plays an important role in establishing cell polarity and the formation of endothelial tight junctions (1-3, 5, 9).
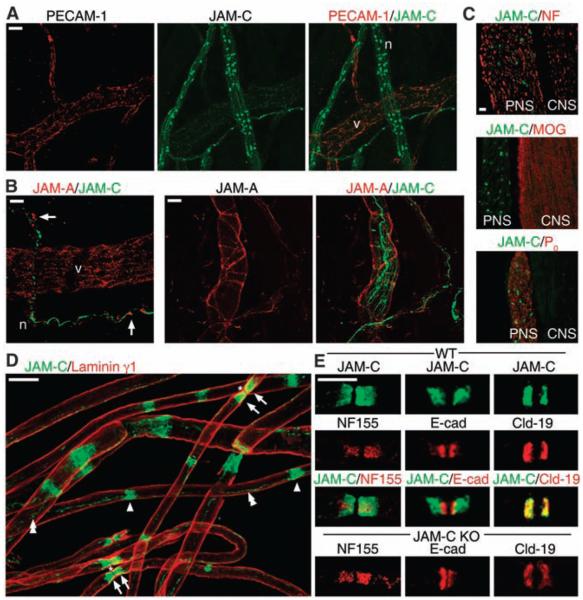
As part of our investigations into the functional role of JAM-C in leukocyte transmigration, we detected in vivo, using immunofluorescence analysis of cremaster muscles from wild-type (WT) mice, low-level expression of JAM-C in microvessels at EC junctions colocalizing with the EC marker platelet endothelial cell adhesion molecule–1 (PECAM-1) (10) (Fig. 1A). In addition, a strong and specific expression of JAM-C was detected at discrete sites within nerve bundles (Fig. 1A and fig. S1). Another member of the JAM family, JAM-A, was also found to be expressed in EC junctions and localized to junctions of perineural cells surrounding JAM-C–positive nerves (Fig. 1B). The costaining of mouse spinal cords (CNS) and its ventral roots [i.e., peripheral nervous system (PNS)] for JAM-C and neurofilament or the CNS- and PNS-specific myelin proteins, myelin oligoden-drocyte glycoprotein (MOG) or protein zero (P0), respectively, demonstrated that neural JAM-C was restricted to the PNS (Fig. 1C).
Fig. 1.
JAM-C is expressed in junctional regions of Schwann cells in peripheral nerves. (A) Confocal images of WT cremaster muscles immunostained for PECAM-1 (red) and JAM-C (green) show expression of JAM-C in nerves (n) and vascular EC junctions (v). (B) Cremaster muscles stained for JAM-A (red) and JAM-C (green) show that JAM-A localizes to EC junctions and surrounds JAM-C–positive nerves (arrows). (C) Longitudinal sections of mouse spinal cord (CNS) and its ventral roots (PNS) immunostained for JAM-C (green) and, in red, neurofilament (NF), P0 (marker for PNS), or MOG (marker for CNS) showing restriction of neural JAM-C expression to the PNS. (D) Teased sciatic nerve fibers stained for JAM-C (green) and laminin γ1 (red). JAM-C is restricted to junctional regions of Schwann cells, i.e., the paranodes (arrows) surrounding the nodes of Ranvier (asterisks), Schmidt-Lanterman incisures (single arrowheads), and mesaxonal bands (double arrowheads). (E) Teased sciatic nerve fibers stained for JAM-C (green) and, in red, neurofascin 155 (NF 155), E-cadherin (E-cad), or claudin-19 (Cld-19) in WT and JAM-C–KO mice showing normal distribution of the latter molecules in KO mice. Scale bars: (A, B, D, E) 10 μm, (C) 20 μm.
In the PNS, myelinating Schwann cells wrap around axons in such a way as to organize the axonal membrane into distinct domains known as nodes of Ranvier (11, 12), sites important for rapid saltatory conduction. To facilitate efficient conduction propagation, tight interactions exist between the axon and the glial cells at regions that flank the nodes of Ranvier (axoglial paranodal junctions) and between adjacent membrane layers of individual glial cells (12). Our observations of teased sciatic nerve fibers immunostained for JAM-C and laminin γ1 indicated that JAM-C was strongly expressed in Schwann cells, at sites characteristic of junctional regions of noncompact myelin. These sites are paranodal regions on either side of the node of Ranvier, from where mesaxonal bands, most likely the inner mesaxon, could be seen connecting the internodal Schmidt-Lanterman incisures (Fig. 1D and schematically illustrated in fig. S2A) (12-14). Analysis of JAM-C expression during development indicated localization at paranodal junctions from postnatal day P5 onward (fig. S3).
