Abstract
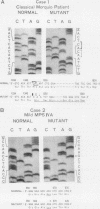
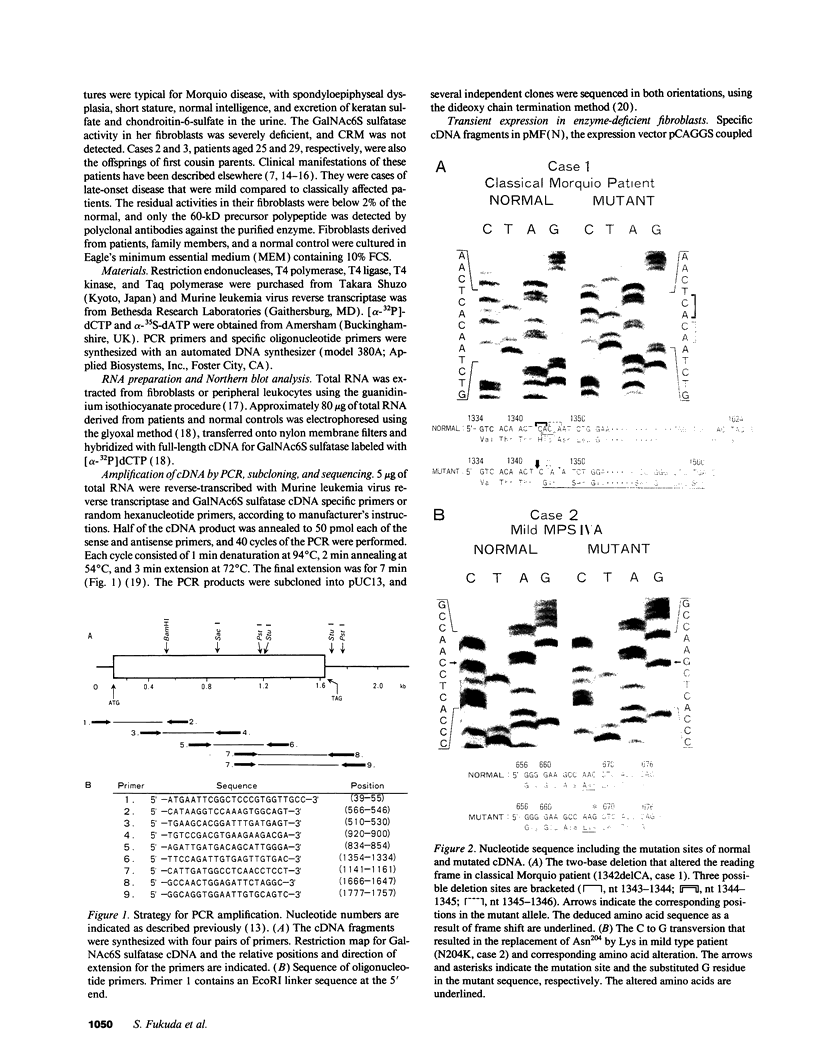
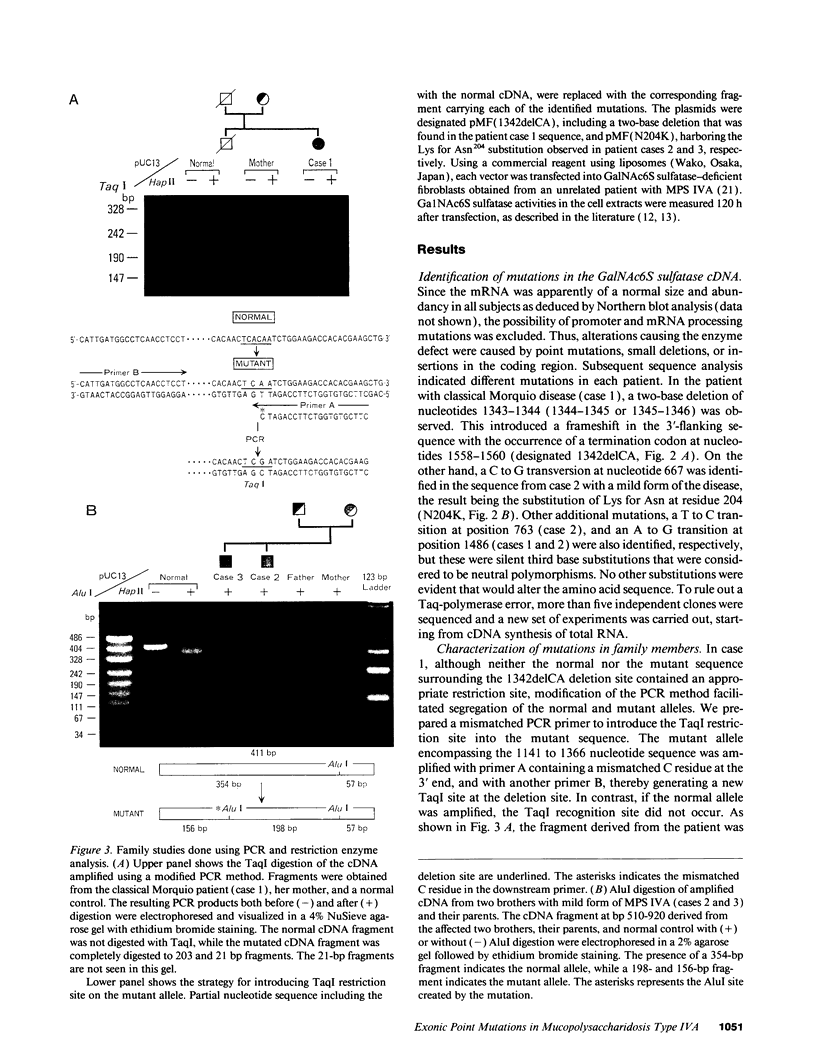
Mucopolysaccharidosis type IVA (MPS IVA) results from a genetic deficiency of N-acetylgalactosamine-6-sulfate (Gal-NAc6S) sulfatase. We have identified two different exonic mutations causing GalNAc6S sulfatase deficiency in two unrelated Japanese families, in one patient with classical Morquio disease, and in two brothers with a mild form of MPS IVA. The nucleotide sequence of the full-length cDNA derived from a patient with classical Morquio disease revealed a two-base deletion at nucleotide position 1343-1344 (1344-1345 or 1345-1346) that altered the reading frame (designated 1342delCA). This mutation, inherited from the proband's consanguineous parents, was revealed by TaqI restriction analysis of a cDNA fragment amplified by the polymerase chain reaction. In the proband with the mild form of the disease, a C to G transversion at nucleotide 667 predicted the substitution of Lys for Asn204 (N204K). Since a new AluI site was created by the N204K mutation, restriction analysis indicated that the affected brothers were homozygous for this mutation, as confirmed by the finding that both their parents had this lesion. Transient expression in GalNAc6S sulfatase deficient fibroblasts of these two mutant alleles showed completely deficient or markedly decreased enzyme activities, thereby indicating that these two mutations were responsible for the enzyme deficiency.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck M., Glössl J., Grubisic A., Spranger J. Heterogeneity of Morquio disease. Clin Genet. 1986 Apr;29(4):325–331. doi: 10.1111/j.1399-0004.1986.tb01262.x. [DOI] [PubMed] [Google Scholar]
- Canning S., Dryja T. P. Short, direct repeats at the breakpoints of deletions of the retinoblastoma gene. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5044–5048. doi: 10.1073/pnas.86.13.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Di Ferrante N., Ginsberg L. C., Donnelly P. V., Di Ferrante D. T., Caskey C. T. Deficiencies of glucosamine-6-sulfate or galactosamine-6-sulfate sulfatases are responsible for different mucopolysaccharidoses. Science. 1978 Jan 6;199(4324):79–81. doi: 10.1126/science.199.4324.79. [DOI] [PubMed] [Google Scholar]
