Abstract
One of the goals of cell-based immune therapy in cancer is the induction of tumor-specific cytotoxic T-lymphocyte (CTL) responses. To achieve this objective, the ability of dendritic cells (DC) to cross-present tumor antigens can be exploited. One of the most efficient pathways for the induction of CTLs by cross-presentation is mediated by immunoglobulins of the IgG class, which are used by DCs to sample antigen in the form of immune complexes via Fc-gamma receptors. Could DCs use an IgE-mediated cross-presentation mechanism in a comparable manner to induce CTLs? We here discuss the potential of two human IgE Fc receptors, FcεRI and FcεRII, to serve as antigen uptake receptors for IgE-mediated cross-presentation. We conclude that the existence of an IgE-mediated cross-presentation pathway would provide a direct link between IgE-driven immune responses and CTL activity.
Keywords: AllergoOncology, Dendritic cells, Antigen presentation, Fc receptors, FcεRI, CD23
Immunological consequences of antigen cross-presentation
Cross-presentation is one of the mechanisms of the adaptive immune system that ensures efficient immune surveillance of tumor antigens, but is also involved in anti-viral and anti-bacterial immunity [1]. This pathway of antigen presentation allows for the generation of MHC class I-restricted CD8+ T cells in response to exogenous antigen, commonly referred to as the induction of cytotoxic T-lymphocyte (CTL) responses [2, 3]. Cross-presentation occurs in parallel to the classical pathway of antigen presentation for exogenous antigen, which induces MHC class II-restricted T-cell responses of the CD4+ T-helper phenotype. Accumulating evidence suggests that CD4+ T-cell responses also play a role in anti-tumor immunity, but the common consensus is that induction of effective CTL responses is the more central event for tumor rejection [4].
The immunological consequences of a cross-presentation event, however, are not restricted to the induction of CTLs. An alternative outcome to the cross-priming of cytotoxic T-cell responses is the induction of cross-tolerance [3]. Cross-tolerance is an important mechanism for the deletion of auto-reactive T cells, but, consequently, CTLs with tumor specificity could be deleted rather than activated via antigen cross-presentation. What are thus the minimal requirements for an IgE-mediated cross-presentation event to efficiently induce CTLs and avoid cross-tolerance?
Cross-presentation as an efficient pathway of CTL induction
The immunological outcome of a cross-presentation event is defined by the type of antigen-presenting cell (APC), the nature of the antigen, and the antigen uptake pathway used for antigen sampling of the extracellular space [1, 3, 5, 6]. A large number of cell types, including professional and non-professional APCs, have been described as cross-presenters for the induction of CD8+ T-cell responses in vitro. Dendritic cells (DCs), however, are undoubtedly the most potent cross-presenting APCs for the induction of CTLs in vivo. Even among DCs, not all subsets are equally capable of performing cross-presentation. CD8+ murine DCs are superior cross-presenters of soluble, bacterial, and viral antigen as well as apoptotic bodies [1, 5]. If the antigen is delivered in the form of an IgG immune complex, the CD8− DC subset acquires cross-presenting abilities [7, 8]. Based on the unique antigen presentation capacities of DCs, it is fair to assume that an IgE-mediated cross-presentation event would induce CTLs most potently if initiated by this cell type [4].
Furthermore, the nature of an antigen defines the antigen uptake mechanism used by DCs and directs the antigen into its antigen-loading compartment [9]. Antigen-loading compartments of DCs are specialized to promote antigen cross-presentation, because their degradative environment favors generation of peptides for loading on MHC complexes rather than their destruction [10, 11]. Several antigen uptake receptors that efficiently shuttle antigen for cross-presentation have been defined, among them DEC-205 [12], the mannose receptor [13] and the family of Fc-gamma receptors (FcγR), which are considered the most potent uptake receptors for the induction of cross-presentation [14]. All known FcγRs are equally potent in promoting cross-presentation [15], and the IgG-mediated cross-presentation pathway requires antigen to be present in the form of an IgG immune complex [7, 8, 16]. In principle, Fc-epsilon receptors (FcεRs) should also be able to use IgE-mediated antigen uptake to initiate cross-presentation comparable to the IgG-mediated FcγR-dependent pathway [17]. The requirement for the efficient induction of CTLs during an IgE-driven immune response via cross-presentation would thus be that DCs express FcεRs for IgE-mediated antigen sampling.