Costaining with neurofascin 155, a molecule involved in the formation of axo-glial paranodal junctions (11), revealed a broader distribution pattern of JAM-C at the paranodal regions (Fig. 1E). Furthermore, JAM-C was more distally located from the node than E-cadherin, a marker of adherens junctions (15), but colocalized with the tight junctional molecule claudin-19 (16) (Fig. 1E). None of the molecules analyzed were mislocalized in JAM-C–knockout (KO) mice [(Fig. 1E and fig. S2B) for the gap junction component connexin 32 (14) and for myelin-associated glycoprotein (MAG) and E-cadherin at incisures].
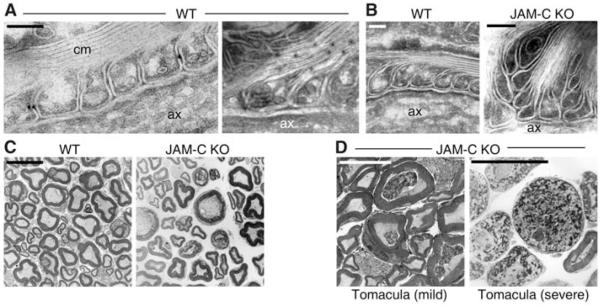
The node of Ranvier is organized on either side by two Schwann cells, whose cytoplasm increases at paranodal regions (noncompact myelin) to form terminal loops that closely interact with the axon (at paranodal junctions) and the lateral myelin lamellae (fig. S4A). Immunogold staining of longitudinal sections of WT sciatic nerve fibers showed that JAM-C was located at the lateral sides of adjacent myelin lamellae of terminal paranodal loops. However, JAM-C was not expressed in the axon or regions of compact myelin and could not be detected at axo-glial paranodal junctions or tight junctional domains (Fig. 2A and fig. S4, A to C). It is interesting that the findings of these studies indicated expression of JAM-C along the whole length of paranodal terminal loops, a distribution pattern that has not been reported for other tight junctional markers, which suggests that colocalization between JAM-C and claudin-19 is only partial, (see Fig. 1E). Expression of JAM-C that is not at the tight junction has also been reported in other cell types (3), which implies that the characteristics of junctional localization of JAM-C may be cell-specific. The ligand with which JAM-C is interacting in this scenario is at present unknown, but it is highly probable that the interaction is mediated by JAM-C/JAM-C autotypic adhesion between adjacent terminal loops, because expression of JAM-B, its major binding partner in endothelial cells, was not detected.
Fig. 2.
JAM-C deficiency alters the integrity of the myelin sheath. (A) Immunoelectron microscopy of longitudinal sections of WT sciatic nerves revealed JAM-C expression at lateral sides of adjacent myelin lamellae in noncompact, paranodal myelin, but not in the axon (ax) or compact myelin (cm). (B) Ultrastructural analysis of longitudinal sections of sciatic nerves from WT and JAM-C–KO mice (16 weeks old) prepared for immunoelectron microscopy (without antibodies to JAM-C) indicated that a proportion of KO terminal loops exhibited an apparent looser adhesion to adjacent loops and axon. (C and D) Ultrastructural analysis of transverse sections of sciatic nerves from WT and JAM-C–KO mice (16 weeks old) revealed abnormalities in the myelin sheath of a proportion of KO fibers (~7%) exhibiting layers of loose myelin that form mild and severe tomacula. Scale bars: (A) 190 nm, (B) 120 nm, and (C and D) 10 μm.
The above immunoelectron studies also identified an important, though mild, morphological defect in JAM-C–KO sciatic nerve fibers. Perfusion-fixed samples indicated a profile of sequential terminal myelin loops that were misaligned, suggesting a defective adhesive contact between these structures in fibers of similar caliber [(Fig. 2B and fig. S4D); 95.8 and 78.9% of the terminal paranodal loops closely interacted with the axon, in WT and JAM-C–KO mice, respectively; for four to six mice with 168 and 279 terminal loops from 15 to 23 nodes of Ranvier counted for WT and KO, respectively]. These findings suggest an important role for JAM-C in maintaining the organization of the paranodal terminal loops, but the relatively low incidence of the noted defect under conditions of JAM-C deletion suggests the existence of compensatory mechanisms in the KO mice, such as enhanced expression and/or function of other adhesion pathways. Furthermore, ultrastructural analysis of transverse sections from sciatic nerves revealed that, whereas most KO fibers showed a normal phenotype (Fig. 2C), a proportion exhibited accumulated layers of loose myelin within the periaxonal space forming tomacula [1.2 ± 0.8 and 6.9 ± 2.1% tomacula formation in WT and KO mice, respectively, P < 0.05, for seven to nine mice with 1571 WT and 4227 KO fibers counted (Fig. 2D)]. This thickening of the myelin sheath is a characteristic feature of nerve fibers from patients with hereditary neuropathy with liability to pressure palsies (HNPP). Together, these observations suggest that the abnormal myelin phenotypes detected in JAM-C–deficient mice are caused by the loss of JAM-C–mediated autotypic interactions between myelin lamellae at the paranodal regions, as well as potentially at Schmidt-Lanterman incisures. Of note, whereas JAM-C–KO mice can exhibit a number of defects such as mega-esophagus or high susceptibility to lung infections (often fatal), and some of them are smaller in size during early phases of their life span (<3 to 8 weeks) (7), the young adult mice chosen for the studies detailed here (ages 8 to 16 weeks) were of normal health status and were matched by weight with the controls.
