Summary
Janus kinases (JAKs) are key effectors in controlling immune responses and maintaining hematopoiesis. SOCS3 (Suppressor of Cytokine Signaling-3) is a major regulator of JAK signaling and here we investigate the molecular basis of its mechanism of action. We found that SOCS3 bound and directly inhibited the catalytic domains of JAK1, JAK2 and TYK2, but not JAK3 via an evolutionarily conserved motif unique to JAKs. Mutation of this motif led to the formation of an active kinase that could not be inhibited by SOCS3. Surprisingly, we found that SOCS3 simultaneously bound JAK and the cytokine receptor to which it is attached, revealing how specificity is generated in SOCS action and explaining why SOCS3 inhibits only a subset of cytokines. Importantly, SOCS3 inhibited JAKs via a non-competitive mechanism, making it a template for the development of specific and effective inhibitors to treat JAK-based immune and proliferative diseases.
Introduction
Both hematopoiesis and the immune response are regulated by the action of cytokines through activation of the Janus kinase-signal transducer and activator of transcription-suppressor of cytokine signaling (JAK-STAT-SOCS) signal transduction pathway (O’Shea and Murray, 2008).
There are four mammalian JAKs (JAK1-3 and TYK2) each consisting of four domains (Figure S1) (Wilks and Harpur, 1994). The N-terminal FERM domain binds constitutively to the appropriate membrane-bound receptor whilst the C-terminal kinase (catalytic) domain phosphorylates substrate proteins. Between these are a non-canonical SH2 domain and a pseudokinase domain, the most distinctive feature of the JAK family. This domain has recently been shown to be catalytically active (Ungureanu et al., 2011) and it regulates the activity of the catalytic domain (Saharinen et al., 2000).
Genetic deletion of each individual JAK leads to various immunological and hematopoietic defects, however aberrant activation of JAKs can be likewise pathological. Three myeloproliferative disorders (Polycythemia Vera, Essential Thrombocythemia and Primary Myelofibrosis) are caused by a single point mutation in JAK2 (JAK2V617F) (James et al., 2005; Levine et al., 2005) which renders the kinase constitutively active and results in cytokine-independent activation of JAK-based signaling pathways. An even more severe phenotype results from activation of JAK by oncogenic fusion, for example TEL-JAK2 which has been studied because of its role in childhood T- and B-cell acute lymphoblastic leukemia. (Lacronique et al., 2000).
In order to prevent aberrant proliferation, JAK activity is regulated in a number of ways. The primary negative regulators of the JAKs are a family of proteins known as the Suppressors of Cytokine Signaling (SOCS) (Endo et al., 1997; Hilton et al., 1998; Naka et al., 1997; Starr et al., 1997) whose expression is induced by JAK-STAT activation and they then inhibit the signaling cascade, creating a negative feedback loop.
All eight SOCS proteins (SOCS1-7 and CIS) contain a central SH2 domain and a C-terminal SOCS box domain (Hilton et al., 1998), which interacts with elongins B and C and Cullin5 to catalyze the ubiquitination of bound signaling proteins (Babon et al., 2009; Kamizono et al., 2001; Zhang et al., 1999). Elegant studies performed by Yoshimura and colleagues (Sasaki et al., 1999; Yasukawa et al., 1999) showed that the two most potent suppressors of signaling, SOCS1 and SOCS3 contain a short motif, upstream of their SH2 domain, known as the KIR (kinase inhibitory region) which allows them to suppress signaling by direct inhibition of JAK catalytic activity. This is their dominant mode-of-action in vivo (Boyle et al., 2007; Zhang et al., 2001). Initial characterization of the KIR noted its amino acid sequence similarity to the activation loop of JAKs (Sasaki et al., 1999; Yasukawa et al., 1999). Like most tyrosine kinases, JAKs contain an activation loop that blocks the catalytic cleft. Autophosphorylation of this loop causes its translocation away from the catalytic site and allows substrate access thus activating the kinase. Consequently, it was proposed that the SH2 domain of SOCS binds the activation loop tyrosine phosphate and the KIR acts as a pseudosubstrate to block the active site (Sasaki et al., 1999; Yasukawa et al., 1999)
Despite the ability of SOCS proteins to bind to and inhibit JAKs, deletion of individual SOCS genes in mice has revealed an exquisite specificity for particular cytokine-receptor combinations rather than specific JAKs. For example SOCS1 inhibits interferon γ signaling without affecting IL-6 signaling while the converse is true for SOCS3 (Alexander et al 1999, Croker et al 2003), yet both cytokine receptor systems utilize the same JAKs (JAK1 and JAK2) (Murray, 2007). Moreover, the binding affinities of the SOCS3 SH2 domain for phosphorylated JAK peptides is several logs lower than that for certain cytokine receptor phosphopeptides and this binding is important in intact cells (Nicholson et al, 2000)
In this study we dissect both the mechanism and specificity of JAK inhibition by SOCS3 using biochemical, structural and kinetic methods and resolve these apparent discrepancies. We show that SOCS3 directly inhibits JAK1, JAK2 and TYK2 but not JAK3 due to the presence of a conserved three-residue motif on the former three JAK family members. By utilizing two distinct binding surfaces, SOCS3 is able to bind to JAK and the cytokine receptor to which it is attached simultaneously, explaining why SOCS3 is specific for cytokines that signal through particular receptors. Intriguingly, inhibition occurs via a mechanism in which SOCS3 does not compete with either ATP or substrate. This makes SOCS3 a non-competitive tyrosine kinase inhibitor and a new template for the future development of a class of small-molecule JAK inhibitors with distinct advantages over the current ATP analogues used to treat JAK-based disease.
Results
SOCS3 inhibits substrate phosphorylation by the kinase domain of JAK2 (JAK2JH1)
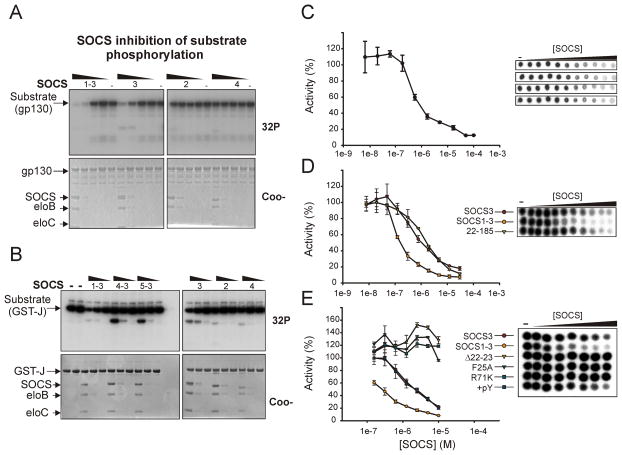
To examine SOCS3 inhibition of JAK kinase activity we developed an in vitro kinase assay consisting of three purified, recombinant components: enzyme (JAK2 catalytic domain, JAK2JH1), substrate (gp130 cytoplasmic domain, gp130cyt) and inhibitor (SOCS3). Various chimeras of SOCS3 were also produced that had the key residues in the KIR, L22-S29, replaced by the corresponding residues of SOCS1, SOCS4 or SOCS5, (these are designated SOCS1-3, SOCS4-3 and SOCS5-3, see Figure S1A). All SOCS proteins were expressed and purified in complex with elongins B and C, their physiological SOCS box ligands, as they provide increased stability and solubility. JAK2JH1 was purified from insect cells. Both SOCS3 and SOCS1-3 inhibited substrate phosphorylation using this system (Figure 1A). Inhibition was not substrate specific as the use of a different substrate, (the activation loop of JAK2 fused to GST, termed GST-J, Figure 1B), led to identical results. In contrast, SOCS2, SOCS4, SOCS4-3 and SOCS5-3 had no effect on phosphorylation of any substrate tested.