FcεRs as antigen uptake structures
The two major IgE Fc receptors, FcεRI and FcεRII [18], are potential candidates for antigen uptake in the context of IgE-mediated cross-presentation. FcεRII, the low-affinity IgE receptor or CD23, was considered the only IgE-binding structure on DCs for a long time. At present, however, it is well established that FcεRI, the high-affinity IgE receptor, is constitutively expressed on human DCs [19, 20]. It is important to note that murine DCs do not express FcεRI at the cell surface, but an inducible form of FcεRI has been described in response to Sendai virus infection [21] and house dust mite allergens [22]. The third human IgE receptor, galectin 3, is a secretory protein that does not exist in a transmembrane form [23]. Thus, galectin 3 by itself cannot be considered an antigen sampling receptor. It is, however, conceivable that galectin 3 uses one of its binding partners at the surface of DCs to indirectly facilitate IgE-mediated antigen uptake and presentation.
FcεRI, the high-affinity IgE receptor, is a member of the immunoglobulin receptor superfamily, like the FcγRs and the IgA-Fc receptor CD89 [24, 25]. The trimeric isoform of FcεRI is the main IgE-binding structure on human DCs in vivo [26]. In contrast to the tetrameric isoform of FcεRI, which is a key structure in immediate type allergic responses and expressed by mast cells and basophils, trimeric FcεRI lacks the beta-chain [19, 20]. Several types of non-professional APCs, among them epithelial cells [27], platelets [28], and neurons [29], have been described to also express trimeric FcεRI. The FcεRI trimer is formed by the classical IgE-binding alpha-chain and a dimer of the common gamma-chain. The latter signal-transducing unit is shared with many other receptors, among them the cell surface FcγRs that facilitate IgG-mediated cross-presentation. This structural feature of FcεRI supports speculations about this receptor being able to shuttle antigen for IgE-mediated cross-presentation comparable to FcγRs, because both types of Fc receptors use ITAM-signals through the common gamma-chain for cell activation.
Surface expression of FcεRI is stabilized by monovalent ligation of the alpha-chain with IgE. Using the binding of its natural ligand to regulate receptor expression levels as well as to prolong IgE stability is a unique characteristic of IgE and FcεRI. This feature clearly distinguishes the IgE-FcεRI system from mechanisms used by IgG and FcγRs to regulate receptor stability as described in a comparative study with FcεRI and FcγRIIIa [30]. Antigen-dependent crosslinking of IgE-loaded FcεRI induces internalization and trafficking of the receptor into endo/lysosomal compartments [20, 31]. Even though the mechanisms of receptor stabilization and internalization are different between IgE and IgG-mediated uptake receptors, the antigen-loading compartment targeted by both uptake pathways is similar, supporting the speculation that an IgE-mediated antigen sampling could induce CTLs.
FcεRII, or CD23, initially was called the low-affinity IgE receptor. It is a member of the C-type lectin superfamily [18]. CD23 has a broad cellular expression pattern and is also found on the cell surface of DCs. This receptor interacts with IgE via its large extracellular globular C-type lectin domain and can form homotrimers via its stalk region. Describing CD23 as having low affinity for IgE is actually misleading because oligomers of CD23 can bind IgE with equal affinity as FcεRI [32]. CD23 has been described as an antigen uptake receptor that can promote MHC class II-restricted antigen presentation [33, 34]. It is currently not entirely clear whether both IgE Fc receptors collaborate to facilitate IgE-mediated immune activation by DCs in humans. Since CD23 is expressed on DCs and can shuttle IgE-antigen complexes into endo/lysosomal compartments, this receptor also meets the minimal requirements to facilitate IgE-mediated cross-presentation.
