Abstract
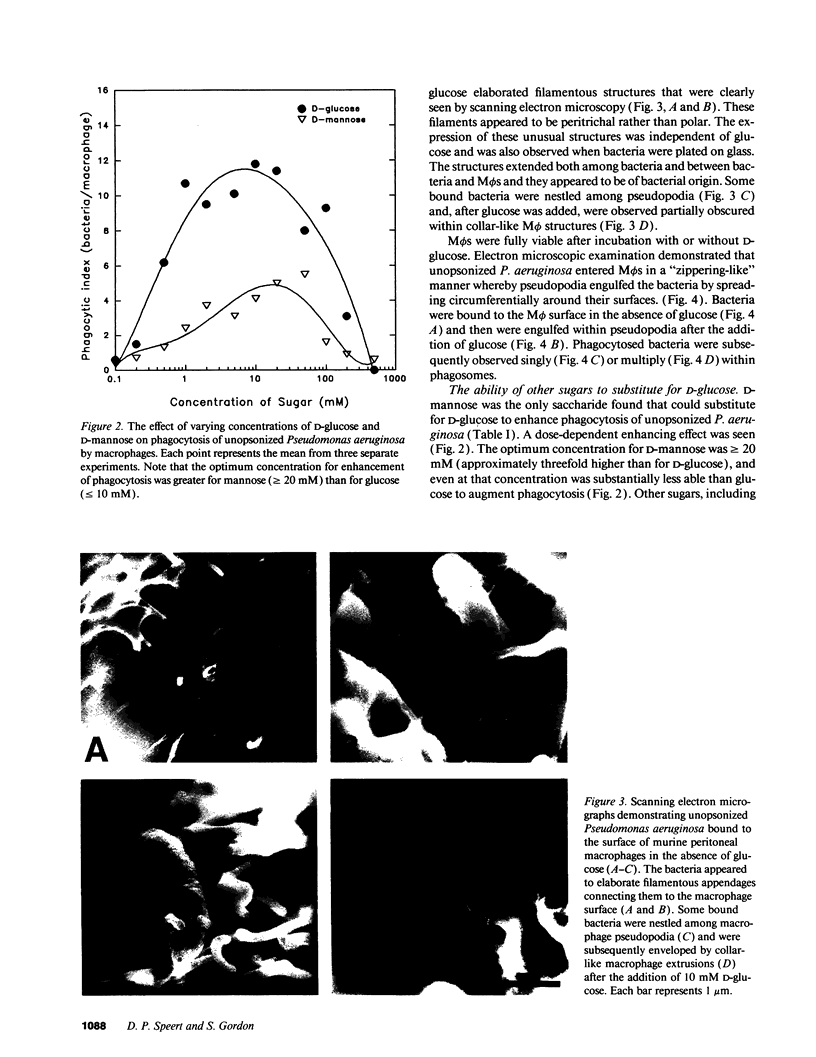
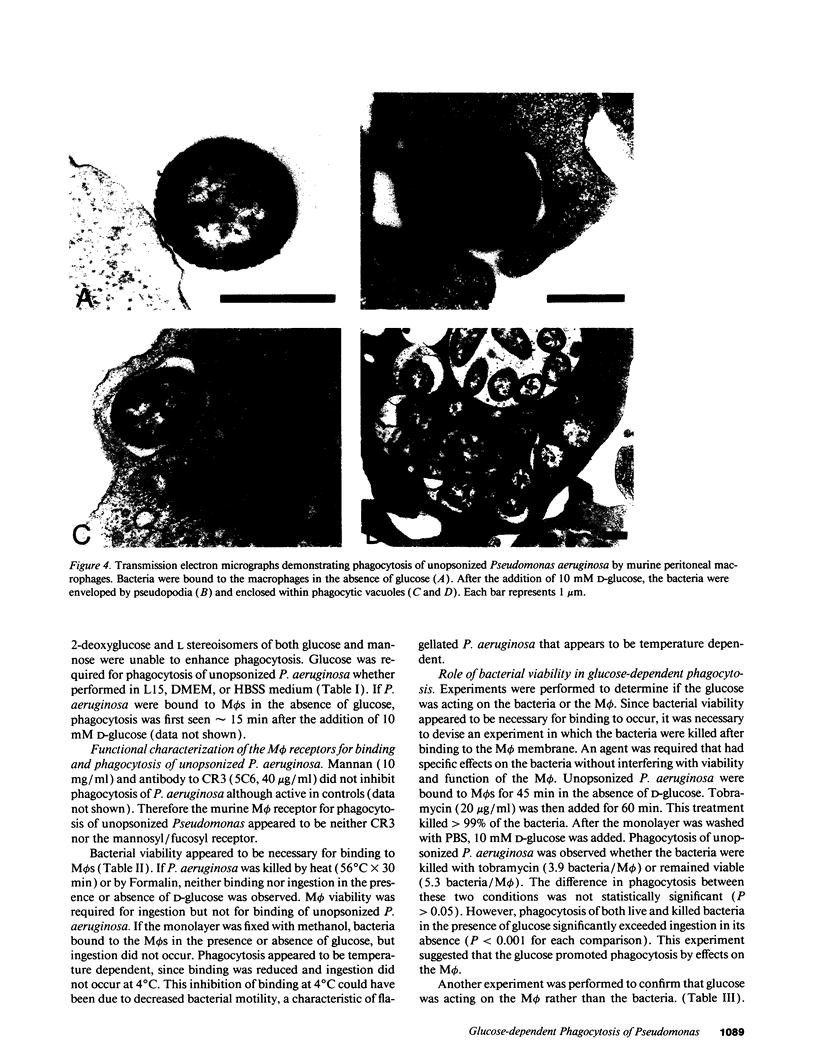
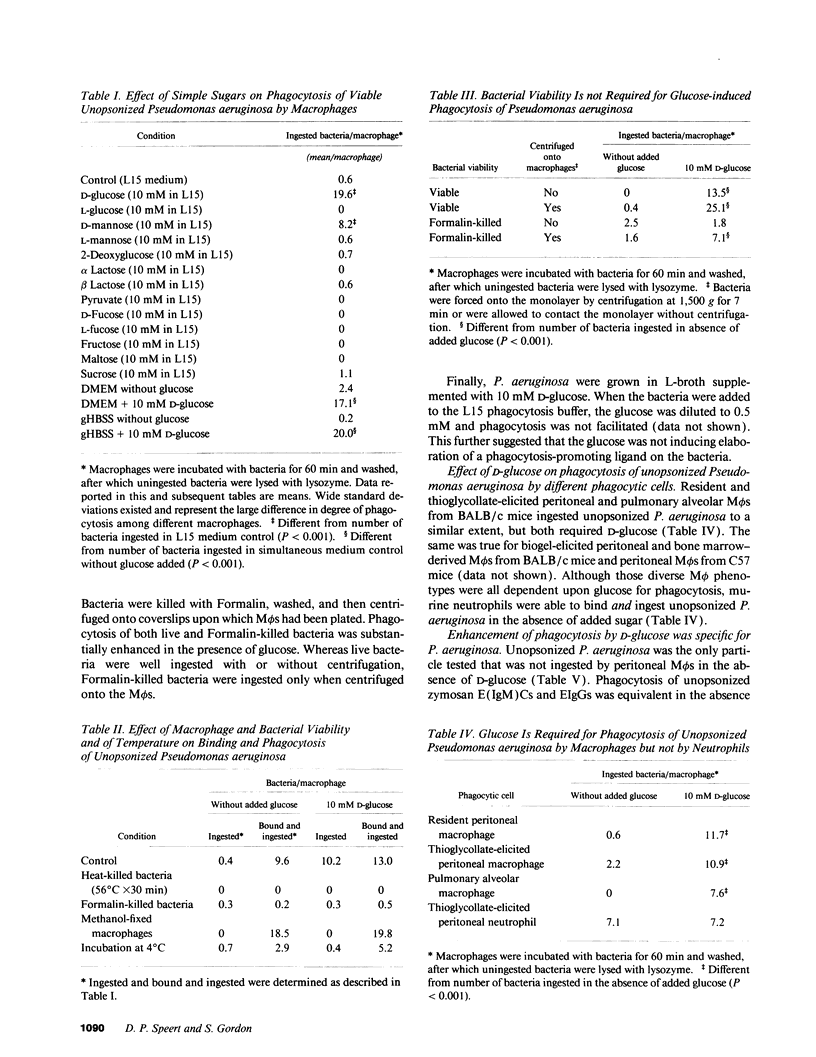
Pseudomonas aeruginosa is an important pulmonary pathogen in cystic fibrosis, but the means by which it evades host defenses is understood poorly. Macrophages (M phi) are critical in protecting the lung and mucosal surfaces against infection and may need to perform their functions in the absence of opsonins before the evolution of an inflammatory response. The purpose of the present study was to define factors that regulate the capacity of macrophages to mediate nonopsonic phagocytosis. Phagocytosis of unopsonized P. aeruginosa by murine peritoneal and pulmonary alveolar M phi s was absolutely dependent upon the presence of glucose; only D-mannose could substitute. Glucose-dependent phagocytosis appears to be selective for P. aeruginosa by M phi s; ingestion of unopsonized zymosan, opsonized P. aeruginosa, EIgG, and E (IgM)C occurred in the presence or absence of glucose as did-ingestion of unopsonized P. aeruginosa by polymorphonuclear leukocytes. M phi binding and phagocytosis of unopsonized P. aeruginosa appeared to occur by a mechanism independent of complement receptor 3 and mannose receptors. Phagocytosis of P. aeruginosa killed by tobramycin or Formalin was glucose dependent, suggesting that the glucose exerted its effects on the M phi rather than the bacteria. The predilection of P. aeruginosa for lower airway disease in patients with cystic fibrosis might be explained in part by the unique dependency upon glucose for M phi phagocytosis.