Figure 1. SOCS3 and SOCS1-3 inhibit substrate phosphorylation by JAK2.
(A) Autoradiography (upper panel) and Coomassie stained (lower panel) SDS-PAGE analysis of JAK2JH1 catalyzed phosphorylation of the gp130 cytoplasmic domain in the presence of serial 10-fold dilutions of SOCS-elonginBC complexes. Both SOCS3 and a SOCS1-3 chimaera were effective inhibitors of substrate phosphorylation. Gradient bars represent decreasing concentration of each SOCS construct. (10μM, 1μM, 100nM, 10nM, left to right)
(B) As in (A) except a different substrate (GST-J) was used as a substrate. SOCS concentration was 5μM, 1μM, 200nM, left to right in each case.
(C) Quantitative kinase inhibition experiments. As in (A) except reactions were spotted onto nitrocellulose filters. The experiment was performed in quadruplicate, raw data shown above and quantitated below. The control reaction (0 SOCS) was normalized to 100%.
(D) SOCS1-3 is a more effective inhibitor of JAK2 than SOCS3. As in (C) except a STAT5b peptide was used as substrate and the reaction spotted onto P81 phosphocellulose paper. Reactions contained 30, 12, 5, 2, 0.8, 0.3, 0.1, 0.04, 0.02, 0.008, 0 μM SOCS (left to right).
(E) SOCS3 constructs that contained mutations in the KIR (F25A), SH2 domain (R71K) or that lacked the first eight residues of the KIR (Δ22–29) did not inhibit JAK2. As in (D). Reactions contained 10, 5, 2.5, 1.2, 0.6, 0.3, 0.15, 0 μM SOCS (left to right).. The addition of gp130 phosphopeptide (pY) did not affect inhibition. Error bars represent +/− range of two experiments. Data are normalized to no-inhibitor controls
As seen in Figure 1, all SOCS constructs were themselves phosphorylated to some extent in these assays, as was elonginC. This was not unexpected as the isolated kinase domain of JAK2, like that of many tyrosine kinases, shows little substrate specificity and will phosphorylate any tyrosines that are solvent exposed and not part of ordered secondary structure. For example we found a synthetic polymer, poly-Glu4Tyr, to be a good substrate for JAK2JH1 phosphorylation. To verify that phosphorylation of SOCS3 was not by itself the cause of decreased gp130cyt phosphorylation, the entire reaction was spotted onto nitrocellulose membranes, allowing total phosphorylation of all components to be quantified. SOCS3 had a clearly titratable inhibitory effect on JAK-catalyzed phosphorylation with an IC50 of ca. 1μM (Figure 1C).
A limiting feature of these assays was that the concentration of SOCS3 required to inhibit JAK2JH1 was similar to the concentration of substrate. To ensure that it was not a SOCS3-substrate interaction that was responsible for inhibiting the phosphorylation reaction we adopted a more robust enzyme-inhibition assay format where [Substrate]≫[Inhibitor]≫[Enzyme]. These assays used high concentrations of a peptide substrate, residues 693–708 of STAT5b (Saharinen et al., 2003; Zhao et al.). SOCS3 inhibited phosphorylation of this peptide substrate with the same IC50 of ca. 1μM (Figure 1D). These results indicate that SOCS3 functions by blocking the ability of JAK2 to phosphorylate protein substrates and is therefore a direct inhibitor of its catalytic activity.
A SOCS1-SOCS3 chimera is a more potent inhibitor than SOCS3
Replacing the KIR of SOCS3 with the corresponding region from SOCS1 resulted in a chimeric construct (termed SOCS1-3) that inhibited JAK2 kinase activity with higher affinity than values of 0.15 +/− .02 and 1.2 +/− 0.1 μM, respectively, see Figure 1D), did wild-type SOCS3 (IC50 despite there only being four residues difference. A truncated construct of SOCS3, SOCS322–185 that lacks the SOCS box and hence elonginB and C binding was equally effective at inhibiting JAK2. These results show that the kinase inhibitory activity of SOCS3 relies solely upon its KIR and extended SH2 domain and not its SOCS box domain. In addition, the KIR from SOCS1, but not SOCS4 or SOCS5, can act as a functional replacement, making it likely that SOCS1 acts in a similar manner to SOCS3.
Both the KIR and SH2 domain of SOCS3 are required for kinase inhibition but phosphotyrosine binding is not
Previous work had highlighted the importance of F25A within the KIR and R71 within the SH2 domain for SOCS3 function (Nicholson et al., 1999; Sasaki et al., 1999). Here we confirm in vitro that deletion of the first eight residues in the KIR (residues 22–29), or mutagenesis of F25 and R71 completely abrogated inhibition (Figure 1E). The KIR in isolation, as a synthetic peptide, could not inhibit JAK2, even at concentrations 100x the IC50 values of the full-length protein (data not shown). The requirement for R71, which directly binds pTyr, implies that SOCS3 may bind the phosphorylated activation loop of JAK2 as part of its inhibitory mechanism. However, the addition of a known high affinity ligand (KD=100nM) for the SOCS3 SH2 domain, murine gp130750–764 (STASTVEpYSTVVHSG) (Nicholson et al., 2000) at a 5-fold molar excess had no effect on JAK inhibition by SOCS3. In addition we were able to form a ternary complex of JAK2JH1:SOCS3:gp130750–764 containing all three components at a stoichiometric ratio as analyzed by gel filtration and rpHPLC (Figure S1). Therefore, whilst R71 may contact JAK2 when bound, these results indicate that phosphopeptide binding by SOCS3 is undisturbed in the presence of JAK2.
SOCS3 inhibits JAK1, JAK2 and TYK2 but not JAK3 due to the presence of a three-residue (GQM) motif in the JAK insertion loop
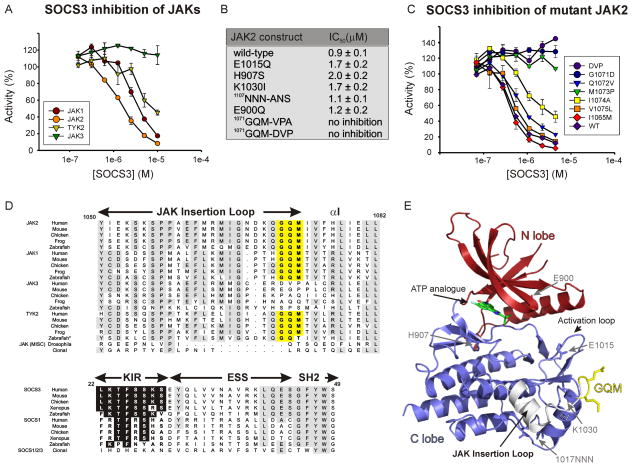
We cloned, expressed and purified the kinase domains of all four JAKs and examined the ability of SOCS3 to inhibit them. SOCS3 inhibited JAK1, JAK2 and TYK2 with IC50 values of 2μM, 1.5μM and 7μM respectively but had no inhibitory effect on JAK3 (Figure 2A).
Figure 2. SOCS3 inhibits JAK1, JAK2 and TYK2 but not JAK3 due to a three residue motif in the JAK insertion loop.
(A) Kinase inhibition assays were performed using the kinase domain of all four JAKs. Only JAK3JH1 was not inhibited by SOCS3. Error bars represent +/− range from two experiments. Data were normalized to no-inhibitor controls
(B) Mutating the GQM motif makes JAK2 non-responsive to SOCS3. All other mutations have no effect.
(C) G1071 and M1073 are absolutely required for SOCS3 inhibition. As in (A) except point mutants of JAK2 were tested.
