Abstract
Object
Emerging research in evoked broadband electrocorticographic (ECoG) measurement from the cortical surface suggests that it might cleanly delineate the functional organization of cortex. The authors sought to demonstrate whether this could be done in a same-session, online manner to identify receptive and expressive language areas.
Methods
The authors assessed the efficacy of simple integration of “χ-band” (76–200 Hz) change in the ECoG signal by implementing a simple band-pass filter to estimate broadband spectral change. Following a brief (less than 10-second) period to characterize baseline activity, χ-band activity was integrated while 7 epileptic patients with implanted ECoG electrodes performed a verb-generation task.
Results
While the patients were performing verb-generation or noun-reading tasks, cortical activation was consistently identified in primary mouth motor area, superior temporal gyrus, and Broca and Wernicke association areas. Maps were robust after a mean time of 47 seconds (using an “activation overlap” measure). Correlation with electrocortical stimulation was not complete and was stronger for noun reading than verb generation.
Conclusions
Broadband ECoG changes can be captured online to identify eloquent cortex. This demonstrates the existence of a powerful new tool for functional mapping in the operative and chronic implant setting.
Keywords: electrocorticography, language, mapping, online mapping, electrocortical stimulation
Electrocorticography has been rapidly evolving as a powerful experimental tool for elucidation of brain function in a wide variety of settings. In particular, recent electrocorticographic and local field potential measurements have revealed the link between neuronal population activity and broadband spectral change in the brain surface electric potential.17,23 This broadband change has been studied primarily by examination of power in higher frequencies, where underlying brain rhythms do not obscure behaviorally modulated broadband changes,3–5,8,13,21,24,34 and more recently by decomposition of spectral change to remove the rhythms.20,25 This understanding that average neuronal population activity can be captured by using the broadband spectral change at frequencies above the influence of the rhythms—the “χ-band”22 (Fig. 1)—means that local cortical function may be captured by simple integration of this broadband for mapping the brain online. This was done for the first time in motor cortex during simple hand movement,18 and the same is done in this article to identify cortical language areas with ECoG (Figs. 2 and 3). Maps could be obtained in only a few minutes, based on as little as 15 seconds of movement.
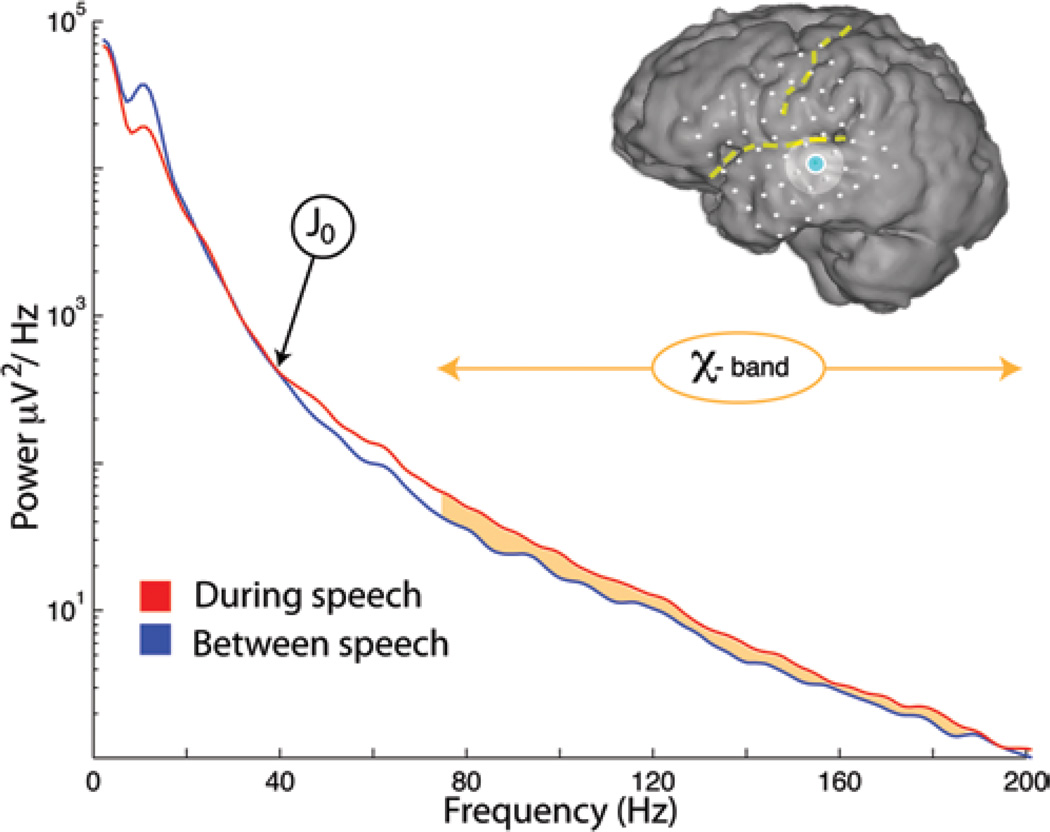
Fig. 1.