In summary, we here conclude that, in humans, both major IgE Fc receptors are expressed on DCs and could serve as antigen uptake structures for shuttling antigen into cross-presentation loading compartments. What is the evidence that this pathway is active in vivo in humans?
IgE-mediated antigen cross-presentation and the induction of CTL responses in vivo
Evidence for an IgE-mediated cross-presentation pathway as a prominent pathway for the induction of CTL responses is scarce, but inflammatory CD8+ T-cell responses have been described in the context of airway hyper-responsiveness (AHR) [35]. In a murine model of ovalbumin-induced AHR, it was demonstrated that CD8-deficient mice develop significantly lower AHR and showed diminished eosinophilic inflammation. Additionally, IL-13 levels in bronchoalveolar lavage fluid were decreased when compared with wild-type mice. All of these responses were restored by adoptive transfer of antigen-primed CD8+ T cells [35].
In general, antigen presentation research in allergy mostly focuses on understanding the role of IgE for the induction of CD4+ T-cell responses and TH2-type cytokine profiles, as this type of T-cell response typifies allergic immune responses. It is therefore conceivable that the prevalence of CD8+/CTL responses as well as their importance for the pathophysiology of delayed type and chronic allergic responses is currently underappreciated. Nonetheless, IgE-mediated antigen cross-presentation as delineated in this comment provides an elegant explanation for the induction of CD8+ T-cell responses in allergy.
Our tools to study mechanistic details of antigen presentation in humans are limited. Contrastingly in murine models, the experimental settings to study MHC class I-restricted antigen-specific cross-presentation are well established. Why has IgE-mediated cross-presentation been missed so far? With regard to IgE-mediated cross-presentation via FcεRI-mediated antigen uptake, the most likely explanation is that species differences in the receptor expression pattern were hiding the phenomenon. Murine DCs do not constitutively express trimeric FcεRI and therefore cannot use this receptor to shuttle exogenous antigen for IgE-mediated classical MHC-II presentation or for cross-presentation. Only recently, the role of FcεRI as an antigen uptake structure for the induction of TH2-type CD4+ T cells was demonstrated with a transgenic animal model that mimics the human expression pattern of the receptor on DCs [36]. Using DCs from this FcεRI-transgenic animal, we were able to demonstrate that FcεRI is indeed a receptor for an IgE-mediated cross-presentation pathway. With the model antigen ovalbumin, we showed that IgE-mediated cross-presentation via FcεRI induces proliferation of OT-I T cells as well as the production of granzyme B (Platzer et al. manuscript under review). If the antigen sampled by the DCs in an IgE-mediated pathway was not soluble but was rather presented to the cell in the form of an IgE immune complex, CD23 might be able to capture the antigen and induce cross-presentation. An alternative explanation as to why IgE-mediated antigen presentation via CD23 has so far not been described might thus be that so far no research has been performed in that direction.