(D) Sequence alignment of the GQM region of all JAKs and the KIR of SOCS3 and SOCS1. Highly conserved residues are shown boxed in grey, the GQM motif in yellow, conserved residues in the KIR in black.
(E) The structure of JAK2, PDB ID 2B7A. The GQM motif is solvent exposed and shown in yellow. The location of mutated residues from (B) are indicated by grey arrows. *Zebrafish JAK2b is grouped with TYK2 in this figure.
A comparison of the sequence of all four kinase domains highlighted a number of residues that were conserved within JAK1, JAK2 and TYK2 but not JAK3. Therefore, a series of mutant JAK2 molecules were produced that replaced wild-type residue(s) with the corresponding residues from JAK3. All mutant kinases were catalytically active with no significant difference in specific activity. The only mutations to effect SOCS3-mediated inhibition were in a three-residue motif 1071–1073GQM (Figure 2B). Mutating this sequence completely abolished the ability of SOCS3 to inhibit JAK2. Depending on the method of sequence/structure alignment, these three residues correspond to either 1043–1045DVP or 1044–1046VPA in JAK3. Both GQM-DVP and GQM-VPA mutants of JAK2 were insensitive to SOCS3.
The GQM motif forms the final three residues of the JAK insertion loop (residues 1052–1073, Figure 2D), an insertion that is specific to JAKs (Haan et al., 2009; Lucet et al., 2006). More detailed mutagenesis showed that the first and third of these residues, G1071 and M1073, were absolutely required for SOCS3 inhibition, while mutation of the central glutamine, Q1072 had only a minor effect (Figure 2C). Mutation of I1074 also had a small effect on the IC50 of SOCS3. The GQM motif is solvent exposed (Figure 2E) as expected if it forms a direct contact with SOCS3. Whilst the GQM sequence is unlikely to represent the entire binding surface on JAK, this data indicates that it is an essential motif which allows JAK to be inhibited by SOCS3..
The GQM motif of JAK1, JAK2 and TYK2 is conserved in all organisms that contain a SOCS1 or SOCS3 homologue
All organisms from insects to mammals contain at least one JAK with vertebrates typically containing four JAKs. A sequence alignment of JAKs from within these organisms (Fig 2D) showed that the GQM motif is conserved in JAK1, JAK2 and TYK2 from all vertebrate species listed with the exception of zebrafish JAK2b which contains a highly similar motif (GQT) at this position. Equally, none of these organisms contained this motif in JAK3 and the corresponding sequence within this region was not conserved. A sequence comparison of SOCS mirrors this phenomenon. Only vertebrates have SOCS1 and SOCS3 homologues and these all have highly similar kinase inhibitory regions. In contrast, insects contain only SOCS4-7 homologues. In summary, this analysis shows that all organisms that contain an expanded JAK system also encode a SOCS protein with a functional KIR.
Mutating the GQM motif in JAK1 leads to prolonged IL-6 signalling in live cells
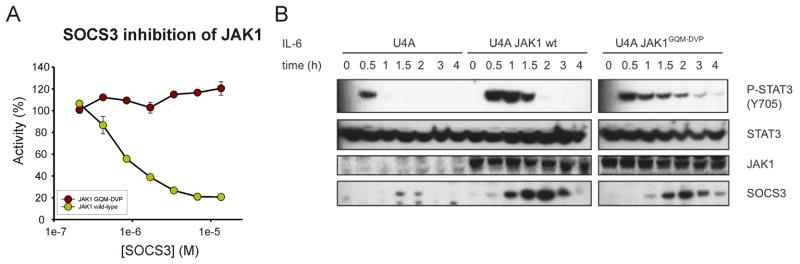
To examine our hypotheses regarding the specificity of SOCS3 action in a physiological setting, we wished to mutate full-length JAK to a form that is impervious to inhibition by SOCS3 and examine the effect this has on IL-6 signalling. As JAK2 is dispensible for IL-6 signalling but JAK1 is not (Murray, 2007), we cloned and expressed JAK1GQM-DVP which we predicted, based on our JAK2 experiments, would be resistant to SOCS inhibition. As shown in Figure 3A, the kinase domain of JAK1 is active in the presence of the GQM-DVP mutation but it cannot be inhibited by SOCS3. We then cloned full-length JAK1WT and JAK1GQM-DVP and transfected these constructs into JAK1−/− human fibrosarcoma (U4A) cells (Guschin et al., 1995). These cells express the gp130 shared co-receptor and hence can be stimulated with a mixture of IL-6 and soluble IL-6Rα (Guschin et al., 1995). As shown in Figure 3B, JAK1GQM-DVP was able to activate STAT3 after IL-6 stimulation, however this activation was prolonged compared to wild-type JAK. pSTAT3 was still detectable four hours post-stimulation in the presence of the ΔGQM mutants compared to only two hours in the presence of WT JAK. These results are identical to those seen in Socs3−/minus; cells which also show a two-fold increase in the persistence of pSTAT3 upon IL-6 stimulation (Croker et al., 2003; Croker et al., 2004) and indicate that SOCS3 inhibition has been completely disrupted in these cells. Collectively, these data demonstrate that the GQM motif is essential for SOCS3 inhibition of JAK, both in vitro and in live cells.
Figure 3. IL-6 induced phosphorylation of STAT3 is prolonged in the presence of JAK1GQM-DVP.
(A) Kinase inhibition assays were performed using the kinase domain of JAK1 and JAK1GQM-DVP. JAK1GQM-DVP was not inhibited by SOCS3. Error bars represent +/− range from two experiments. Data were normalized to no-inhibitor controls.
(B) Wild-type and mutant (GQM-DVP) JAK1 constructs were transfected into JAK1−/− (U4A) cells. Cells were stimulated with IL-6 plus s-IL-6Rα for 15 min and then harvested at the indicated times. Lysates were analyzed by immunoblotting with antibodies specific for phosphorylated STAT3, total STAT3, SOCS3 and JAK1.
NMR analysis reveals that SOCS3 can interact with JAK2 and cytokine receptor simultaneously, via two adjacent binding surfaces
Using NMR, we mapped the surface of SOCS3 that binds to JAK2 by chemical shift perturbation. Both 1H-15N HMQC and 1H-13C HMQC spectra were recorded using 250μM labeled SOCS22–185 +/− 500μM unlabeled JAK2JH1. The gp130 peptide was present in all experiments as it leads to increased solubility of SOCS3 but does not interfere with JAK inhibition (Figure 1).