Capturing the frequency power spectrum of the electrical potential during speech from an auditory electrode in Patient 1. (Superior temporal gyrus, Brodmann area 42, Talairach coordinates −70, −15, 7 mm, shown in blue on inset brain.) During periods when the patient was resting between speech periods, there was a characteristic 1/f falloff in power across all frequencies, with a superimposed “rhythmic” peak in the power spectrum at approximately 15 Hz (the region of the θ/α/β rhythms). During periods when the patient was speaking, there was a decrease in power in the rhythm and a broad increase in power of the 1/f phenomenon across all frequencies. Filtering the signal above the intersection between the speech and rest spectra (the “primary junction”—J022) captures this broadband phenomenon, the “χ-band” (orange), which has been shown to correlate with local processing in the brain.
Fig. 2.
The verb-generation task. Patients were visually presented with printed noun cues on a screen at the bedside. In response to each cue (times shown by gray bars on the audio trace), they produced verbs related to each noun. While they performed the task, audio (middle black trace) was recorded in addition to the differentially referenced cortical surface electric potential (jagged blue trace on lower right). The χ-band was extracted from the raw potential. The example shown (middle blue trace) shows activity in Patient 1 from an electrode in the Broca area (Brodmann area 45, inferior frontal gyrus, Talairach location −60, 32, 12 mm), over time in seconds (from right to left). The correlation between the χ-band activity and the onset of speech is clear. Interestingly, clinical stimulation at this site did not interrupt simple object naming.
Fig. 3.
The relationship between function and anatomy. A and B: The anatomy in Patient 2 is shown in A, with the non-shaded region representing the area of the exposed craniotomy shown in B and the inset. Yellow lines indicate the Sylvian fissure and the central sulcus. The blue dot is an electrode site in the Broca area (Brodmann area 44, Talairach location −58, 9, 10 mm). The orange dot is an electrode site on the superior temporal gyrus (Brodmann area 22, location −64, −14, 4 mm). The white dots are the locations of the remainder of the electrode sites. The locations of the electrodes on the exposed brain surface (B) were determined from photographs with the electrode array in situ (inset). C: Traces showing the χ-band activity at the blue site (blue trace) and the orange site (orange trace). Simultaneous audio recording is also shown. Note the relationship between activity at the blue, speech production, site (speech onset) and orange, auditory, site (following speech onset). Interestingly, clinical stimulation did not interrupt object naming at either of these sites.
In 2001 and 2005, Crone et al.5 and Sinai et al.34 demonstrated that a distributed network of language areas was revealed in high-frequency ECoG during language production. Canolty and colleagues4 then demonstrated that these areas are sequentially activated and reflect different aspects of receptive, expressive, and auditory processing. In their ECoG study explicitly comparing picture naming with verb generation,30 Edwards and colleagues7 found that, although the semantic activations exhibited a high degree of overlap between the two, the strength of activation during picture naming was less intense and less widespread than during verb generation. In this article, we examine the rapidly evoked cortical activity in the χ-band during verb generation for rapid online mapping of language function. By quantifying the stability of the evoked maps, we are able to demonstrate that robust, reliable mapping can be performed in less than a minute. This suggests that rapid online electrocorticographic mapping can be used as a convenient adjuvant tool for functional localization in the operative suite.
Methods
Patients
Seven patients with intractable epilepsy (Table 1) underwent craniotomy for temporary placement of subdural electrode arrays to localize seizure foci, prior to resection (Fig. 3). These electrode arrays were typically placed for 5–7 days, with the duration and location of placement determined solely by clinical indication. Patients provided informed consent through a protocol approved by the University of Washington institutional review board.
TABLE 1.
Patient demographic and clinical characteristics*
| Patient No. |
Age (yrs) | Grid Location | Seizure Focus |
|---|---|---|---|
| 1 | 42, M | lt frontotemporal | lt temporal |
| 2 | 18, F | lt frontotemporal | lt frontal |
| 3 | 31, M | lt frontotemporal-parietal | lt temporal |
| 4 | 18, F | lt frontal | lt frontal |
| 5 | 34, F | lt frontal | lt frontal |
| 6 | 27, F | lt frontotemporal-parietal | lt frontal |
| 7 | 21, M | lt frontotemporal | lt temporal |
All 7 patients were right handed.