- Fujimoto A., Horwitz A. L. Biochemical defect of non-keratan-sulfate-excreting Morquio syndrome. Am J Med Genet. 1983 Jun;15(2):265–273. doi: 10.1002/ajmg.1320150210. [DOI] [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., McBride L. J., Koepf S. M., Caskey C. T. Identification of mutations leading to the Lesch-Nyhan syndrome by automated direct DNA sequencing of in vitro amplified cDNA. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1919–1923. doi: 10.1073/pnas.86.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi Y., Aparicio J. M., Takiguchi M., Akaboshi I., Yoshino M., Mori M., Matsuda I. Molecular basis of argininemia. Identification of two discrete frame-shift deletions in the liver-type arginase gene. J Clin Invest. 1990 Jul;86(1):347–350. doi: 10.1172/JCI114707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hecht J. T., Scott C. I., Jr, Smith T. K., Williams J. C. Mild manifestations of the Morquio syndrome. Am J Med Genet. 1984 Jun;18(2):369–371. doi: 10.1002/ajmg.1320180222. [DOI] [PubMed] [Google Scholar]
- Horwitz A. L., Dorfman A. The enzymic defect in Morquio's disease: the specificity of N-acetylhexosamine sulfatases. Biochem Biophys Res Commun. 1978 Feb 28;80(4):819–825. doi: 10.1016/0006-291x(78)91318-9. [DOI] [PubMed] [Google Scholar]
- Masue M., Sukegawa K., Orii T., Hashimoto T. N-acetylgalactosamine-6-sulfate sulfatase in human placenta: purification and characteristics. J Biochem. 1991 Dec;110(6):965–970. doi: 10.1093/oxfordjournals.jbchem.a123697. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Singh J., Di Ferrante N., Niebes P., Tavella D. N-acetylgalactosamine-6-sulfate sulfatase in man. Absence of the enzyme in Morquio disease. J Clin Invest. 1976 Apr;57(4):1036–1040. doi: 10.1172/JCI108345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukegawa K., Orii T. Residual activity in fibroblasts from two brothers with the late-onset form of N-acetylgalactosamine-6-sulphate sulphatase deficiency. J Inherit Metab Dis. 1982;5(4):231–232. doi: 10.1007/BF02179150. [DOI] [PubMed] [Google Scholar]
- Tomatsu S., Fukuda S., Masue M., Sukegawa K., Fukao T., Yamagishi A., Hori T., Iwata H., Ogawa T., Nakashima Y. Morquio disease: isolation, characterization and expression of full-length cDNA for human N-acetylgalactosamine-6-sulfate sulfatase. Biochem Biophys Res Commun. 1991 Dec 16;181(2):677–683. doi: 10.1016/0006-291x(91)91244-7. [DOI] [PubMed] [Google Scholar]