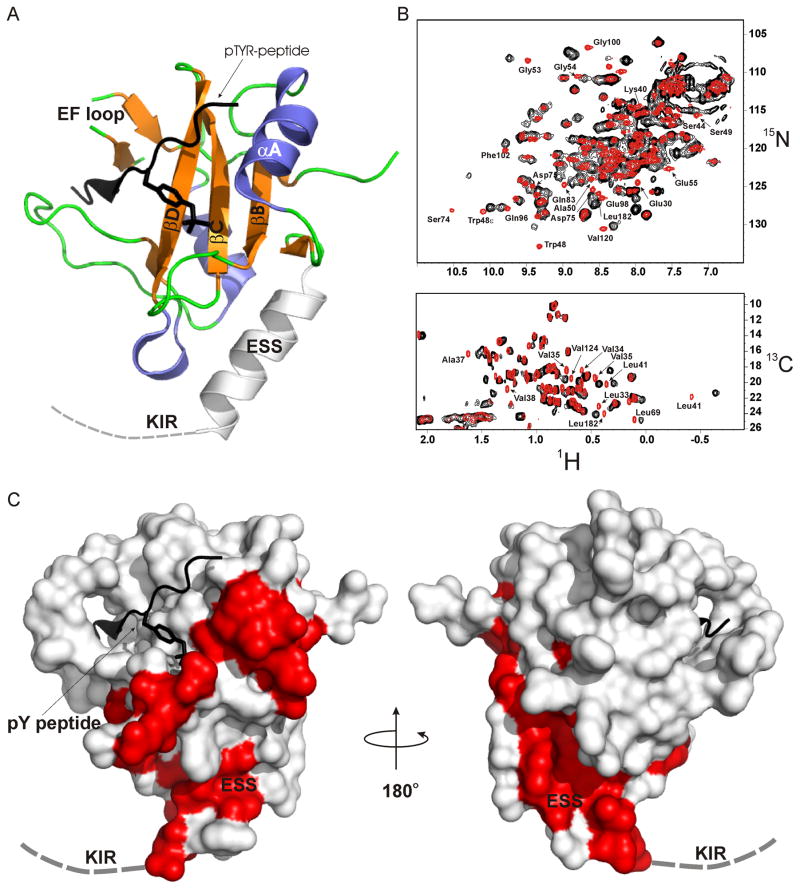
The 15N-HMQC spectrum of SOCS3 was well dispersed with narrow line-widths however the addition of a 2-fold molar excess of unlabelled JAK2 led to intense line broadening and widespread chemical shift perturbation (Figure 4B), consistent with the formation of a 52 kDa SOCS3-JAK2 complex. No line broadening or peak shifts were seen upon addition of JAK2GQM-DVP which is not susceptible to SOCS inhibition (Figure S2A). Likewise, SOCS329–185 which lacks the KIR, showed no interaction with wild-type JAK2 (Figure S2B). The SOCS3-JAK2 complex was in slow exchange and hence could not be assigned, however the surface of SOCS3 that interacts with JAK2 could be mapped by identifying resonances from the SOCS3 spectra that shift when in complex with JAK2. In order to avoid false positives, only non-overlapped peaks that shift by more than one peak-width (0.1 and 0.6 ppm in the 1H and 15N dimensions respectively) were considered in this analysis. A number of residues, including K22-S29 (the first eight residues of the KIR) had to be excluded from analysis on this basis (they are unstructured in the absence of JAK2 and hence their resonances reside in a heavily overlapped region of the spectrum). Nevertheless, 21 backbone and two sidechain amides were identified that shifted in the presence of JAK2. Several of these shifts were large, for example S74 had a chemical shift perturbation of > δAV= 0.67 (δAV=((Δδ1H2 + (Δδ15N/5)2/2)½. Repeating this analysis on the methyl region of 1H-13C-HMQC spectra identified a further 10 residues whose methyl groups shifted in the presence of JAK2. The combination of these data mapped a 30 residue binding surface on SOCS3 (Table S2). This surface is centered on the extended SH2 subdomain (ESS) helix (Figure 4) and includes its junction with the SH2 domain proper, the N-terminal portion of helix αA, the BC loop and the DE loop.
Figure 4. NMR analysis identifies the surface of SOCS3 that interacts with JAK2.
(A) Ribbon diagram of the structure of SOCS322–185 (PDB ID 2HMH) with important secondary structural motifs indicated. The dashed line indicates the first seven residues of the KIR which are unstructured in the absence of JAK.
(B) 1H-15N and 1H-13C HMQC analysis of the SOCS3-JAK2JH1 interaction are shown in upper and lower panels respectively. The SOCS spectra are in red and the SOCS3-JAK2JH1 spectrum in black.
(C) Surface diagram of SOCS3. Residues whose resonances shift in the presence of JAK2JH1 highlighted in red. The orientation of SOCS3 on the left is identical to that in (A).
Of the 10 methyl containing residues identified, six are within the ESS helix and five of these have solvent-exposed sidechains in the unbound state, making it likely they represent part of the true binding surface. The other residue, L41, forms the junction with the SH2 domain and appears to anchor the ESS helix to the core of the SH2 domain by a number of hydrophobic interactions. This residue contains the most upfield shifted resonance in the SOCS3 spectra (δ-CH3 −0.4 ppm) due to ring current effects from Y47, F80 and F102. This shifts even further upfield in the presence of JAK2, suggesting that a subtle conformation change in this region moves the Leu sidechain closer to one of these three aromatic groups.
The mapped interaction surface is adjacent to one end of the pTyr binding groove. Residues that shift upon JAK binding include several (T52-G54) around the pY-2 pocket as well as several in the BC loop (R71-D75), including R71, which forms a salt bridge with pTyr. However, residues that display characteristic chemical shift perturbations when the gp130 peptide is bound (T89, D107, R94, L178) (Babon et al., 2006), maintain these characteristic chemical shift positions in the presence of JAK2. In addition, it is clear from chromatographic analysis that the gp130 phosphopeptide remains bound in the presence of JAK2 (Figure S1).
Taken together, these results indicate that the JAK2 binding surface on SOCS3 borders but does not overlap the phosphotyrosine binding groove. This surface can be defined as consisting of the KIR, ESS helix and the edge of the pTyr binding groove. By binding JAK and specific cytokine receptors (which are already associated with JAK) simultaneously, SOCS3 becomes part of a high affinity ternary complex. A model in which this ternary complex underpins the specificity of SOCS3 (Figure S2) will be discussed.
SOCS3 is a non-competitive inhibitor of JAK2
The model for the mechanism of JAK inhibition by SOCS3 has been that the KIR acts as a pseudosubstrate and thereby blocks access to the active site (Yasukawa et al., 1999). Kinases have two substrates: ATP (the phosphate donor) and a tyrosine-containing substrate (the phosphate acceptor). If SOCS3 acts as a pseudosubstrate then this implies that it will compete with the binding of one or both of these substrates. This can be addressed by performing steady-state enzyme kinetics in the presence of SOCS3 (Cornish-Bowden, 2004).
Kinetic experiments were performed at 25°C, using an enzyme:substrate ratio > 1:1000 (10nM enzyme:0.01–5mM STAT peptide). Under these conditions, product formation was linear with time for >45 minutes, although two timepoints (7.5 and 15 min) were taken in all experiments to ensure this was the case. Results were quantified using scintillation counting and phosphorimaging. When the ATP concentration was varied (0–1mM), the STAT substrate concentration was fixed at 1.6 mM. Conversely, when the STAT peptide concentration was varied (0–2.5mM), the ATP concentration was fixed at 2 mM. JAK2JH1 had KMATP = 140μM and KMpeptide = 0.6mM under these conditions. Initial reaction velocity was plotted against substrate concentration at various concentrations of inhibitor (Figure 5).
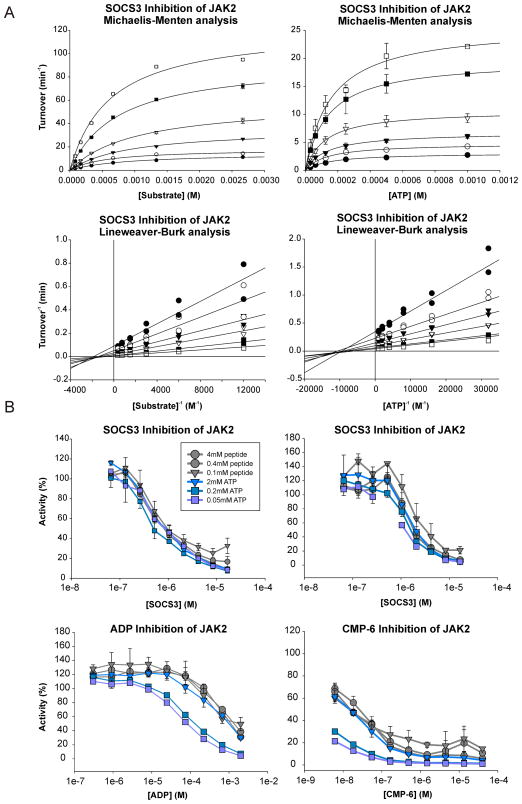
Figure 5. SOCS3 is a non-competitive inhibitor of JAK2.
