Abstract
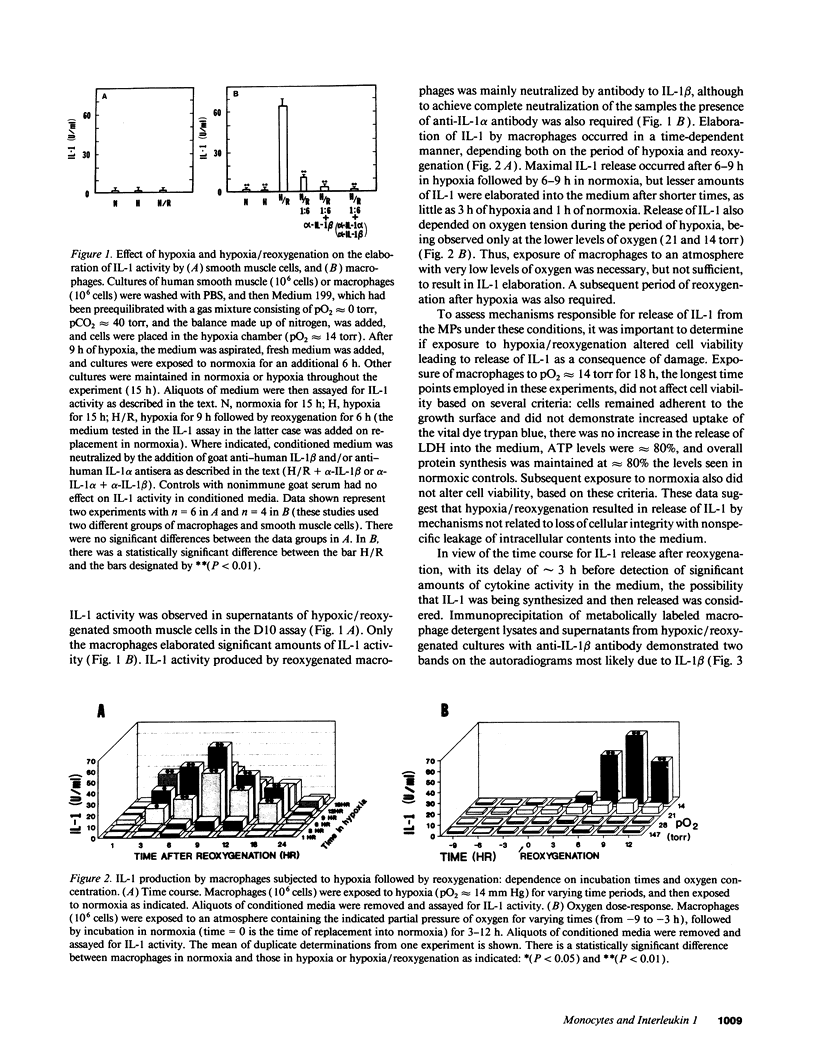
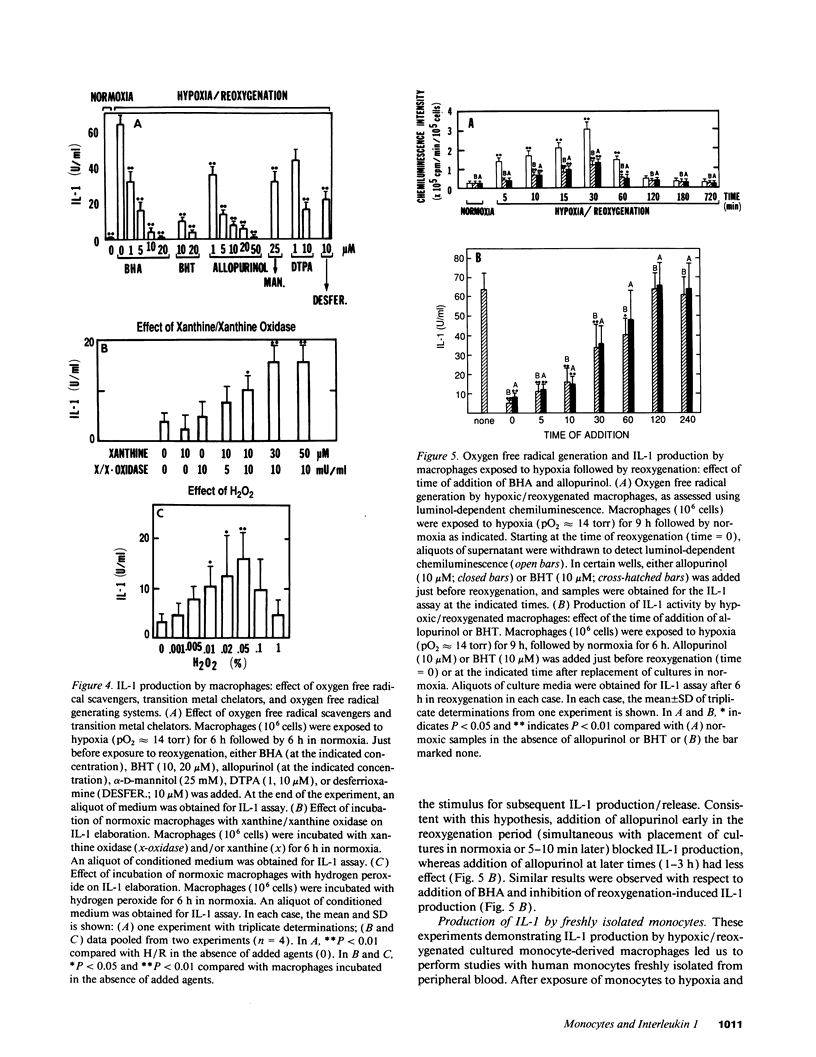
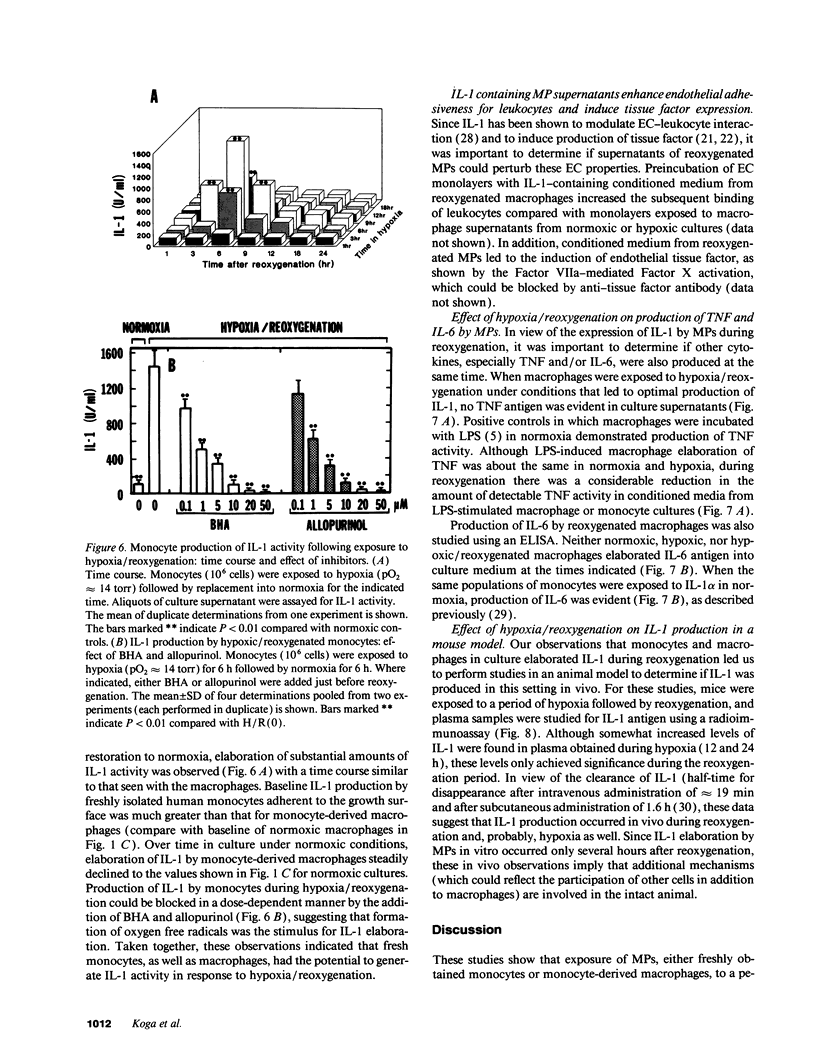
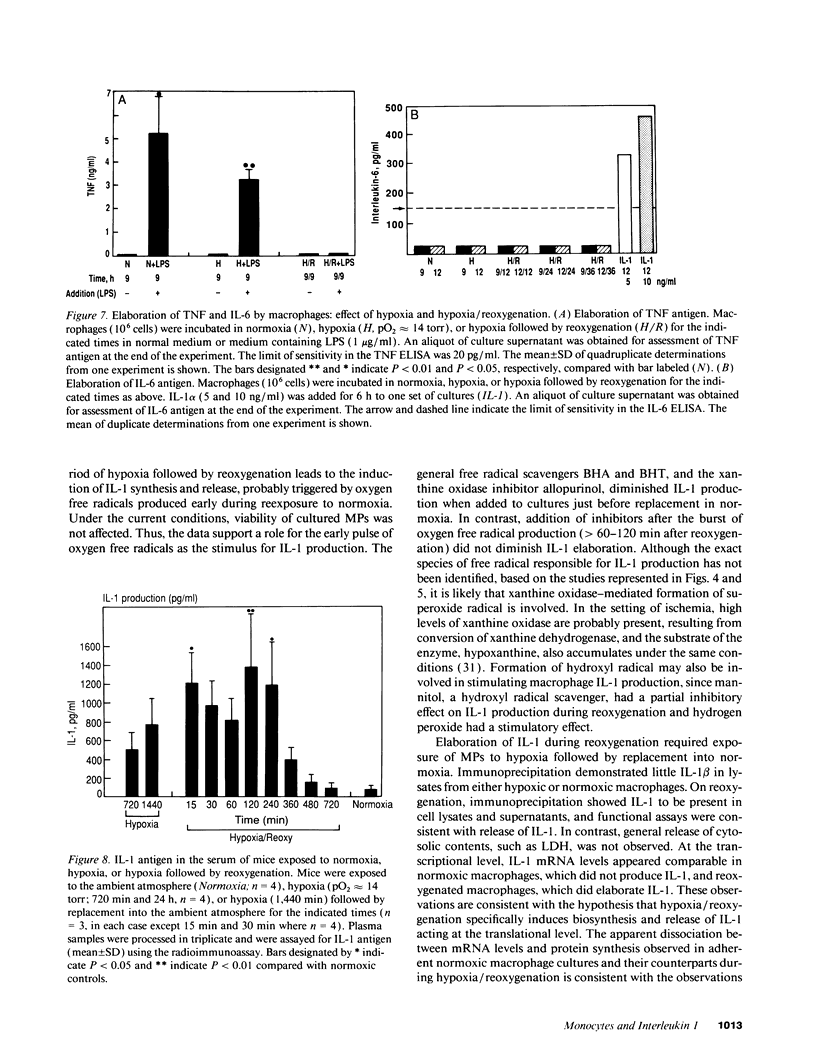
To examine the possible involvement of cytokines in reperfusion injury, we have studied production of IL-1 by human vascular cells, including smooth muscle and mononuclear phagocytes. Exposure of cells to hypoxia (pO2 approximately 14 torr) followed by reoxygenation led to significant release of IL-1 only from the mononuclear phagocytes. Elaboration of IL-1 was dependent on the oxygen tension and duration of hypoxia (optimal at lower pO2s, approximately 14-20 torr, and after 9 h), as well as the time in reoxygenation (maximal IL-1 release at 6-9 h). Although a period of hypoxia was necessary for subsequent IL-1 production during reoxygenation of either peripheral blood monocytes or cultured monocyte-derived macrophages, no IL-1 release occurred during the hypoxic exposure. IL-1 released during reoxygenation was newly synthesized, and its production was triggered by the generation of oxygen free radicals, as it could be blocked by the addition of either allopurinol or free radical scavengers to cultures and could be stimulated in part by low concentrations of hydrogen peroxide or xanthine/xanthine oxidase. The potential pathophysiological effects of IL-1-containing supernatants from reoxygenated macrophages was shown by their induction of endothelial tissue factor and enhancement of endothelial adhesiveness for neutrophils, both of which could be blocked by anti-IL-1 antibody. The relevance of IL-1 to hypoxia/reoxygenation