Full text
PDF

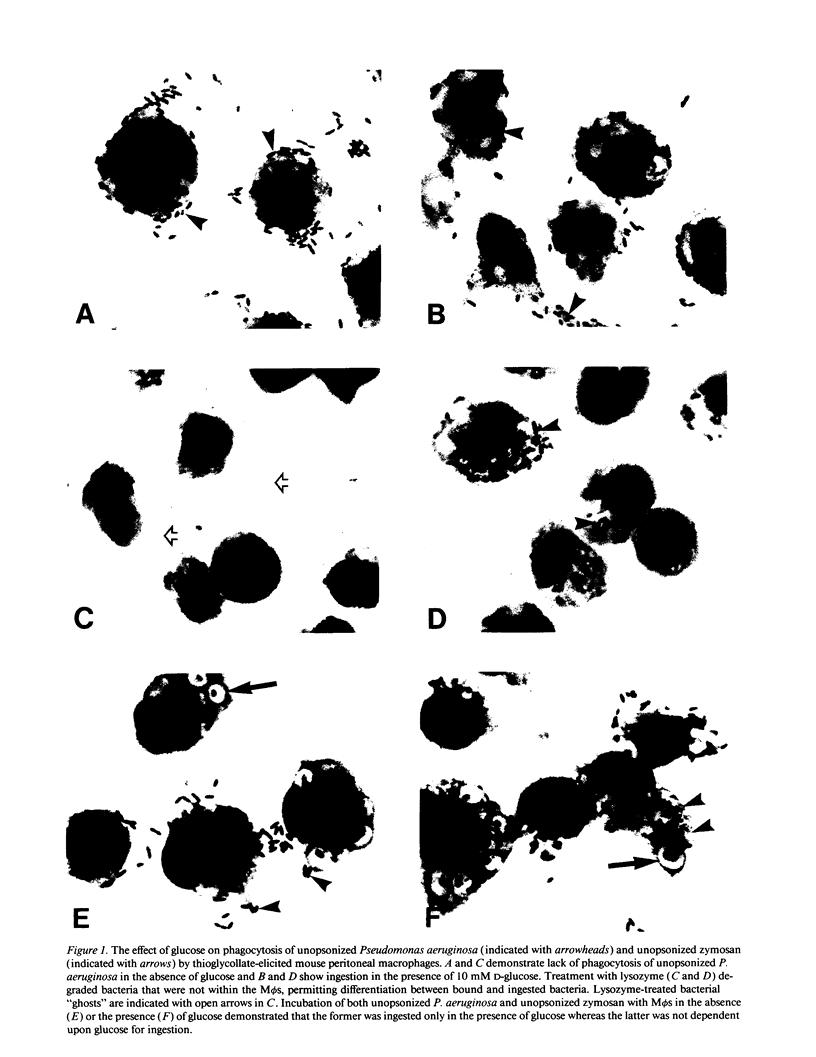


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabral D. A., Loh B. A., Speert D. P. Mucoid Pseudomonas aeruginosa resists nonopsonic phagocytosis by human neutrophils and macrophages. Pediatr Res. 1987 Oct;22(4):429–431. doi: 10.1203/00006450-198710000-00013. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., MacPherson G. G., Gordon S. Interaction of human monocytes, macrophages, and polymorphonuclear leukocytes with zymosan in vitro. Role of type 3 complement receptors and macrophage-derived complement. J Clin Invest. 1985 Dec;76(6):2368–2376. doi: 10.1172/JCI112249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Heffron F., Falkow S. Epithelial cell surfaces induce Salmonella proteins required for bacterial adherence and invasion. Science. 1989 Feb 17;243(4893):940–943. doi: 10.1126/science.2919285. [DOI] [PubMed] [Google Scholar]
- Gilboa-Garber N. Pseudomonas aeruginosa lectins. Methods Enzymol. 1982;83:378–385. doi: 10.1016/0076-6879(82)83034-6. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- Michl J., Ohlbaum D. J., Silverstein S. C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages II. Dissociation of the inhibitory effects of 2-deoxyglucose on phagocytosis and ATP generation. J Exp Med. 1976 Dec 1;144(6):1484–1493. doi: 10.1084/jem.144.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Ohlbaum D. J., Silverstein S. C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages. I. Description of the inhibitory effect. J Exp Med. 1976 Dec 1;144(6):1465–1483. doi: 10.1084/jem.144.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeser J. R., Chambaz A., Golliard M., Link-Amster H., Fryder V., Kolodziejczyk E. Adhesion of colonization factor antigen II-positive enterotoxigenic Escherichia coli strains to human enterocytelike differentiated HT-29 cells: a basis for host-pathogen interactions in the gut. Infect Immun. 1989 Dec;57(12):3727–3734. doi: 10.1128/iai.57.12.3727-3734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P., Curi R., Gordon S., Newsholme E. A. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem J. 1986 Oct 1;239(1):121–125. doi: 10.1042/bj2390121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranchych W., Sastry P. A., Volpel K., Loh B. A., Speert D. P. Fimbriae (pili): molecular basis of Pseudomonas aeruginosa adherence. Clin Invest Med. 1986;9(2):113–118. [PubMed] [Google Scholar]