(A) Michaelis-Menten (upper panels) and Lineweaver-Burk (lower panels) analysis of SOCS3 inhibition of JAK2JH1. ATP (left) and peptide substrate (right) titrations show that SOCS3 alters Vmax but not Km, indicating non-competitive inhibition. Inhibition experiments were performed using 10nM JAK and 0–8μM SOCS3 with varying concentrations of ATP and substrate. Lineweaver-Burk curves that intersect on the abscissa indicate non-competitive inhibition. Error bars represent +/− range from two experiments.
(B) JAK2 inhibition experiments were performed in the presence of SOCS3 (upper panels, 2 independent experiments) or in the presence of ATP competitive inhibitors ADP and CMP-6 (lower panels). The IC50 values of SOCS3 were unaffected by ATP concentration unlike the IC50 values of ADP and CMP-6. Error bars represent +/− range from two experiments. Data are normalized to no-inhibitor controls.
Surprisingly, these analyses showed that SOCS3 is a non-competitive inhibitor of JAK2JH1, with respect to both ATP and substrate. This was apparent by linear least-squares fitting of the data to a mixed inhibition model using Sigmaplot as well as by Lineweaver-Burk reciprocal analyses (Figure 5, lower panels). Lines that intersect on the abscissa indicate non-competitive inhibition. Dixon plot analyses (Dixon, 1953) of these data are shown in Figure S3A. These analyses were performed on three separate occasions, each time in duplicate with different preparations of both enzyme and inhibitor. Fitting of the data yields Ki = 1.5 ± 0.7 μM and 1.2 ± 0.3 μM vs. substrate and ATP, respectively. These results can be contrasted both qualitatively and quantitatively to identical experiments performed using ADP as inhibitor (Figure S3B) which gives rise to the expected ATP-competitive inhibition curves. One attribute of non-competitive inhibition is that the IC50 is not affected by substrate concentration. As shown in Figure 5, SOCS3 inhibited JAK with identical IC50 values at ATP and substrate concentrations that varied by 40-fold. In contrast, the IC50 of the ATP-competitive inhibitors ADP and CMP-6 increased in the presence of high ATP concentration, but not in the presence of high substrate concentration, as expected. Collectively, these results show that SOCS3 is a non-competitive inhibitor of JAK2 and therefore imply that it does not act by blocking the active site of the kinase.
Mechanism of SOCS3 mediated suppression of JAK/STAT signaling
In considering the molecular mechanism of SOCS inhibition of JAK we thought it most likely that SOCS3 was directly inhibiting phosphate transfer. Many kinases have the ability to catalyse the transfer of a phosphate moiety to a water molecule, rather than to tyrosine, thereby acting as an ATPase. We reasoned that if the mode-of-action of SOCS3 is to inhibit phosphate transfer then it should also inhibit phosphate transfer to water and hence the ability of JAK2 to act as an ATPase. Therefore, we measured the ATPase activity of JAK2JH1 in the presence and absence of SOCS3.
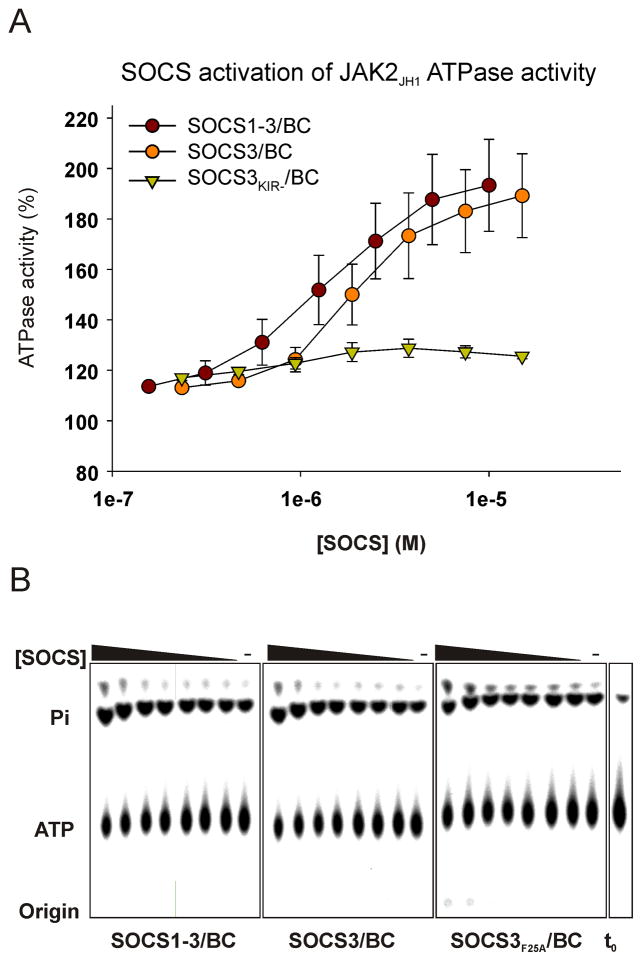
As shown in Figure 6, we unexpectedly observed a small, but reproducible, activation of JAK2 ATPase activity in the presence of SOCS3. SOCS3 and SOCS1-3 stimulated the ATPase activity of JAK2 by nearly 2-fold. SOCS3F25A had no effect. This activity titrated with an apparent EC50 of 2μM. These results indicate that SOCS3 specifically inhibits the ability of JAK to transfer phosphate to tyrosine but does not inhibit its ability to hydrolyse ATP and transfer phosphate to water. Our favored molecular model of inhibition, incorporating this information, will be discussed.
Figure 6. SOCS3 has an activating effect on JAK2 JH1 ATPase activity.
Assays were analyzed by TLC (lower panels) and quantified (upper panel). In control reactions (right hand lane of each panel) JAK2JH1 hydrolysed ATP at 0.05 s−1 under these conditions compared to 0.09 s−1 in the presence of 15μM SOCS3. Error bars: +/− range, two experiments. Data are normalized to no inhibitor controls.
As the rate limiting step of a number of kinases is product (ADP) release (Adams, 2001), we wished to rule out the possibility that SOCS3 may act by stabilizing a JAK-ADP (Enzyme-Product) complex. Such a mechanism implies that JAK would be insensitive to the presence of SOCS3 during the first round of catalysis, when ADP is absent. However, single turnover experiments showed that SOCS3 was still a potent inhibitor of JAK under these conditions (Figure S4A). In addition, we did not observe any synergistic effect when a combination of SOCS3 and ADP were used in standard kinase inhibition experiments (Figure S4B).
Collectively, these results show that ATP is still hydrolyzed by JAK in the presence of SOCS3 and thereby confirm that SOCS3 does not compete with ATP for binding. Therefore, inhibition of JAK by SOCS3 will not be affected by a high intracellular ATP concentration.
DISCUSSION
The prevailing model of SOCS3 action has been that it is recruited to specific cytokine receptors by its SH2 domain and once there would eventually engage JAK using both its SH2 domain and KIR. The SH2 domain would bind the phosphorylated activation loop of JAK whilst the KIR would then block ATP binding (Sasaki et al., 1999). We now show that SOCS3 interacts with both JAK and the gp130 receptor simultaneously by utilizing two adjacent binding surfaces and that ATP binding by JAK is unaffected. Such a mode-of-action explains the specificity of SOCS3 and has important consequences both biologically and therapeutically on a number of fronts as now discussed.