in vivo was suggested by the presence of circulating nanogram amounts of this cytokine in the plasma of mice during the reoxygenation period following a hypoxia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beuscher H. U., Günther C., Röllinghoff M. IL-1 beta is secreted by activated murine macrophages as biologically inactive precursor. J Immunol. 1990 Mar 15;144(6):2179–2183. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984 Aug 1;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Colletti L. M., Remick D. G., Burtch G. D., Kunkel S. L., Strieter R. M., Campbell D. A., Jr Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990 Jun;85(6):1936–1943. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci W. S., Brock T. A., Gimbrone M. A., Jr, Alexander R. W. Nonlinear relationship between alpha 1-adrenergic receptor occupancy and norepinephrine-stimulated calcium flux in cultured vascular smooth muscle cells. Mol Pharmacol. 1985 May;27(5):517–524. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Flaherty J. T., Weisfeldt M. L. Reperfusion injury. Free Radic Biol Med. 1988;5(5-6):409–419. doi: 10.1016/0891-5849(88)90115-3. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Gordon D., Lu P. L. A smooth muscle-specific monoclonal antibody recognizes smooth muscle actin isozymes. J Cell Biol. 1985 Mar;100(3):807–813. doi: 10.1083/jcb.100.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Gunther S., Alexander R. W., Atkinson W. J., Gimbrone M. A., Jr Functional angiotensin II receptors in cultured vascular smooth muscle cells. J Cell Biol. 1982 Feb;92(2):289–298. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins S. J., Humphreys M. Simple, sensitive and specific bioassay of interleukin-1. J Immunol Methods. 1989 Jun 21;120(2):271–276. doi: 10.1016/0022-1759(89)90252-4. [DOI] [PubMed] [Google Scholar]