Tasks
Offline
Patients participated in a simple verb-generation task, where nouns (approximately 2.5 cm high, and 8–12 cm wide) were presented on a screen approximately 1 m from the patient, at the bedside. The patient’s task was to speak a verb that was connected to the noun: for example, if the cue read “ball”, the patient might say “kick”, or if the cue read “bee”, the patient might say “fly” (Fig. 2). In between each 1.6-second cue was a blank-screen 1.6-second interstimulus interval. Patients 3–6 also performed a control task, where they read each noun as it appeared on the screen, rather than producing an associated verb.
Online
Patient 3 performed the same verb-generation task except reading from a list of words held in his left hand, instead of reading a list of words from the computer screen (Fig. 4). The signal processing, as described below, was computed online using a custom-made BCI2000 module.
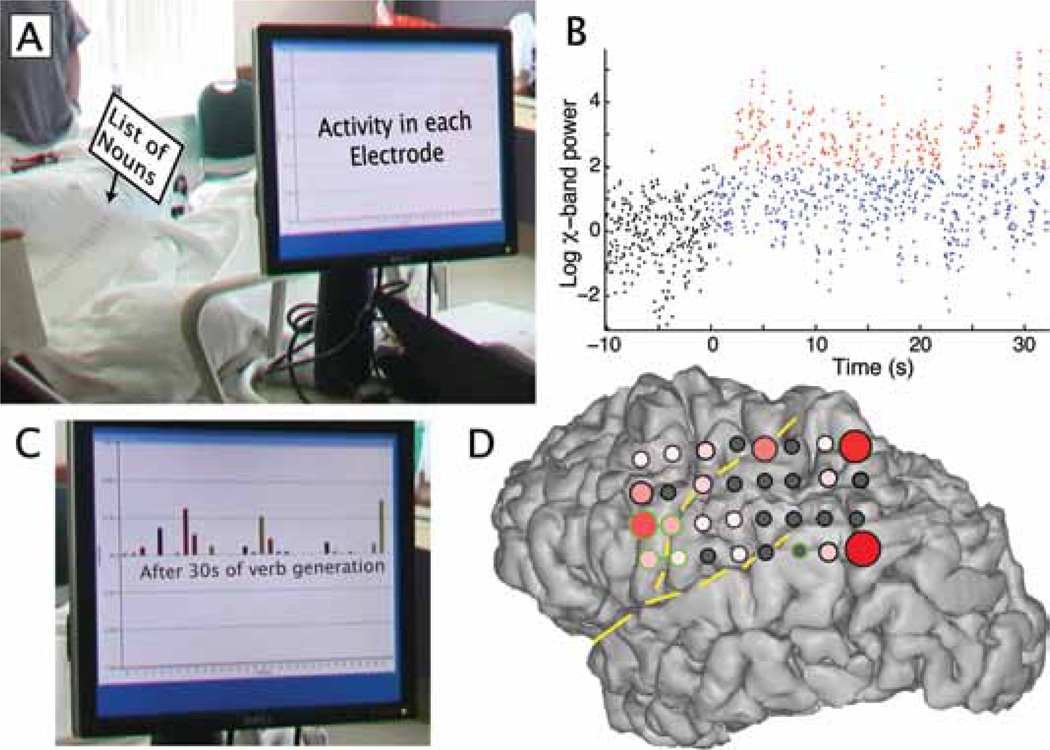
Fig. 4.
Demonstration of online mapping at the bedside—Patient 3. The patient held a list of nouns in his left hand, reading associated verbs at his own pace (A). The χ-band activity in each ECoG electrode is integrated over time (B). This is done by first normalizing the log power between 76 and 200 Hz in z-score units of a short preactivity baseline (samples in black). During the task, data above 2 z-score units (red) is integrated over time. The integrated activity at each electrode can be appreciated online (C). The interpolated activity is plotted on a rendered brain (D), with green circles and bars denoting ECS pair sites that interrupted response in a picture-naming task. (The posterior single site involved an electrode that was not measured with ECoG online.)