Firstly, the ability of SOCS3 to bind to JAK and simultaneously to the receptor to which it is attached, leads to an unusual ternary complex in which each moiety is directly bound to the other two. For such a ternary complex to dissociate at least two direct interactions must be broken, consequently the overall affinity of such a complex is higher than any of the individual associations. It follows therefore, that cytokines that utilize receptors with SOCS3 binding sites (eg IL-6) will be effectively inhibited by SOCS3 (Wormald et al., 2006), whilst cytokines that signal through receptors that lack such a site (eg IFN-γ) will not, even though they may signal through the same JAKs.
Importantly, we show that whilst SOCS3 can inhibit JAK1, JAK2 and TYK2 in the absence of receptor, it does so with relatively poor (micromolar) affinity. Therefore, when artificially over-expressed, SOCS3 can be predicted to inhibit signaling via any cytokine that utilizes JAK1, JAK2 or TYK2, however when present at physiological levels, SOCS3 will only inhibit cytokines that signal through particular receptors. This has been seen in numerous studies and explains why SOCS3 shows detectable, but greatly impaired, inhibition of JAK-STAT signalling through gp130Y757F compared to wild-type gp130 (Jenkins et al., 2005; Nicholson et al., 2000). Likewise, it has been shown that IFN-γ signaling is only poorly suppressed by SOCS1 when its binding site on the IFN-γ receptor (pTyr441) has been mutated (Starr et al., 2009) and we suggest that SOCS1 and SOCS3 both share this mechanism of receptor dependence to gain high specificity and efficacy towards particular cytokines.
Even in the absence of receptor, SOCS3 is highly specific towards JAKs, rather than other tyrosine kinases. This is highlighted by the fact that it displays selectivity even within the JAK family. Selectivity arises from the fact that SOCS3 interacts with a motif within the JAK insertion loop. By interacting with this region, it is able to specifically target JAKs (over other kinases) and, by targeting the GQM motif, it is able to distinguish JAK1, JAK2 and TYK2 from JAK3. An evolutionary comparison of JAK and SOCS sequences is telling in this regard. Only vertebrates have evolved an expanded JAK system (four JAKs) and it seems they have also evolved the ability to directly and specifically inhibit three of them. Although there may be another protein that functions to inhibit JAK3, it is unlikely to do so via the same mechanism since JAK3 shows no evolutionary conservation in the GQM-equivalent region. No other human kinases contain GQM at this position and therefore SOCS3 would not be expected to directly inhibit any other kinases in the genome. This is exemplified by the fact that, in intact cells, JAK1 becomes unresponsive to SOCS3 if the GQM motif is mutated, even though it remains tethered next to the kinase on the gp130 receptor. This indicates that JAK3, which lacks GQM, will not be inhibited by SOCS3 even if they were to be bound to the same receptor complex.
The SOCS3 binding site on the gp130 receptor, pY757, is also the binding site of the phosphatase SHP-2 which is involved in stimulating the Ras/ERK and PI-3K/Akt signaling pathways (Neel et al., 2003). Mutating this site in mice leads to a Th1-biased immune response, autoimmune arthritis and the development of gastric adenoma due to the combined effect of dysregulating both the JAK-STAT and SHP2-ERK pathways (Ernst and Jenkins, 2004) In this context, our JAKGQM-DVP mutant provides a useful resource as, unlike the gp130Y757F system, only JAK/STAT signaling (and not SHP2-ERK signaling) is dysregulated.
Therapeutically, our data have important consequences as, to our knowledge, SOCS3 is the only biological kinase inhibitor that acts non-competitively as regards both ATP and substrate. Other inhibitors act by competitive mechanisms, either by blocking the active site directly, for example p27KIP1 (Russo et al., 1996) and RKIP (Yeung et al., 1999), or disrupting it allosterically for example JIP (Barr et al., 2004; Heo et al., 2004). By virtue of its non-competitive mechanism, the inhibitory function of SOCS3 is unaffected by high intracellular ATP concentration. Structure-guided drug design has historically targeted the ATP binding site as the most amenable for inhibitor development. All current JAK inhibitors (Ghoreschi et al., 2009) are ATP analogues or competitors and bind to the active site of the enzyme, which has two major drawbacks: (A) ATP in the cell, which can be as high as 10mM, competes with inhibitor binding and leads to reduced efficacy in vivo and (B) the ATP binding site of tyrosine kinases are all structurally similar and thus specificity is difficult to achieve. In contrast, a non-competitive JAK inhibitor would retain its potency in vivo and may take advantage of the greater structural variation in regions outside the ATP binding site to gain greater specificity for JAK over the rest of the kinome (Cozza et al., 2009). As a specific, non-competitive JAK inhibitor that does not bind to the active site of the enzyme, SOCS3 is the ideal template for the development of a new class of therapeutic JAK inhibitors.
Having targeted JAK via both the receptor to which it is attached and its GQM motif, what then is the molecular mechanism of SOCS3? The non-competitive nature of inhibition by SOCS3, and that fact that it does not block phosphate transfer to water, implies that it does not block or destroy the structure of the kinase active site. We propose a model in which SOCS3 binding alters the conformation of JAK in such a way that the distance between the ATP terminal phosphate and the acceptor tyrosine hydroxyl group, or their relative geometry, is affected. The GQM motif is located within 8 Å of the substrate binding site of JAK2 and therefore SOCS3 binding may distort its location. Even a small shift in their relative positions could dramatically affect phosphate transfer from ATP to the tyrosine hydroxyl as these moieties need to be positioned within 3 Å to allow nucleophilic attack within the developing transition state (Madhusudan et al., 1994). Only the structure of a SOCS-JAK complex will enable this hypothesis to be examined.
By regulating cytokine signaling, SOCS3 plays a key role in maintaining the hematopoietic system and controlling the immune response. Our results reveal the basis for both the efficacy and specificity of SOCS3 action and explain how it is able to regulate signaling via a particular subset of cytokines. Finally, our experiments show that unlike all currently available JAK inhibitors, SOCS3 inhibits JAK via a mechanism in which it is not affected by high intracellular ATP levels thereby suggesting it is the ideal template upon which to base the development of a new class of therapeutic JAK inhibitors.
Experimental Procedures
Cloning and Expression
All SOCS3 constructs lack the first 21 amino acids and have the PEST motif (Residues 129–163) replaced by a Gly-Serx4 linker, these modifications enhancing its stability and solubility (Babon et al., 2006; Babon et al., 2005). This parent construct, SOCS322–225ΔPEST, was used as a template for all further mutagenesis and is henceforth referred to as SOCS3. Co-expression and purification of SOCS3 with elongins B and C was as previously described (Babon et al., 2008) Plasmids encoding SOCS2 and SOCS4 were kind gifts of Alex Bullock (SGC, Oxford, U.K). The sequence of all constructs is given in supplemental data.
JAK1, JAK2, JAK3 and TYK2 (kinase domains) were cloned into pFASTBAC (Invitrogen) and expressed as 6xHIS-tagged proteins. JAK2 mutants were produced using oligonucleotide-directed PCR mutagenesis. JAK2 was also expressed as a GST fusion by cloning into pDEST-20 (Invitrogen). All JAK constructs were expressed and purified as previously described (Lucet et al., 2006) except that JAK2JH1 used for NMR analysis was expressed in the presence of 0.4μM of the JAK inhibitor 2-(1,1-Dimethylethyl)-9-fluoro-3,6-dihydro-7H-benz[h]-imidaz[4,5-f]isoquinolin-7-one (EMD biosciences) to increase yield. As we were unable to completely displace this inhibitor following purification, all other assays used JAK expressed in the absence of this inhibitor.