- Kudo S., Mizuno K., Hirai Y., Shimizu T. Clearance and tissue distribution of recombinant human interleukin 1 beta in rats. Cancer Res. 1990 Sep 15;50(18):5751–5755. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loike J. D., Kozler V. F., Silverstein S. C. Increased ATP and creatine phosphate turnover in phagocytosing mouse peritoneal macrophages. J Biol Chem. 1979 Oct 10;254(19):9558–9564. [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Tucker A. M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J Cell Biol. 1988 Apr;106(4):1375–1384. doi: 10.1083/jcb.106.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury C. P., Teppo A. M. Circulating tumour necrosis factor-alpha (cachectin) in myocardial infarction. J Intern Med. 1989 May;225(5):333–336. doi: 10.1111/j.1365-2796.1989.tb00090.x. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Moorhouse P. C., Grootveld M., Halliwell B., Quinlan J. G., Gutteridge J. M. Allopurinol and oxypurinol are hydroxyl radical scavengers. FEBS Lett. 1987 Mar 9;213(1):23–28. doi: 10.1016/0014-5793(87)81458-8. [DOI] [PubMed] [Google Scholar]
- Narayanan R., Tare N. S., Benjamin W. R., Gubler U. A sensitive technique to monitor gene transfer and expression in bone marrow stem cells. Exp Hematol. 1989 Aug;17(7):832–835. [PubMed] [Google Scholar]