Recordings
Platinum electrode arrays (Ad-Tech) were placed as 8 × 8 electrode arrays in each patient. Each electrode array was embedded in a sheet of Silastic, with a 1-cm interelectrode distance (each electrode had a 2.3-mm-diameter surface exposed to the brain surface) (Fig. 3). The electrocorticographic signal was split, with one set of leads fed into a clinical electroencephalography system and the other amplified at the bedside with Synamps2 (Neuroscan) biosignal amplifiers. Stimuli were presented and electrocorticographic signals were acquired from the Neuroscan system using the general-purpose BCI2000 software environment.33 The sampling frequency was 1000 Hz, and the hardware band-passed the signal between 0.3 and 200 Hz. Audio was recorded using a Logitech USB desktop microphone at 11 kHz, and synchronized with a trigger in the BCI2000 program.
Signal Processing
The “online” technique employed was as follows: Ten seconds of baseline data were collected prior to movement cue, noun, or object presentation. Data were band-passed using a Butterworth filter for the χ-band (76–200 Hz)18 with notch filters at 120 and 180 Hz to remove ambient noise.
For the traces shown in Figs. 2 and 3, the log of this band-passed signal was taken, put in z-score units of an 8-second pretask baseline, smoothed, and re-exponentiated to obtain the traces shown.
Log χ-band power was calculated for the duration of the task in 80-msec windows of data (each window overlapping by 40 msec). The data were then put in z-score units from an 8-second pretask baseline (subtracting the mean log χ-band power from the baseline period and then dividing by the standard deviation of the log χ-band power from the baseline period.) This puts the data in natural units of significance because the filtered χ-band power has a log-normal distribution.
To calculate the brain activity associated with the verb-generation task, we defined the “accumulation time,” τa. The cumulative χ-band activity as a function of τa was calculated as follows: at each point in time, in each electrode, if the normalized log χ-band power was above 2 (that is, 2 SDs above the mean of the baseline), then the activity was increased by 1. Because it is expected to be above 2 SDs 0.023% of the time, 0.023 was subtracted from the cumulative activity in each channel at each point in time. These activations quickly converge to a stable map, as shown in Fig. 4. A custom signal-processing module was created for BCI2000 to analyze the raw potential and present accumulated activity online at the bedside (for example, Fig. 5C). For offline analysis, MATLAB (The MathWorks, Inc.) was used.
Fig. 5.
Accumulation of χ-band activity to obtain a stable activation map. A: Cumulative activity integrated over time in Patient 1 during the verb-generation task. The distribution quickly converges on a stable pattern. The activation meets stability criteria at “accumulation time” τa = 33 seconds. Most of the gray regions of complete inactivity correspond to sites over a previous resection cavity. Electrodes were numbered as follows: 1, top right; 8, top left; 48, bottom right. B: Distribution of activity plotted on the brain surface at 33, 66, and 99 seconds. The activity is represented by the diameter and degree of red at each site. Note that the pattern is very strongly conserved after τa reaches the stability criterion. C: Graph showing the accumulation overlap (AO, a synthesis of the spatial correlation in the representation 3 seconds behind and 9 seconds ahead of each time point); the accumulation time (τa, in seconds) is deemed to have reached the stability criterion when the AO reaches 0.99 (denoted by circle).
We calculated τa necessary to obtain a stable activation map by estimating another quantity, which we call “activation overlap” (AO); AO is a metric that estimates stability by calculating the spatial correlations between the activity at τa compared with τa − 3 seconds and τa + 9 seconds. These 2 measures are in turn combined by taking the square-root of the product of the correlations:
where XE (t) is the χ-band activity in electrode E at time t.
Brain Surface Rendering and Coregistration
Brain surfaces were obtained by extracting gray and white matter volumes from the clinical MR imaging in standardized Talairach coordinates, resliced to 1-mm voxel resolution using SPM5.1 These gray and white volumes were then combined and smoothed in 3 dimensions and rendered brain surfaces were obtained from isoluminant contours on the volumes using the MATLAB “isosurface” function. A smoothed cortical surface hull, with the sulci filled in and gyri blunted, is also obtained by applying stronger smoothing. Electrode positions were projected orthonormally from a sagittal radiograph or sagittal CT scout images to the surface of the hull.12,19 These electrode positions can then be plotted to the extracted cortical surface.
Electrocortical Stimulation
Electrocortical stimulation mapping of language27 was performed extraoperatively for clinical purposes in all cases using the same electrode array placed for seizure localization and ECoG recordings. Pulses of 5–10 mA were passed between paired electrodes for up to 3 seconds while the patient performed a standard object-naming task. It is known that when repeated stimulation of a specific site consistently evokes disruption in object naming, resection within 2 cm of this site may lead to a postoperative language deficit.27 Stimulation was performed pairwise, and was not redundant enough to establish which electrode of a given pair was responsible for interruption of function. The stimulation mapping protocol generally did not survey the entire electrode array, but was halted in most cases when a surgical margin was established.