Cloning and over-expression of JAK1 in JAK1−/− cell lines
The JAK1-deficient human fibrosarcoma cell line U4A has been described previously (McKendry, 1991; Guschin, 1995) and was maintained in Dulbecco’s modified Eagle’s medium, supplemented with 10% (v/v) heat-inactivated fetal calf serum and hygromycin (250 μg/mL). The mutation of murine JAK1 resulting in the amino acid mutations GQM>DVP for expression in U4A cells was produced using oligonucleotide directed PCR mutagenesis. Wild-type and mutant JAK1 cDNAs were cloned into the puromycin-resistant plasmid pEF-IRES-P (Hobbs, 1998) and transfected into the U4A cell line using FuGENE HD Transfection Reagent (Roche), according to the manufacturer’s instructions. Stable cell lines over-expressing either wild-type JAK1 or the JAK1GQM-DVP mutant were selected using puromycin (2 μg/mL) and tested for JAK1 expression by Western blot with an antibody toJAK1 (Santa Cruz).
Cytokine stimulation and Western blotting
U4A cells and their derivatives (2 × 106) were plated overnight in 6-well plates and pulsed with 400 ng/mL human recombinant IL-6 and 500 ng/mL sIL-6Rα (R&D Systems, MN) for 15 min. Cells were washed in PBS and lysed for 30 min in 50 μL ice-cold KALB lysis buffer (150 mM NaCl, 50 mM Tris [pH 7.5], 1% [v/v] Triton X-100, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF, 1mM PMSF) containing protease inhibitors (Complete cocktail tablets; Roche). Lysates were cleared by centrifugation (13,000 ×g) for 10 min at 4°C and supernatants boiled in 4 × reducing sample buffer. A 15 μL sample was separated by SDS-PAGE, transferred onto polyvinylidene difluoride membranes (Millipore), and examined for phosphorylated STAT3 (Cell Signalling), total STAT3 (Santa Cruz) and JAK1 expression by Western blot.
JAK2 kinase inhibition assays using protein substrates
1mg/mL protein substrate was incubated with 50nM JAK2JH1 at 25°C for 30 min in 20mM Tris pH 8.0, 100mM NaCl, 1mM DTT, 2mM ATP and 4mM MgCl2 (kinase buffer)and various concentrations of SOCS-elonginBC complexes. 1μCi 32P-γ-ATP was included to allow visualization of phosphorylation via autoradiography and phosphorimaging. Following incubation, the reactions were either boiled and subjected to analysis by SDS-PAGE or terminated with 50mM EDTA and spotted onto a nitrocellulose membrane. Membranes were washed extensively (4×200ml, 15 mins) with PBS and subsequently exposed to a phosphorimager plate (Fuji).
JAK2 kinase inhibition assays using peptide substrates
0–2mM substrate peptide was incubated with 10nM JAK2JH1 at 25°C for 10–20 minutes in kinase buffer and 1μCi 32P-γ-ATP. Following incubation, the reactions were spotted onto P81 phosphocellulose paper and quenched in 5% H3PO4. The paper was washed extensively (4×200ml, 15 mins) with 5% H3PO4 and exposed to a phosphorimager plate (Fuji).
Steady-State Kinetics
Michaelis/Menten analysis requires the use of a high enzyme-to-substrate ratio so that product formation is linearly proportional to time and product inhibition is negligible. Substrate concentration must be ≥ KM to approach saturation and allow accurate determination of Vmax. Therefore, 2nM JAK2JH1 was used to phosphorylate 0–5mM STAT5b peptide in these assays. Inhibitor, SOCS3-elonginBC, was included at 0–10μM final concentration. Reactions were performed in kinase buffer except that both ATP and STAT5b peptide were titrated independently, 0.1 mg/ml BSA and 1μCi 32P-γ-ATP were added at 25°C. 7.5 and 15 min timepoints were used to ensure that product formation was linear with time. When ATP was titrated, STAT5b was at 1.6 mM (~KM), when STAT5b was titrated ATP was at 2mM (~15xKm). Following incubation, the reactions were spotted onto P81 phosphocellulose paper (Millipore) and treated as described in the previous paragraph. Control experiments showed that retention onto P81 paper was linear to > 5mM peptide.
ATPase assays
ATPase assays were performed by incubating 0.25 μM JAK2JH1 in kinase buffer (except that ATP concentration was 0.1mM) and 1μCi 32P-γ-ATP for 30 minutes at 25°C. SOCS3-elonginBC was present in the reaction at 0–10μM concentration. Reactions were stopped with 25mM EDTA, spotted onto PEI-cellulose TLC plates and developed in 1M LiCl, 1M formic acid for 45 minutes, air-dried and exposed to a phosphorimager plate.
NMR
SOFAST-HMQC experiments were recorded at 37°C in 20mM MES, 1mM DTT on a Bruker Avance 600 MHz spectrometer equipped with cryoprobe.
Supplementary Material
Highlights.
SOCS3 inhibits the catalytic activities of JAK1, JAK2 and TYK2, but not JAK3.
A three-residue motif, specific to JAK kinases is crucial for its interaction with SOCS3
SOCS3 binds JAK and cytokine receptor simultaneously.
SOCS3 is a non-competitive JAK inhibitor
Acknowledgments
We thank S. Yao for assistance with NMR, A. Bullock for plasmids, J-G. Zhang for recombinant IL-6 and the ACRF Biomolecular Resource Facility for sequencing. We are grateful to P. Hertzog for the gift of U4A cells. This work was supported in part by the National Health and Medical Research Council of Australia (program grant nos. 461269 and 487922 and project grant no. 1011804), the U.S. National Institutes of Health, USA (grant no. CA22556), the Victorian State Government Operational Infrastructure Support Grant and the NHMRC Independent Research Institutes Infrastructure Support Scheme (361646). J.J.B., R.S.N. and N.A.N. acknowledge fellowship support from the National Health and Medical Research Council, J.M.M from the Australian Research Council. L.N.V acknowledges scholarship support from the Leukaemia Foundation of Australia and the Australian Stem Cell Centre.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JA. Kinetic and catalytic mechanisms of protein kinases. Chem Rev. 2001;101:2271–2290. doi: 10.1021/cr000230w. [DOI] [PubMed] [Google Scholar]
- Babon JJ, McManus EJ, Yao S, DeSouza DP, Mielke LA, Sprigg NS, Willson TA, Hilton DJ, Nicola NA, Baca M, et al. The structure of SOCS3 reveals the basis of the extended SH2 domain function and identifies an unstructured insertion that regulates stability. Mol Cell. 2006;22:205–216. doi: 10.1016/j.molcel.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Babon JJ, Sabo JK, Soetopo A, Yao S, Bailey MF, Zhang JG, Nicola NA, Norton RS. The SOCS box domain of SOCS3: structure and interaction with the elonginBC-cullin5 ubiquitin ligase. J Mol Biol. 2008;381:928–940. doi: 10.1016/j.jmb.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babon JJ, Sabo JK, Zhang JG, Nicola NA, Norton RS. The SOCS box encodes a hierarchy of affinities for Cullin5: implications for ubiquitin ligase formation and cytokine signalling suppression. J Mol Biol. 2009;387:162–174. doi: 10.1016/j.jmb.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babon JJ, Yao S, DeSouza DP, Harrison CF, Fabri LJ, Liepinsh E, Scrofani SD, Baca M, Norton RS. Secondary structure assignment of mouse SOCS3 by NMR defines the domain boundaries and identifies an unstructured insertion in the SH2 domain. Febs J. 2005;272:6120–6130. doi: 10.1111/j.1742-4658.2005.05010.x. [DOI] [PubMed] [Google Scholar]
