Abstract
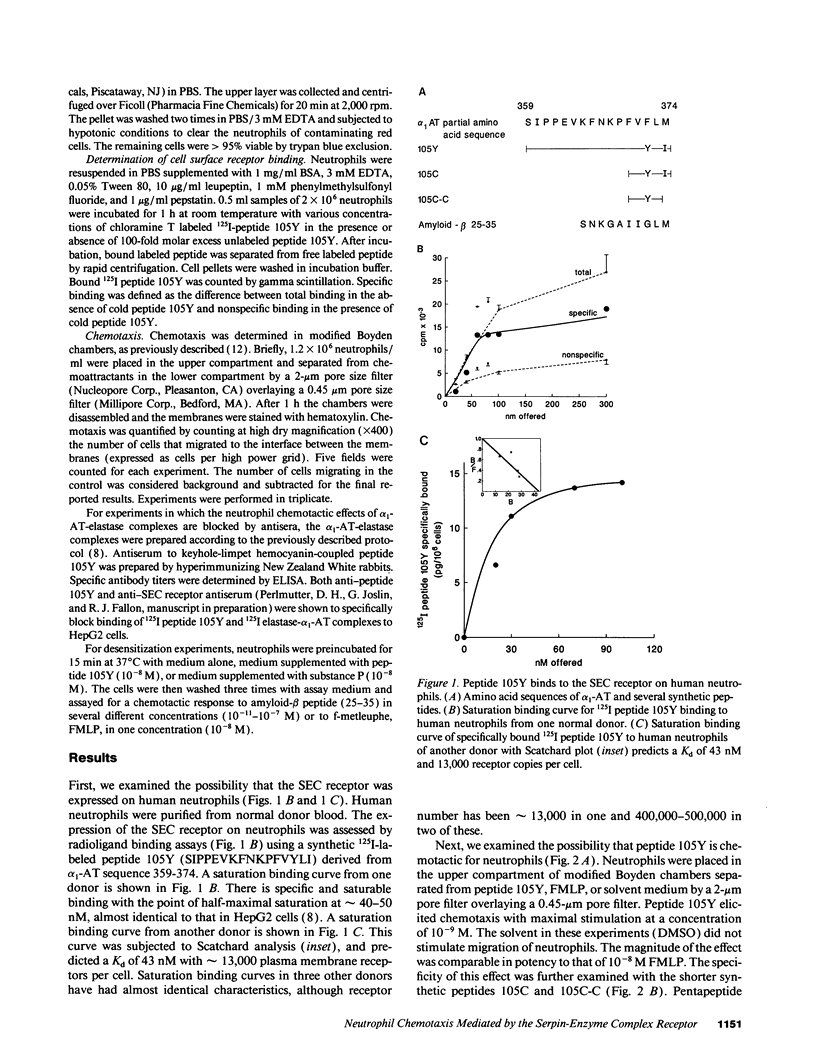
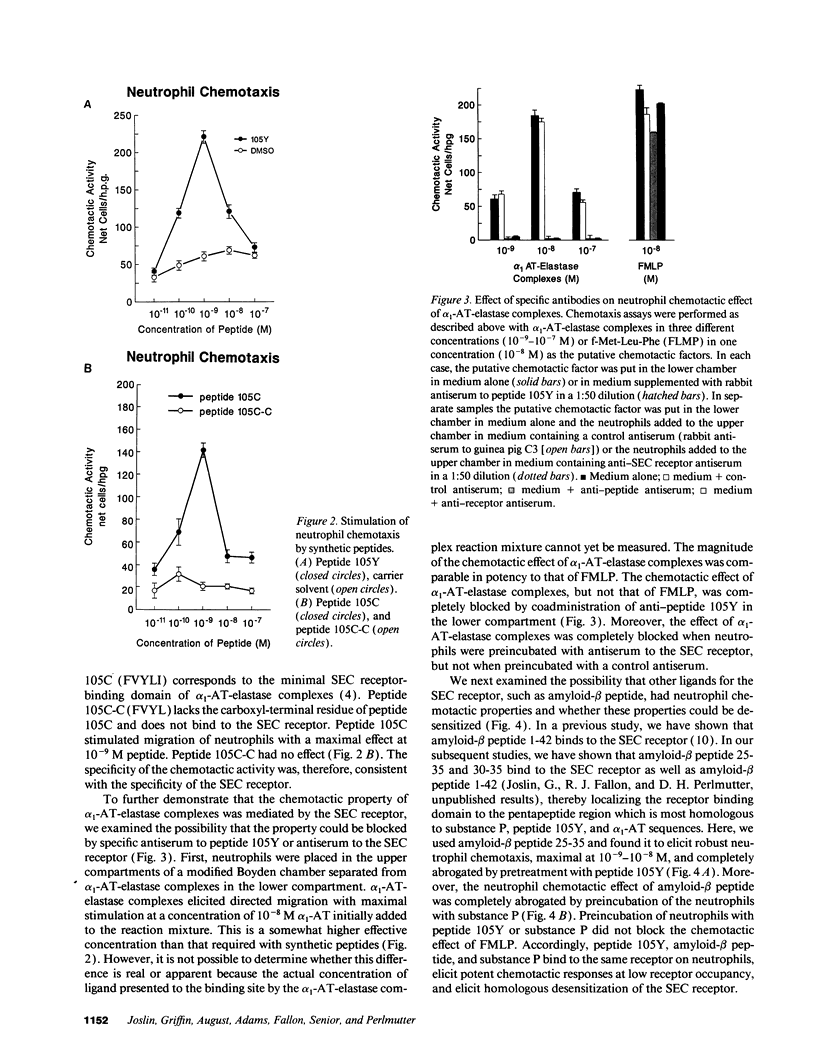
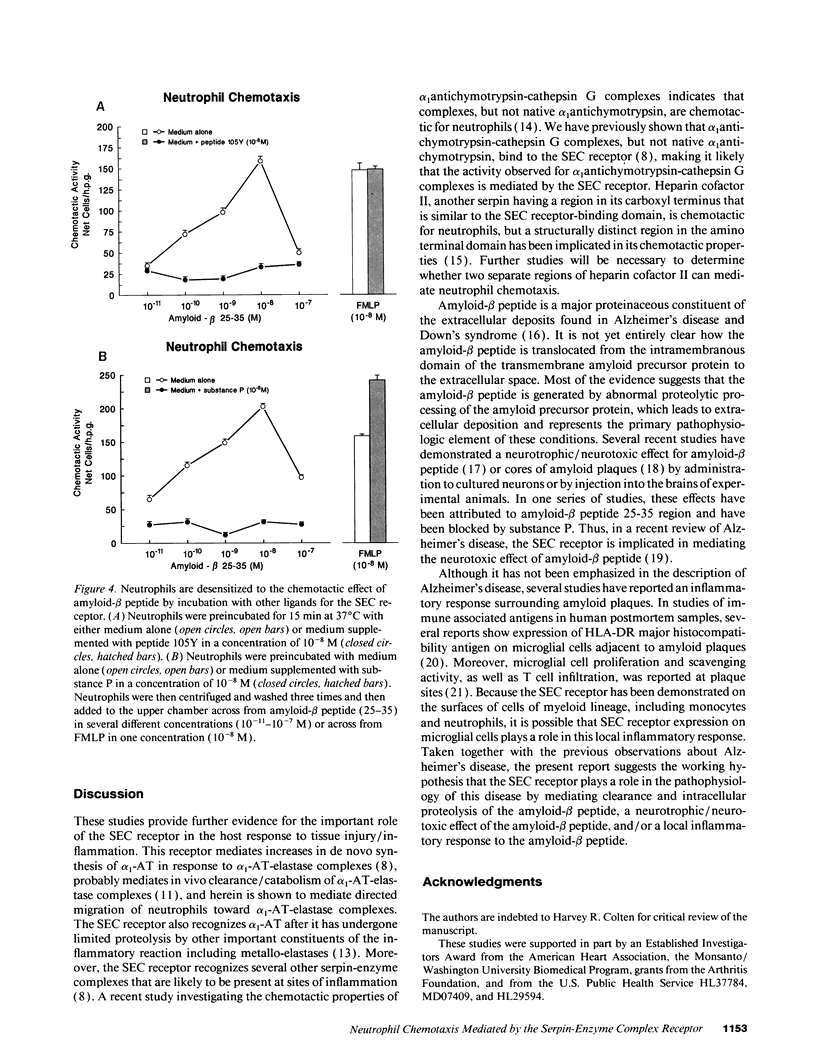
The serpin-enzyme complex (SEC) receptor mediates catabolism of alpha 1-antitrypsin (alpha 1-AT)-elastase complexes and increases in synthesis of alpha 1-AT in cell culture. The SEC receptor recognizes a pentapeptide domain on alpha 1-AT-elastase complexes (alpha 1-AT 370-374), and the same domain in several other serpins, amyloid-beta peptide, substance P, and other tachykinins. Thus, it has also been implicated in the biological properties of these ligands, including the neurotoxic effect of amyloid-beta peptide. In this study, we examined the possibility that the SEC receptor mediates the previously described neutrophil chemotactic activity of alpha 1-AT-elastase complexes, and whether the other ligands for the SEC receptor have neutrophil chemotactic activity. The results show that 125I-peptide 105Y (based on alpha 1-AT 359-374) binds specifically and saturably to human neutrophils, and the characteristics of this binding are almost identical to that of monocytes and hepatoma-derived hepatocytes. Peptide 105Y and amyloid-beta peptide mediate chemotaxis for neutrophils with maximal stimulation at 1-10 nM. Mutant or deleted forms of peptide 105Y, which do not bind to the SEC receptor, have no effect. The neutrophil chemotactic effect of alpha 1-AT-elastase complexes is blocked by antiserum to peptide 105Y and by antiserum to the SEC receptor, but not by control antiserum. Preincubation of neutrophils with peptide 105Y or substance P completely blocks the chemotactic activity of amyloid-beta peptide, but not that of FMLP. These results, therefore, indicate that the SEC receptor can be modulated by homologous