Results
Behaviorally, all patients were able to perform the verb-generation task without difficulty. While produced verbs often occurred during the interstimulus period following the appropriate noun cue, the temporal sequence of verb production and χ-band activity was clearly associated (Fig. 2).
The χ-band broadband spectral change was easily and robustly extracted from the electrocorticographic signal (Figs. 1–3). Language areas were identified using the online mapping protocol during this period, and included receptive, expressive, and auditory processing areas (Figs. 2–7). Stable maps were identified using the criterion AO ≥ 0.99, and for Patients 1–6, this was reached in τa = 18, 51, 45, 33, 108, and 27 seconds, respectively (mean 47 seconds; Fig. 5; Patient 7 did not reach threshold within the abbreviated time of participation, ~ 30 seconds). The online implementation at the bedside rapidly produced a stable cortical surface map (Fig. 4).
Fig. 7.
Activity during noun reading (details as in Fig. 6). Note that in Patient 6, the top half of the grid was not surveyed by stimulation.
As demonstrated in Figs. 6 and 7, these maps of language-associated activity are focused in classic receptive and expressive language areas and mouth and face motor areas, as well as auditory processing areas. In addition, there was frequently activity in postcentral, dorsolateral parietal sites, which is not classically described, but is clearly present in previous electrocorticographic studies.4,5,7,34
Fig. 6.
Activity during verb generation and its relationship to stimulation. The dashed yellow lines denote the central sulcus and Sylvian fissure. The χ-band activity during the verb association task is shown at time τa that satisfies the stability criterion (AO ≥ 0.99, see Fig. 4) and is reflected by the diameter and degree of red at each site. Gray indicates that the activity was less than 5% of the maximum from the array. Black circles around an electrode indicate ECS without impairment of object naming. Green circles with a bar between them indicate where pairwise stimulation interrupted picture naming. In Patient 7, recording was cut short prior to reaching the stability criterion, so the map shown is for the maximum obtained τa. Pt = Patient.
Electrocortical stimulation sites were obtained in each patient to identify areas where object naming was interrupted (Figs. 6 and 7). Of the 2 language tasks, noun reading is closer to picture naming than verb generation. A comparison of noun-reading–associated electrocorticographic change and of the 35 positive stimulation electrode pairs, revealed that at least 1 electrode from the pair demonstrated significant χ-band change in 31 cases (significance: > 0.05 of the maximum, sensitivity of 89%). Of 137 stimulation-negative electrodes, 47 had significant χ-band change (specificity of 66%). There was no statistically significant difference in the amount of activation at significant χ-band electrodes that were ECS-positive sites and ECS-negative sites (p = 0.98, t-test). For verb generation, 46 of 62 ECS-positive electrode pairs had significant χ-band change at 1 or more sites (sensitivity of 74%), and 128 of 248 ECS-negative electrodes had significant χ-band change (48%). There was no statistically significant difference in amount of activation at verb-generation–significant χ-band electrodes that were ECS-positive sites and those that were ECS-negative sites (p = 0.29, t-test).
Interestingly, as with many dominant temporal lobe resections, Patient 6, who underwent resection of the left middle and inferior temporal gyri 4 cm from the pole, had a verbal memory impairment and mild word-finding deficit postoperatively. The resection included a single site that was ECoG positive and ECS negative. This does not necessarily imply that the ECoG site is the one responsible for creating the deficit because it may result from the medial extent of the resection into regions not surveyed by ECoG or ECS, but this result does underscore the incomplete nature of any method in predicting more subtle, but measurable, postsurgical deficits.28
Discussion
This electrocorticographic method demonstrates that the widespread cortical language areas identified in ECoG studies4,5,7,34 can be rapidly and robustly identified online using a verb-generation task. Integration of the χ-band range of frequencies captures broadband spectral change associated with local cortical activity and is easily mapped to the brain surface.
We have introduced a metric of robustness, the “activation overlap”, which quantified the stability of the full activation map over time. This allows us to quantify the amount of time needed to establish a clinically reliable map of cortical activity at approximately 45 seconds (range 18–108 seconds, and following an 8-second baseline period). In the setting of an awake craniotomy, this is an acceptable amount of time. Combined with the result that individual motor modalities can be identified in 15 seconds,18 this suggests that a full set of clinical mapping using electrocorticography could be reasonably accomplished in 5–10 minutes. If used as a preliminary complement to ECS, it could reduce the overall amount of time needed to isolate eloquent cortex prior to resection.