- Barr RK, Boehm I, Attwood PV, Watt PM, Bogoyevitch MA. The critical features and the mechanism of inhibition of a kinase interaction motif-based peptide inhibitor of JNK. J Biol Chem. 2004;279:36327–36338. doi: 10.1074/jbc.M402181200. [DOI] [PubMed] [Google Scholar]
- Boyle K, Egan P, Rakar S, Willson TA, Wicks IP, Metcalf D, Hilton DJ, Nicola NA, Alexander WS, Roberts AW, Robb L. The SOCS box of suppressor of cytokine signaling-3 contributes to the control of G-CSF responsiveness in vivo. Blood. 2007;110:1466–1474. doi: 10.1182/blood-2007-03-079178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozza G, Bortolato A, Menta E, Cavalletti E, Spinelli S, Moro S. ATP non-competitive Ser/Thr kinase inhibitors as potential anticancer agents. Anticancer Agents Med Chem. 2009;9:778–786. doi: 10.2174/187152009789056930. [DOI] [PubMed] [Google Scholar]
- Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- Croker BA, Metcalf D, Robb L, Wei W, Mifsud S, DiRago L, Cluse LA, Sutherland KD, Hartley L, Williams E, et al. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20:153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- Dixon M. The determination of enzyme inhibitor constants. Biochem J. 1953;55:170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- Ernst M, Jenkins BJ. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 2004;20:23–32. doi: 10.1016/j.tig.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, O’Shea JJ. Selectivity and therapeutic inhibition of kinases: to be or not to be? Nat Immunol. 2009;10:356–360. doi: 10.1038/ni.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan C, Kroy DC, Wuller S, Sommer U, Nocker T, Rolvering C, Behrmann I, Heinrich PC, Haan S. An unusual insertion in Jak2 is crucial for kinase activity and differentially affects cytokine responses. J Immunol. 2009;182:2969–2977. doi: 10.4049/jimmunol.0800572. [DOI] [PubMed] [Google Scholar]
- Heo YS, Kim SK, Seo CI, Kim YK, Sung BJ, Lee HS, Lee JI, Park SY, Kim JH, Hwang KY, et al. Structural basis for the selective inhibition of JNK1 by the scaffolding protein JIP1 and SP600125. Embo J. 2004;23:2185–2195. doi: 10.1038/sj.emboj.7600212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton DJ, Richardson RT, Alexander WS, Viney EM, Willson TA, Sprigg NS, Starr R, Nicholson SE, Metcalf D, Nicola NA. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci U S A. 1998;95:114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- Jenkins BJ, Roberts AW, Najdovska M, Grail D, Ernst M. The threshold of gp130-dependent STAT3 signaling is critical for normal regulation of hematopoiesis. Blood. 2005;105:3512–3520. doi: 10.1182/blood-2004-09-3751. [DOI] [PubMed] [Google Scholar]
- Kamizono S, Hanada T, Yasukawa H, Minoguchi S, Kato R, Minoguchi M, Hattori K, Hatakeyama S, Yada M, Morita S, et al. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J Biol Chem. 2001;276:12530–12538. doi: 10.1074/jbc.M010074200. [DOI] [PubMed] [Google Scholar]
- Lacronique V, Boureux A, Monni R, Dumon S, Mauchauffe M, Mayeux P, Gouilleux F, Berger R, Gisselbrecht S, Ghysdael J, Bernard OA. Transforming properties of chimeric TEL-JAK proteins in Ba/F3 cells. Blood. 2000;95:2076–2083. [PubMed] [Google Scholar]
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Lucet IS, Fantino E, Styles M, Bamert R, Patel O, Broughton SE, Walter M, Burns CJ, Treutlein H, Wilks AF, Rossjohn J. The structural basis of Janus kinase 2 inhibition by a potent and specific pan-Janus kinase inhibitor. Blood. 2006;107:176–183. doi: 10.1182/blood-2005-06-2413. Epub 2005 Sep 2020. [DOI] [PubMed] [Google Scholar]
- Madhusudan, Trafny EA, Xuong NH, Adams JA, Ten Eyck LF, Taylor SS, Sowadski JM. cAMP-dependent protein kinase: crystallographic insights into substrate recognition and phosphotransfer. Protein Sci. 1994;3:176–187. doi: 10.1002/pro.5560030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, et al. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Nicholson SE, De Souza D, Fabri LJ, Corbin J, Willson TA, Zhang JG, Silva A, Asimakis M, Farley A, Nash AD, et al. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc Natl Acad Sci U S A. 2000;97:6493–6498. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson SE, Willson TA, Farley A, Starr R, Zhang JG, Baca M, Alexander WS, Metcalf D, Hilton DJ, Nicola NA. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. Embo J. 1999;18:375–385. doi: 10.1093/emboj/18.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo AA, Jeffrey PD, Patten AK, Massague J, Pavletich NP. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- Saharinen P, Takaluoma K, Silvennoinen O. Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol Cell Biol. 2000;20:3387–3395. doi: 10.1128/mcb.20.10.3387-3395.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen P, Vihinen M, Silvennoinen O. Autoinhibition of Jak2 tyrosine kinase is dependent on specific regions in its pseudokinase domain. Mol Biol Cell. 2003;14:1448–1459. doi: 10.1091/mbc.E02-06-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Yasukawa H, Suzuki A, Kamizono S, Syoda T, Kinjyo I, Sasaki M, Johnston JA, Yoshimura A. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells. 1999;4:339–351. doi: 10.1046/j.1365-2443.1999.00263.x. [DOI] [PubMed] [Google Scholar]
- Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- Ungureanu D, Wu J, Pekkala T, Niranjan Y, Young C, Jensen ON, Xu CF, Neubert TA, Skoda RC, Hubbard SR, Silvennoinen O. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat Struct Mol Biol. 2011;18:971–6. doi: 10.1038/nsmb.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks AF, Harpur AG. Cytokine signal transduction and the JAK family of protein tyrosine kinases. Bioessays. 1994;16:313–320. doi: 10.1002/bies.950160505. [DOI] [PubMed] [Google Scholar]
- Wormald S, Zhang JG, Krebs DL, Mielke LA, Silver J, Alexander WS, Speed TP, Nicola NA, Hilton DJ. The comparative roles of suppressor of cytokine signaling-1 and -3 in the inhibition and desensitization of cytokine signaling. J Biol Chem. 2006;281:11135–11143. doi: 10.1074/jbc.M509595200. [DOI] [PubMed] [Google Scholar]
- Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, Wakioka T, Ohtsuka S, Imaizumi T, Matsuda T, Ihle JN, Yoshimura A. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. Embo J. 1999;18:1309–1320. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, et al. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature. 1999;401:173–177. doi: 10.1038/43686. [DOI] [PubMed] [Google Scholar]
- Zhang JG, Farley A, Nicholson SE, Willson TA, Zugaro LM, Simpson RJ, Moritz RL, Cary D, Richardson R, Hausmann G, et al. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci U S A. 1999;96:2071–2076. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JG, Metcalf D, Rakar S, Asimakis M, Greenhalgh CJ, Willson TA, Starr R, Nicholson SE, Carter W, Alexander WS, et al. The SOCS box of suppressor of cytokine signaling-1 is important for inhibition of cytokine action in vivo. Proc Natl Acad Sci U S A. 2001;98:13261–13265. doi: 10.1073/pnas.231486498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Ma Y, Seemann J, Huang LJ. A regulating role of the JAK2 FERM domain in hyperactivation of JAK2(V617F) Biochem J. 426:91–98. doi: 10.1042/BJ20090615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.