desensitization and raise the possibility that pharmacological manipulation of this receptor will modify the local tissue response to inflammation/injury and the neuropathologic reaction of Alzheimer's disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banda M. J., Rice A. G., Griffin G. L., Senior R. M. Alpha 1-proteinase inhibitor is a neutrophil chemoattractant after proteolytic inactivation by macrophage elastase. J Biol Chem. 1988 Mar 25;263(9):4481–4484. [PubMed] [Google Scholar]
- Banda M. J., Rice A. G., Griffin G. L., Senior R. M. The inhibitory complex of human alpha 1-proteinase inhibitor and human leukocyte elastase is a neutrophil chemoattractant. J Exp Med. 1988 May 1;167(5):1608–1615. doi: 10.1084/jem.167.5.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey-Morel C., Perlmutter D. H. Effect of pseudomonas elastase on human mononuclear phagocyte alpha 1-antitrypsin expression. Pediatr Res. 1991 Feb;29(2):133–140. doi: 10.1203/00006450-199102000-00005. [DOI] [PubMed] [Google Scholar]
- Church F. C., Pratt C. W., Hoffman M. Leukocyte chemoattractant peptides from the serpin heparin cofactor II. J Biol Chem. 1991 Jan 15;266(2):704–709. [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Chang D., Griffin G. L., Heinrikson R. L., Kaiser E. T. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4584–4587. doi: 10.1073/pnas.78.7.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschy S. A., Baird A., Cole G. M. Effects of injected Alzheimer beta-amyloid cores in rat brain. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8362–8366. doi: 10.1073/pnas.88.19.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Savill J. S., Meagher L. The neutrophil. Curr Opin Immunol. 1989 Oct;2(1):10–18. doi: 10.1016/0952-7915(89)90091-5. [DOI] [PubMed] [Google Scholar]