- Nawroth P. P., Handley D. A., Esmon C. T., Stern D. M. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986 May;83(10):3460–3464. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Gerlach H., Esposito C., Pasagian-Macaulay A., Brett J., Stern D. Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties. J Clin Invest. 1990 Apr;85(4):1090–1098. doi: 10.1172/JCI114540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987 May;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichle H., Langner D., Wendt P., Blümel G. Endotoxin-induced generation of oxygen free radicals in freshly drawn human blood. Prog Clin Biol Res. 1987;236A:419–426. [PubMed] [Google Scholar]
- Repine J. E., Cheronis J. C., Rodell T. C., Linas S. L., Patt A. Pulmonary oxygen toxicity and ischemia-reperfusion injury. A mechanism in common involving xanthine oxidase and neutrophils. Am Rev Respir Dis. 1987 Aug;136(2):483–485. doi: 10.1164/ajrccm/136.2.483. [DOI] [PubMed] [Google Scholar]
- Rubartelli A., Cozzolino F., Talio M., Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990 May;9(5):1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler R., Clark B. D., Dinarello C. A. Dissociation between interleukin-1 beta mRNA and protein synthesis in human peripheral blood mononuclear cells. J Biol Chem. 1990 Jun 25;265(18):10232–10237. [PubMed] [Google Scholar]
- Schindler R., Gelfand J. A., Dinarello C. A. Recombinant C5a stimulates transcription rather than translation of interleukin-1 (IL-1) and tumor necrosis factor: translational signal provided by lipopolysaccharide or IL-1 itself. Blood. 1990 Oct 15;76(8):1631–1638. [PubMed] [Google Scholar]