Summary and perspectives
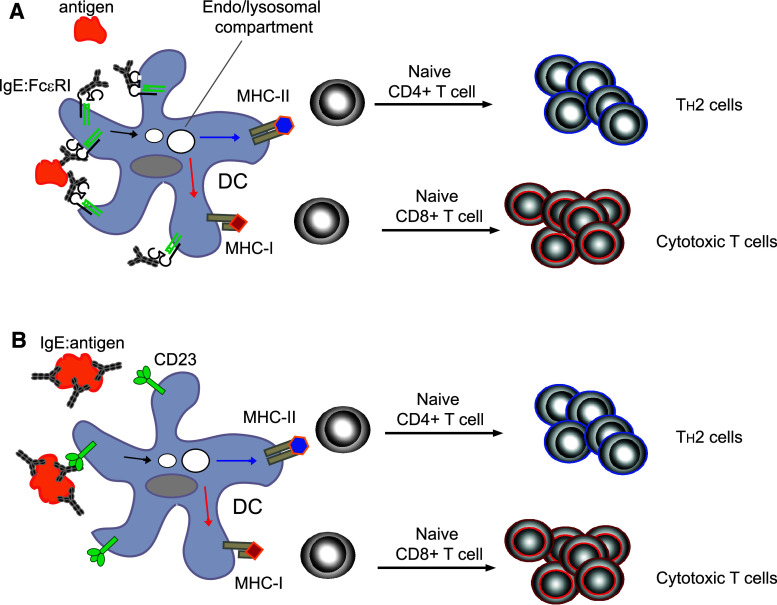
We here speculate that DCs could use FcεR-dependent antigen uptake pathways to promote IgE-mediated cross-presentation comparable to IgG-mediated cross-presentation, and thus link IgE-mediated immune activation to the induction of CTL responses (Fig. 1). The existence of an IgE-mediated cross-presentation pathway not only provides an elegant mechanistic explanation for CD8+ T-cell responses described in allergy but would also support the emerging field of AllergoOncology that claims an important role for IgE-mediated immunity during tumor responses [37]. Pertinent questions to be addressed at this point are: (1) how does IgE-mediated antigen cross-presentation work? and (2) how prevalent is this type of immune activation during the induction of CTLs in humans? The IgG-mediated pathway of cross-presentation is studied for its potential to induce specific CTL responses with a high relevance for cell-based cancer therapy [38]. Antigen targeting via IgE and eliciting CTLs via the IgE-mediated cross-presentation pathway might be an equally attractive therapeutic approach. However, it is important to keep in mind that the outcome of an IgE-mediated cross-presentation event potentially ranges from the induction of CTLs to the induction of tolerance [3]. In light of this broad spectrum of physiological consequences of an IgE-mediated cross-presentation event, it is of outmost importance to develop a better understanding of this pathway of cross-presentation.
Fig. 1.
Dendritic cells induce IgE-mediated immune responses after FcεR-mediated antigen sampling. a FcεRI is stabilized at the cell surface by ligation with monomeric IgE. Soluble antigen induces crosslinking of FcεRI pre-loaded with IgE. Consequently, antigen is shuttled to endo/lysosomal compartments where antigenic peptides are generated and loaded onto MHC class II and MHC class I molecules. The immunological consequences of this antigen presentation event can thus include the generation of CD4+ T helper cells with a TH2 phenotype via the classical presentation pathway as well as the generation of CD8+ cytotoxic T cells via a cross-presentation pathway. b Dendritic cells likely use CD23 to sense IgE immune complexes, since there is no evidence for CD23 serving as a binding structure for monomeric IgE on this cell type. Antigen in form of an IgE immune complex enters the endo/lysosomal sorting pathway in a manner that is comparable to soluble antigen in the FcεRI-mediated antigen sampling pathway. While responsive to different types of antigen, the immunological outcome of IgE-receptor-mediated antigen uptake could be identical for FcεRI and CD23
Acknowledgments
We apologize to colleagues whose work was not cited in this manuscript due to space limitations. This work is supported by the National Institutes of Health grant AI075037 (to E.F.) and the American Cancer Society Research Scholar Award (to S.J.T).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
This paper is part of the Symposium in Writing: AllergoOncology: The role of Th2 responses in cancer.