- Itagaki S., McGeer P. L., Akiyama H., Zhu S., Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989 Oct;24(3):173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- Joslin G., Fallon R. J., Bullock J., Adams S. P., Perlmutter D. H. The SEC receptor recognizes a pentapeptide neodomain of alpha 1-antitrypsin-protease complexes. J Biol Chem. 1991 Jun 15;266(17):11282–11288. [PubMed] [Google Scholar]
- Joslin G., Krause J. E., Hershey A. D., Adams S. P., Fallon R. J., Perlmutter D. H. Amyloid-beta peptide, substance P, and bombesin bind to the serpin-enzyme complex receptor. J Biol Chem. 1991 Nov 15;266(32):21897–21902. [PubMed] [Google Scholar]
- Mast A. E., Enghild J. J., Pizzo S. V., Salvesen G. Analysis of the plasma elimination kinetics and conformational stabilities of native, proteinase-complexed, and reactive site cleaved serpins: comparison of alpha 1-proteinase inhibitor, alpha 1-antichymotrypsin, antithrombin III, alpha 2-antiplasmin, angiotensinogen, and ovalbumin. Biochemistry. 1991 Feb 12;30(6):1723–1730. doi: 10.1021/bi00220a039. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Glover G. I., Rivetna M., Schasteen C. S., Fallon R. J. Identification of a serpin-enzyme complex receptor on human hepatoma cells and human monocytes. Proc Natl Acad Sci U S A. 1990 May;87(10):3753–3757. doi: 10.1073/pnas.87.10.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Joslin G., Nelson P., Schasteen C., Adams S. P., Fallon R. J. Endocytosis and degradation of alpha 1-antitrypsin-protease complexes is mediated by the serpin-enzyme complex (SEC) receptor. J Biol Chem. 1990 Oct 5;265(28):16713–16716. [PubMed] [Google Scholar]