Direct comparison between ECS and χ-band activity is problematic in our study because the behaviors from our studies were different from the picture-naming task used during ECS. The semantic activations associated with verb generation and picture naming are known to exhibit a high degree of overlap, but with less intense and less widespread activation for naming than verb generation.7,11,29 We found that a simple noun-reading task elicited χ-band activity that was more sensitive (89% vs 74%) and specific (66% vs 48%) for capturing ECS-positive stimulation than verb generation was. This is similar to the specificity found in longer mapping sessions using high-frequency ECoG in previous studies.34
Electrocortical stimulation mapping has long been the gold standard in clinical language mapping,29 but there are significant reasons to explore electric potential–based alternatives. Stimulation mapping can be coarse in its ability to localize eloquent cortex. For example, sensorimotor mapping by ECS has been associated with motor activation at sites distant from the precentral gyrus.26 Similarly, stimulation of the basal temporal cortex, including fusiform gyrus and inferior temporal gyrus, is associated with disruption of standard language tasks, but resection of these sites is not associated with postoperative language deficit.14,16 It is important to consider these limitations in the specificity of ECS when comparing the maps generated by ECS and ECoG. In some settings, critical language sites may also be missed by ECS.32 From a practical standpoint, ECS is limited as it requires a significant amount of time intraoperatively and requires persistent attention from the patient, and might be accelerated with online ECoG. Because stimulation mapping can induce seizures, a medical risk to the patient, acceleration with electrocorticographic mapping is in some sense safer.
Passive signal-based mapping techniques provide a different type of information than ECS, with some potential advantages for the study of cortical neural networks, which is why signal-based mapping modalities, such as ECoG, magnetoencephalography, and fMR imaging, are now under such intense investigation. The most obvious of these advantages is that signal-based mapping techniques identify language sites as they are naturally activated and do not disrupt the neural populations being examined. While the all-or-none nature of ECS simplifies the clinical mapping process and identifies input and output structures that are critical for language function, ECS is incapable of examining the spatial and temporal relationships of cortical activation across the brain. Electrocorticography, on the other hand, enables analysis of multiple channels at a single point in time. Furthermore, the temporal profile of ECoG corresponds closely with stimulus and behavioral response (which is not the case in fMR imaging), making it well suited for studying activation relationships between different cortical sites.
The optimal task paradigm for clinically-relevant signal-based language mapping is unknown. In the fMR imaging study by Rutten and colleagues,31 fMR imaging signals generated by verb generation, picture naming, verbal fluency, and sentence comprehension tasks were examined and compared with ECS. Not surprisingly, this study reported that the correlation between ECS and fMR imaging depended heavily on statistical thresholding and the language task used. Identification of posterior temporal language cortex (the classical Wernicke area) may be more challenging as some have suggested that activity here is diminished relative to other cortical language sites.6,9,15 Rutten and colleagues31 found that sentence comprehension most reliably activated temporoparietal cortices and yielded the highest sensitivity of all tasks. To achieve the highest sensitivity for signal-based mapping modalities, it is likely that a combination of different tasks will need to be used, and further investigation into which tasks will yield the most clinically relevant language maps is necessary. Functional MR imaging as an independent modality carries some of the same limitations we found in this small study, namely that activation does not appear to correspond to ECS-identified “essential” language areas.10
In their 2009 paper, Edwards and colleagues7 found that verb generation more dramatically activated nonmotor language areas than did a simple object-naming task. Because we chose a verb-generation task for this study, the comparison with ECS during object naming was not robust outside of the motor cortex, as might be expected from the finding of Edwards et al. Although the common adage is that ECS with object naming is the gold standard for functional localization of language, this may not be the case when more subtle neuropsychological follow-up is used to characterize function. It is unclear whether postoperative verbal fluency deficits after dominant temporal resection are due to lateral cortical resection of language cortex that was not identified by ECS mapping, or due to dominant mesial temporal resection.28 If it is due to cortical resection, a verb-generation or other language task may be a more sensitive measure2 than simple naming. We propose that, as with other signal-based mapping methods such as fMR imaging,10 a battery of tasks would be most appropriate and that an optimal future study would examine both ECoG change and ECS in conjunction with cortical resection data and comprehensive follow-up neuropsychological testing.
Conclusions
In this article, we have examined the evoked cortical activity in the χ-band during verb generation and noun reading for online mapping of language function. We demonstrate that robust, reliable mapping can be performed in less than a minute. This suggests that online electrocorticographic mapping can be used as a convenient adjuvant tool for functional localization in the operative suite.