References
- 1.Amigorena S, Savina A. Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr Opin Immunol. 2010;22(1):109–117. doi: 10.1016/j.coi.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Bevan MJ. Cross-priming. Nat Immunol. 2006;7(4):363–365. doi: 10.1038/ni0406-363. [DOI] [PubMed] [Google Scholar]
- 3.Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. 2010;10(6):403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 4.Robson NC, Hoves S, Maraskovsky E, Schnurr M. Presentation of tumour antigens by dendritic cells and challenges faced. Curr Opin Immunol. 2010;22(1):137–144. doi: 10.1016/j.coi.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Segura E, Villadangos JA. Antigen presentation by dendritic cells in vivo. Curr Opin Immunol. 2009;21(1):105–110. doi: 10.1016/j.coi.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nat Rev Immunol. 2008;8(8):607–618. doi: 10.1038/nri2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker K, Qiao SW, Kuo TT, Aveson VG, Platzer B, Andersen JT, Sandlie I, Chen Z, de Haar C, Lencer WI, Fiebiger E, Blumberg RS. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc Natl Acad Sci USA. 2011;108(24):9927–9932. doi: 10.1073/pnas.1019037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(−) dendritic cells in vivo. J Exp Med. 2002;196(6):817–827. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgdorf S, Kurts C. Endocytosis mechanisms and the cell biology of antigen presentation. Curr Opin Immunol. 2008;20(1):89–95. doi: 10.1016/j.coi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during cross presentation by dendritic cells. Cell. 2006;126(1):205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 11.van Montfoort N, Camps MG, Khan S, Filippov DV, Weterings JJ, Griffith JM, Geuze HJ, van Hall T, Verbeek JS, Melief CJ, Ossendorp F. Antigen storage compartments in mature dendritic cells facilitate prolonged cytotoxic T lymphocyte cross-priming capacity. Proc Natl Acad Sci USA. 2009;106(16):6730–6735. doi: 10.1073/pnas.0900969106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrimpton RE, Butler M, Morel AS, Eren E, Hue SS, Ritter MA. CD205 (DEC-205): a recognition receptor for apoptotic and necrotic self. Mol Immunol. 2009;46(6):1229–1239. doi: 10.1016/j.molimm.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9(5):558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 14.Amigorena S, Bonnerot C. Fc receptors for IgG and antigen presentation on MHC class I and class II molecules. Semin Immunol. 1999;11(6):385–390. doi: 10.1006/smim.1999.0196. [DOI] [PubMed] [Google Scholar]
- 15.de Jong JM, Schuurhuis DH, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Ossendorp F, Toes RE, Verbeek JS. Murine Fc receptors for IgG are redundant in facilitating presentation of immune complex derived antigen to CD8+ T cells in vivo. Mol Immunol. 2006;43(13):2045–2050. doi: 10.1016/j.molimm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189(2):371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harbers SO, Crocker A, Catalano G, D’Agati V, Jung S, Desai DD, Clynes R. Antibody-enhanced cross-presentation of self antigen breaks T cell tolerance. J Clin Invest. 2007;117(5):1361–1369. doi: 10.1172/JCI29470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8(3):205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 19.Maurer D, Fiebiger E, Ebner C, Reininger B, Fischer GF, Wichlas S, Jouvin M-H, Schmitt-Egenolf M, Kraft D, Kinet J-P, Stingl G. Peripheral blood dendritic cells express FceRI as a complex composed of FceRIa- and FceRIg-chains and can use this receptor for IgE-mediated allergen presentation. J Immunol. 1996;157:607–613. [PubMed] [Google Scholar]
- 20.Maurer D, Fiebiger E, Reininger B, Wolff-Winiski B, Jouvin M-H, Kilgus O, Kinet J-P, Stingl G. Expression of functional high affinity immunoglobulin E receptors (FceRI) on monocytes of atopic individuals. J Exp Med. 1994;179:745–750. doi: 10.1084/jem.179.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grayson MH, Cheung D, Rohlfing MM, Kitchens R, Spiegel DE, Tucker J, Battaile JT, Alevy Y, Yan L, Agapov E, Kim EY, Holtzman MJ. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J Exp Med. 2007;204(11):2759–2769. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207(10):2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platzer B, Ruiter F, van der Mee J, Fiebiger E (2011) Soluble IgE receptors—Elements of the IgE network. Immunol Lett. doi:10.1016/j.imlet.2011.08.004 [DOI] [PMC free article] [PubMed]