- Perlmutter D. H., Punsal P. I. Distinct and additive effects of elastase and endotoxin on expression of alpha 1 proteinase inhibitor in mononuclear phagocytes. J Biol Chem. 1988 Nov 5;263(31):16499–16503. [PubMed] [Google Scholar]
- Perlmutter D. H., Travis J., Punsal P. I. Elastase regulates the synthesis of its inhibitor, alpha 1-proteinase inhibitor, and exaggerates the defect in homozygous PiZZ alpha 1 PI deficiency. J Clin Invest. 1988 Jun;81(6):1774–1780. doi: 10.1172/JCI113519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J., Fedak D., Dubin A., Mast A., Travis J. Proteolytic inactivation of alpha-1-anti-chymotrypsin. Sites of cleavage and generation of chemotactic activity. J Biol Chem. 1991 Nov 15;266(32):21482–21487. [PubMed] [Google Scholar]
- Pratt C. W., Church F. C., Pizzo S. V. In vivo catabolism of heparin cofactor II and its complex with thrombin: evidence for a common receptor-mediated clearance pathway for three serine proteinase inhibitors. Arch Biochem Biophys. 1988 Apr;262(1):111–117. doi: 10.1016/0003-9861(88)90173-7. [DOI] [PubMed] [Google Scholar]
- Rogers J., Luber-Narod J., Styren S. D., Civin W. H. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiol Aging. 1988 Jul-Aug;9(4):339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Amyloid beta protein precursor and the pathogenesis of Alzheimer's disease. Cell. 1989 Aug 25;58(4):611–612. doi: 10.1016/0092-8674(89)90093-7. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Duffy L. K., Kirschner D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990 Oct 12;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Mesulam M. M. Seminars in medicine of the Beth Israel Hospital, Boston. beta-Amyloid and the pathogenesis of Alzheimer's disease. N Engl J Med. 1991 Dec 26;325(26):1849–1857. doi: 10.1056/NEJM199112263252605. [DOI] [PubMed] [Google Scholar]