Acknowledgments
The authors thank the patients and staff at Harborview Medical Center, Seattle, for their time and dedication, as well as Dora Hermes and George A. Ojemann for helpful discussions.
This work was supported by the National Aeronautics and Space Administration Graduate Student Researchers Program and the National Institute of General Medical Sciences Medical Scientist Training Program.
Abbreviations used in this paper
- AO
activation overlap
- ECoG
electrocorticography
- ECS
electrocortical stimulation
- fMR
functional MR
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Miller, Ojemann. Acquisition of data: all authors. Analysis and interpretation of data: Miller, Abel. Drafting the article: Miller. Critically revising the article: Miller, Abel, Hebb. Reviewed final version of the manuscript and approved it for submission: all authors. Statistical analysis: Miller. Administrative/technical/material support: Hebb, Ojemann. Study supervision: Hebb, Ojemann.
References
- 1.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Binder JR, Gross WL, Allendorfer JB, Bonilha L, Chapin J, Edwards JC, et al. Mapping anterior temporal lobe language areas with fMRI: a multicenter normative study. Neuroimage. 2011;54:1465–1475. doi: 10.1016/j.neuroimage.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brindley GS, Craggs MD. The electrical activity in the motor cortex that accompanies voluntary movement. J Physiol. 1972;223:28P–29P. [PubMed] [Google Scholar]
- 4.Canolty RT, Soltani M, Dalal SS, Edwards E, Dronkers NF, Nagarajan SS, et al. Spatiotemporal dynamics of word processing in the human brain. Front Neurosci. 2007;1:185–196. doi: 10.3389/neuro.01.1.1.014.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crone NE, Hao L, Hart J, Jr, Boatman D, Lesser RP, Irizarry R, et al. Electrocorticographic gamma activity during word production in spoken and sign language. Neurology. 2001;57:2045–2053. doi: 10.1212/wnl.57.11.2045. [DOI] [PubMed] [Google Scholar]
- 6.Damasio AR. Aphasia. N Engl J Med. 1992;326:531–539. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- 7.Edwards E, Nagarajan SS, Dalal SS, Canolty RT, Kirsch HE, Barbaro NM, et al. Spatiotemporal imaging of cortical activation during verb generation and picture naming. Neuroimage. 2010;50:291–301. doi: 10.1016/j.neuroimage.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards E, Soltani M, Kim W, Dalal SS, Nagarajan SS, Berger MS, et al. Comparison of time-frequency responses and the event-related potential to auditory speech stimuli in human cortex. J Neurophysiol. 2009;102:377–386. doi: 10.1152/jn.90954.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etard O, Mellet E, Papathanassiou D, Benali K, Houdé O, Mazoyer B, et al. Picture naming without Broca’s and Wernicke’s area. Neuroreport. 2000;11:617–622. doi: 10.1097/00001756-200002280-00036. [DOI] [PubMed] [Google Scholar]
- 10.Giussani C, Roux FE, Ojemann J, Sganzerla EP, Pirillo D, Papagno C. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery. 2010;66:113–120. doi: 10.1227/01.NEU.0000360392.15450.C9. [DOI] [PubMed] [Google Scholar]
- 11.Herholz K, Reulen HJ, von Stockhausen HM, Thiel A, Ilmberger J, Kessler J, et al. Preoperative activation and intraoperative stimulation of language-related areas in patients with glioma. Neurosurgery. 1997;41:1253–1262. doi: 10.1097/00006123-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Hermes D, Miller KJ, Noordmans HJ, Vansteensel MJ, Ramsey NF. Automated electrocorticographic electrode localization on individually rendered brain surfaces. J Neurosci Methods. 2010;185:293–298. doi: 10.1016/j.jneumeth.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs J, Kahana MJ. Direct brain recordings fuel advances in cognitive electrophysiology. Trends Cogn Sci. 2010;14:162–171. doi: 10.1016/j.tics.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krauss GL, Fisher R, Plate C, Hart J, Jr, Uematsu S, Gordon B, et al. Cognitive effects of resecting basal temporal language areas. Epilepsia. 1996;37:476–483. doi: 10.1111/j.1528-1157.1996.tb00594.x. [DOI] [PubMed] [Google Scholar]
- 15.Lehéricy S, Cohen L, Bazin B, Samson S, Giacomini E, Rougetet R, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54:1625–1633. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- 16.Lüders H, Lesser RP, Hahn J, Dinner DS, Morris HH, Wyllie E, et al. Basal temporal language area. Brain. 1991;114:743–754. doi: 10.1093/brain/114.2.743. [DOI] [PubMed] [Google Scholar]