- 24.Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 2007;7(5):365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 25.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 26.Maurer D, Stingl G. Immunoglobulin E-binding structures on antigen-presenting cells present in skin and blood. J Invest Dermatol. 1995;104(5):707–710. doi: 10.1111/1523-1747.ep12606958. [DOI] [PubMed] [Google Scholar]
- 27.Untersmayr E, Bises G, Starkl P, Bevins CL, Scheiner O, Boltz-Nitulescu G, Wrba F, Jensen-Jarolim E. The high affinity IgE receptor Fc epsilonRI is expressed by human intestinal epithelial cells. PLoS One. 2010;5(2):e9023. doi: 10.1371/journal.pone.0009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasegawa S, Pawankar R, Suzuki K, Nakahata T, Furukawa S, Okumura K, Ra C. Functional expression of the high affinity receptor for IgE (FcepsilonRI) in human platelets and its’ intracellular expression in human megakaryocytes. Blood. 1999;93(8):2543–2551. [PubMed] [Google Scholar]
- 29.Andoh T, Kuraishi Y. Expression of Fc epsilon receptor I on primary sensory neurons in mice. Neuroreport. 2004;15(13):2029–2031. doi: 10.1097/00001756-200409150-00007. [DOI] [PubMed] [Google Scholar]
- 30.Kubota T, Mukai K, Minegishi Y, Karasuyama H. Different stabilities of the structurally related receptors for IgE and IgG on the cell surface are determined by length of the stalk region in their alpha-chains. J Immunol. 2006;176(11):7008–7014. doi: 10.4049/jimmunol.176.11.7008. [DOI] [PubMed] [Google Scholar]
- 31.Maurer D, Fiebiger E, Reininger B, Ebner C, Petzelbauer P, Shi GP, Chapman HA, Stingl G. Fc epsilon receptor I on dendritic cells delivers IgE-bound multivalent antigens into a cathepsin S-dependent pathway of MHC class II presentation. J Immunol. 1998;161(6):2731–2739. [PubMed] [Google Scholar]
- 32.Kilmon MA, Ghirlando R, Strub MP, Beavil RL, Gould HJ, Conrad DH. Regulation of IgE production requires oligomerization of CD23. J Immunol. 2001;167(6):3139–3145. doi: 10.4049/jimmunol.167.6.3139. [DOI] [PubMed] [Google Scholar]
- 33.Mudde GC, Bheekha R, Bruijnzeel-Koomen CA. Consequences of IgE/CD23-mediated antigen presentation in allergy. Immunol Today. 1995;16(8):380–383. doi: 10.1016/0167-5699(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 34.Santamaria LF, Bheekha R, van Reijsen FC, Perez Soler MT, Suter M, Bruijnzeel-Koomen CA, Mudde GC. Antigen focusing by specific monomeric immunoglobulin E bound to CD23 on Epstein-Barr virus-transformed B cells. Hum Immunol. 1993;37(1):23–30. doi: 10.1016/0198-8859(93)90139-R. [DOI] [PubMed] [Google Scholar]
- 35.Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, Dakhama A, Ott VL, Gelfand EW. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat Med. 2004;10(8):865–869. doi: 10.1038/nm1081. [DOI] [PubMed] [Google Scholar]
- 36.Sallmann E, Reininger B, Brandt S, Duschek N, Hoflehner E, Garner-Spitzer E, Platzer B, Dehlink E, Hammer M, Holcmann M, Oettgen HC, Wiedermann U, Sibilia M, Fiebiger E, Rot A, Maurer D. High-Affinity IgE Receptors on Dendritic Cells Exacerbate Th2-Dependent Inflammation. J Immunol. 2011;187(1):164–171. doi: 10.4049/jimmunol.1003392. [DOI] [PubMed] [Google Scholar]
- 37.Jensen-Jarolim E, Achatz G, Turner MC, Karagiannis S, Legrand F, Capron M, Penichet ML, Rodriguez JA, Siccardi AG, Vangelista L, Riemer AB, Gould H. AllergoOncology: the role of IgE-mediated allergy in cancer. Allergy. 2008;63(10):1255–1266. doi: 10.1111/j.1398-9995.2008.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhodapkar KM, Krasovsky J, Williamson B, Dhodapkar MV. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J Exp Med. 2002;195(1):125–133. doi: 10.1084/jem.20011097. [DOI] [PMC free article] [PubMed] [Google Scholar]