- Sherry B., Cerami A. Cachectin/tumor necrosis factor exerts endocrine, paracrine, and autocrine control of inflammatory responses. J Cell Biol. 1988 Oct;107(4):1269–1277. doi: 10.1083/jcb.107.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. M., Bank I., Nawroth P. P., Cassimeris J., Kisiel W., Fenton J. W., 2nd, Dinarello C., Chess L., Jaffe E. A. Self-regulation of procoagulant events on the endothelial cell surface. J Exp Med. 1985 Oct 1;162(4):1223–1235. doi: 10.1084/jem.162.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttles J., Carruth L. M., Mizel S. B. Detection of IL-1 alpha and IL-1 beta in the supernatants of paraformaldehyde-treated human monocytes. Evidence against a membrane form of IL-1. J Immunol. 1990 Jan 1;144(1):170–174. [PubMed] [Google Scholar]
- TOTTER J. R., DE DUGROS E. C., RIVEIRO C. The use of chemiluminescent compounds as possible indicators of radical production during xanthine oxidase action. J Biol Chem. 1960 Jun;235:1839–1842. [PubMed] [Google Scholar]
- Tosato G., Jones K. D. Interleukin-1 induces interleukin-6 production in peripheral blood monocytes. Blood. 1990 Mar 15;75(6):1305–1310. [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Weitz J. I., Huang A. J., Levin S. M., Silverstein S. C., Loike J. D. Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7734–7738. doi: 10.1073/pnas.85.20.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Crowley J. J., Chan J. C., Raffin T. A. Attenuation of LPS-induced neutrophil thromboxane b2 release and chemiluminescence. J Cell Physiol. 1991 Feb;146(2):264–269. doi: 10.1002/jcp.1041460211. [DOI] [PubMed] [Google Scholar]