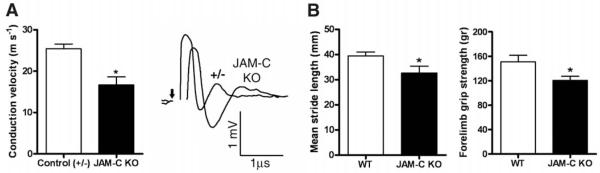
Because defective JAM-C–mediated adhesion may directly or indirectly lead to reduced paranodal sealing, we assessed whether JAM-C–KO mice exhibited neurological defects. KO sciatic nerves displayed reduced conduction velocity (Fig. 3A) with a lag and lower amplitudes in their compound action potentials (CAPs) compared with control nerves. As the clustering of voltagegated sodium channels at the node of Ranvier was not affected by JAM-C deficiency (fig. S2B), these results suggest that the reduced conduction velocity of at least a subpopulation of myelinated fibers was a functional consequence of the abnormal neuronal morphology. Furthermore, JAM-C–KO mice exhibited significantly shorter stride length than littermate controls, as well as reduced forepaw grip strength, indicating muscle weakness (Fig. 3B). These findings imply that JAM-C deficiency not only leads to reduced paranodal sealing but may also contribute to axonal loss.
Fig. 3.
JAM-C–deficient mice exhibit electrophysiological and behavioral defects. (A) Isolated sciatic nerves from control littermates (+/−) and JAM-C–KO mice revealed significantly reduced conduction velocities in the KO (*P < 0.05, n = four to eight nerves) and a lag in their CAPs with reduced amplitudes compared with control nerves. (B) JAM-C–KO mice exhibited significantly reduced stride length (*P < 0.05, n = 13 to 18 mice) and grip strength (*P < 0.05, n = 8 to 9 mice). Experiments were performed by a blinded examiner.
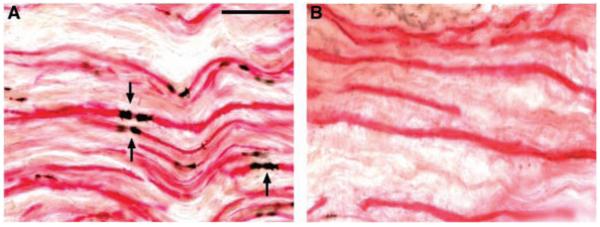
In a final series of experiments, we investigated the expression of JAM-C in human peripheral nerves. In all sural nerve sections from healthy nerves, JAM-C was clearly localized to paranodal regions (Fig. 4A; from six healthy nerves obtained for grafting during surgery). Sural nerve biopsies obtained from patients with clinical and histopathological diagnosis of chronic inflammatory demyelinating polyneuropathy were also investigated for JAM-C expression and were found to exhibit a significantly reduced number of JAM-C–positive paranodal regions, in accord with the demyelinated state of the nerves [(Fig. 4B and fig. S5C); n = 5]. These patients exhibited progressive motor and sensory distal limb neuropathy of moderate severity and slowed nerve conduction velocities. Reduced numbers of JAM-C–positive paranodal regions were also found in regenerating fibers of post-traumatic neuromas from patients with upper limb nerve injuries (fig. S5). Although the present findings indicate that reduced JAM-C expression is in accord with demyelination, additional studies are needed in acquired and inherited demyelinating neuropathies to investigate the possible association of JAM-C expression with causation or progression of neuropathies. Furthermore, whereas at present there are no known neuropathic conditions associated with JAM-C mutations, the role of JAM-C in vascular biology strongly suggests that future studies in this line should focus on neuropathies associated with vasculopathy. Peripheral neuropathies and vasculopathy are observed in a number of acquired clinical conditions, including diabetes, systemic lupus erythematosus (SLE), Behçet’s disease, and Sjögren’s syndrome, although the underlying pathological mechanisms are complex in these conditions.