- 17.Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29:13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller KJ, denNijs M, Shenoy P, Miller JW, Rao RP, Ojemann JG. Real-time functional brain mapping using electrocorticography. Neuroimage. 2007;37:504–507. doi: 10.1016/j.neuroimage.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Miller KJ, Hebb AO, Hermes D, den Nijs M, Ojemann JG, Rao RPN. Brain surface electrode co-registration using MRI and x-ray. Conf Proc IEEE Eng Med Biol Soc. 2010;1:6015–6018. doi: 10.1109/IEMBS.2010.5627597. [DOI] [PubMed] [Google Scholar]
- 20.Miller KJ, Hermes D, Honey CJ, Sharma M, Rao RP, den Nijs M, et al. Dynamic modulation of local population activity by rhythm phase in human occipital cortex during a visual search task. Front Hum Neurosci. 2010;4:197. doi: 10.3389/fnhum.2010.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller KJ, Schalk G, Fetz EE, den Nijs M, Ojemann JG, Rao RP. Cortical activity during motor execution, motor imagery, and imagery-based online feedback. Proc Natl Acad Sci U S A. 2010;107:4430–4435. doi: 10.1073/pnas.0913697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller KJ, Shenoy P, den Nijs M, Sorensen LB, Rao RN, Ojemann JG. Beyond the gamma band: the role of high-frequency features in movement classification. IEEE Trans Biomed Eng. 2008;55:1634–1637. doi: 10.1109/TBME.2008.918569. [DOI] [PubMed] [Google Scholar]
- 23.Miller KJ, Sorensen LB, Ojemann JG, den Nijs M. Power-law scaling in the brain surface electric potential. PLoS Comput Biol. 2009;5 doi: 10.1371/journal.pcbi.1000609. e1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller KJ, Weaver KE, Ojemann JG. Direct electrophysiological measurement of human default network areas. Proc Natl Acad Sci U S A. 2009;106:12174–12177. doi: 10.1073/pnas.0902071106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller KJ, Zanos S, Fetz EE, den Nijs M, Ojemann JG. Decoupling the cortical power spectrum reveals real-time representation of individual finger movements in humans. J Neurosci. 2009;29:3132–3137. doi: 10.1523/JNEUROSCI.5506-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nii Y, Uematsu S, Lesser RP, Gordon B. Does the central sulcus divide motor and sensory functions? Cortical mapping of human hand areas as revealed by electrical stimulation through subdural grid electrodes. Neurology. 1996;46:360–367. doi: 10.1212/wnl.46.2.360. [DOI] [PubMed] [Google Scholar]
- 27.Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- 28.Ojemann GA, Dodrill CB. Verbal memory deficits after left temporal lobectomy for epilepsy. Mechanism and intraoperative prediction. J Neurosurg. 1985;62:101–107. doi: 10.3171/jns.1985.62.1.0101. [DOI] [PubMed] [Google Scholar]
- 29.Ojemann JG, Ojemann GA, Lettich E. Cortical stimulation mapping of language cortex by using a verb generation task: effects of learning and comparison to mapping based on object naming. J Neurosurg. 2002;97:33–38. doi: 10.3171/jns.2002.97.1.0033. [DOI] [PubMed] [Google Scholar]
- 30.Petersen SE, Fox PT, Posner MI, Mintum MA, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. In: Balota DA, Marsh EJ, editors. Cognitive Psychology: Key Readings. New York: Psychology Press; 2004. pp. 109–118. [Google Scholar]
- 31.Rutten GJ, Ramsey NF, van Rijen PC, Noordmans HJ, van Veelen CW. Development of a functional magnetic resonance imaging protocol for intraoperative localization of critical temporoparietal language areas. Ann Neurol. 2002;51:350–360. doi: 10.1002/ana.10117. [DOI] [PubMed] [Google Scholar]
- 32.Rutten GJ, van Rijen PC, van Veelen CW, Ramsey NF. Language area localization with three-dimensional functional magnetic resonance imaging matches intrasulcal electrostimulation in Broca’s area. Ann Neurol. 1999;46:405–408. doi: 10.1002/1531-8249(199909)46:3<405::aid-ana17>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 33.Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004;51:1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 34.Sinai A, Bowers CW, Crainiceanu CM, Boatman D, Gordon B, Lesser RP, et al. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005;128(Pt 7):1556–1570. doi: 10.1093/brain/awh491. [DOI] [PubMed] [Google Scholar]