- Rosen H., Gordon S. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J Exp Med. 1987 Dec 1;166(6):1685–1701. doi: 10.1084/jem.166.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P., Eftekhar F., Puterman M. L. Nonopsonic phagocytosis of strains of Pseudomonas aeruginosa from cystic fibrosis patients. Infect Immun. 1984 Mar;43(3):1006–1011. doi: 10.1128/iai.43.3.1006-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P. Host defenses in patients with cystic fibrosis: modulation by Pseudomonas aeruginosa. Surv Synth Pathol Res. 1985;4(1):14–33. doi: 10.1159/000156962. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Loh B. A., Cabral D. A., Salit I. E. Nonopsonic phagocytosis of nonmucoid Pseudomonas aeruginosa by human neutrophils and monocyte-derived macrophages is correlated with bacterial piliation and hydrophobicity. Infect Immun. 1986 Jul;53(1):207–212. doi: 10.1128/iai.53.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P., Wright S. D., Silverstein S. C., Mah B. Functional characterization of macrophage receptors for in vitro phagocytosis of unopsonized Pseudomonas aeruginosa. J Clin Invest. 1988 Sep;82(3):872–879. doi: 10.1172/JCI113692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Silverstein S. C. Role of 2-deoxy-D-glucose in the inhibition of phagocytosis by mouse peritoneal macrophage. Biochim Biophys Acta. 1985 May 30;845(2):204–215. doi: 10.1016/0167-4889(85)90178-8. [DOI] [PubMed] [Google Scholar]
- Valeyre D., Soler P., Basset G., Loiseau P., Pre J., Turbie P., Battesti J. P., Georges R. Glucose, K+, and albumin concentrations in the alveolar milieu of normal humans and pulmonary sarcoidosis patients. Am Rev Respir Dis. 1991 May;143(5 Pt 1):1096–1101. doi: 10.1164/ajrccm/143.5_Pt_1.1096. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Craigmyle L. S., Silverstein S. C. Fibronectin and serum amyloid P component stimulate C3b- and C3bi-mediated phagocytosis in cultured human monocytes. J Exp Med. 1983 Oct 1;158(4):1338–1343. doi: 10.1084/jem.158.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Tumor-promoting phorbol esters stimulate C3b and C3b' receptor-mediated phagocytosis in cultured human monocytes. J Exp Med. 1982 Oct 1;156(4):1149–1164. doi: 10.1084/jem.156.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Fujita K., Yokota T. Adherence characteristics to human small intestinal mucosa of Escherichia coli isolated from patients with diarrhea or urinary tract infections. J Infect Dis. 1990 Oct;162(4):896–908. doi: 10.1093/infdis/162.4.896. [DOI] [PubMed] [Google Scholar]