Fig. 4.
JAM-C is expressed in human peripheral nerves. Double-staining of human sural nerves for JAM-C (black) and neurofilament (red) obtained from a healthy limb (A) and a patient with a clinical and histopathological diagnosis of chronic inflammatory demyelinating polyneuropathy (B). JAM-C is expressed at paranodal regions (arrows) in healthy nerves, and the number of JAM-C–positive paranodes was significantly reduced in nerves from patients in accordance with the demyelinated state of the fibers (P < 0.01, for six healthy subjects and five patients with neuropathy; the images shown are representative of all samples analyzed). Scale bar, 50 μm.
Among the molecules at autotypic junctions within Schwann cells studied in detail, e.g., E-cadherin, claudin 19, and connexin 32, the phenotypes of mutant models can be varied (16-18). In this study, we show that deletion of JAM-C causes a phenotype resembling a peripheral neuropathy with liability to pressure palsies. Collectively, these findings describe a previously unknown role for JAM-C and provide a strong platform for further investigations into the role of JAM-C in myelin sheath integrity and function under both physiological and pathological conditions.
Supplementary Material
Acknowledgments
We thank J. Greenwood and P. Brophy for critical comments; P. Hanchoz for expert technical assistance; L. Sorokin, D. R. Colman, S. J. Piddlesden, and E. Dejana for valuable reagents; and I. Horresh for technical advice. This work was funded by the British Heart Foundation (grant PG/03/123/16102 to S.N., in the form of a Ph.D. studentship for C.S.); the Wellcome Trust, UK (grant 064920 to S.N.); the Swiss National Science Foundation (grant 31-109402 to P.M.); the Juvenile Diabetes Research Foundation International (grants 1-2005-46 and 1-2007-158 to P.M.); and the Swiss National Science Foundation (grants 310000-112551/I and 3100AO-100697 to M.A.-L. and B.A.I., respectively).
Footnotes
www.sciencemag.org/cgi/content/full/318/5855/1472/DC1 Materials and Methods, Figs S1 to S5, References
References and Notes
- 1.Mandell KJ, Parkos CA. Adv. Drug Deliv. Rev. 2005;57:857. doi: 10.1016/j.addr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Ebnet K, Suzuki A, Ohno S, Vestweber D. J. Cell Sci. 2004;117:19. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 3.Bradfield PF, Nourshargh S, Aurrand-Lions M, Imhof BA. Arterioscler. Thromb. Vasc. Biol. 2007;27:2104. doi: 10.1161/ATVBAHA.107.147694. [DOI] [PubMed] [Google Scholar]
- 4.Arrate MP, Rodriguez JM, Tran TM, Brock TA, Cunningham SA. J. Biol. Chem. 2001;276:45826. doi: 10.1074/jbc.M105972200. [DOI] [PubMed] [Google Scholar]
- 5.Aurrand-Lions M, Duncan L, Ballestrem C, Imhof BA. J. Biol. Chem. 2001;276:2733. doi: 10.1074/jbc.M005458200. [DOI] [PubMed] [Google Scholar]
- 6.Aurrand-Lions M, et al. J. Immunol. 2005;174:6406. doi: 10.4049/jimmunol.174.10.6406. [DOI] [PubMed] [Google Scholar]
- 7.Imhof BA, et al. J. Pathol. 2007;212:198. doi: 10.1002/path.2163. [DOI] [PubMed] [Google Scholar]
- 8.Santoso S, et al. J. Exp. Med. 2002;196:679. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Nature. 2004;431:320. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- 10.Materials and methods are available as supporting material on Science Online.
- 11.Sherman DL, Brophy PJ. Nat. Rev. Neurosci. 2005;6:683. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 12.Poliak S, Peles E. Nat. Rev. Neurosci. 2003;4:968. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- 13.Salzer JL. Neuron. 2003;40:297. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- 14.Scherer SS, Arroyo EJ. J. Peripher. Nerv. Syst. 2002;7:1. doi: 10.1046/j.1529-8027.2002.02001.x. [DOI] [PubMed] [Google Scholar]
- 15.Fannon AM, et al. J. Cell Biol. 1995;129:189. doi: 10.1083/jcb.129.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto T, et al. J. Cell Biol. 2005;169:527. doi: 10.1083/jcb.200501154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young P, et al. Mol. Cell. Neurosci. 2002;21:341. doi: 10.1006/mcne.2002.1177. [DOI] [PubMed] [Google Scholar]
- 18.Scherer SS, et al. Glia. 1998;24:8. doi: 10.1002/(sici)1098-1136(199809)24:1<8::aid-glia2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.