Abstract
Many psychological and physiological studies with simple stimuli have suggested that perceptual learning specifically enhances the response of primary sensory cortex to task-relevant stimuli. The aim of this study was to determine whether auditory discrimination training on complex tasks enhances primary auditory cortex responses to a target sequence relative to non-target and novel sequences. We collected responses from more than 2,000 sites in 31 rats trained on one of six discrimination tasks that differed primarily in the similarity of the target and distractor sequences. Unlike training with simple stimuli, long-term training with complex stimuli did not generate target specific enhancement in any of the groups. Instead, cortical receptive field size decreased, latency decreased, and paired pulse depression decreased in rats trained on the tasks of intermediate difficulty while tasks that were too easy or too difficult either did not alter or degraded cortical responses. These results suggest an inverted-U function relating neural plasticity and task difficulty.
Keywords: task difficulty, sequence learning, cortical plasticity, auditory cortex, operant training
Performance on visual, auditory or somatosensory discrimination tasks improves with practice and is generally specific for the trained stimulus (Ball and Sekuler, 1982; Recanzone et al., 1992a; Ahissar and Hochstein, 1997; Fahle, 1997; Wright et al., 1997; Irvine et al., 2000; Karmarkar and Buonomano, 2003; van Wassenhove and Nagarajan, 2007). In many cases, the improved performance is correlated with expanded cortical maps, receptive field selectivity or improved signal to noise ratio that is stimulus specific (Karni and Sagi, 1991; Recanzone et al., 1992b; Recanzone et al., 1993; Zohary et al., 1994; Elbert et al., 1995; Schoups et al., 2001; Fritz et al., 2003; Rutkowski and Weinberger, 2005) but see (Brown et al., 2004; Ghose, 2004). Motivation is believed to regulate learning and plasticity (Bao et al., 2004; Blake et al., 2006), although previous experiments have shown that plasticity and learning effects in animal models and human individuals can be achieved using passive exposure to stimuli (Dinse et al., 2003; Lotze et al., 2003; Frenkel et al., 2006) but see (Recanzone et al., 1993; Bakin and Weinberger, 1996; Irvine et al., 2000; Bao et al., 2001). Cortical acetylcholine, which is released during operant training, modulates both learning and plasticity (Orsetti et al., 1996; Himmelheber et al., 2000). Repeatedly pairing simple tones with electrical stimulation of the cholinergic nucleus basalis (NB) results in receptive field, map, and temporal plasticity similar to that observed after operant training (Kilgard and Merzenich, 1998b, a; Bao et al., 2004). When a high tone, a low tone, and a noise burst separated by 100 ms (HLN) was paired with NB stimulation, the majority of primary auditory cortex (A1) neurons exhibited response facilitation that was specific to the paired sequence. The observations that exposure to complex stimuli can generate order and interval specific plasticity suggests these forms of plasticity could contribute to learning of natural stimuli (Kilgard and Merzenich, 1998b, 2002). Order and interval specific responses to behaviorally relevant vocalizations in birds, bats, monkeys and ferrets further support this hypothesis (Fitzpatrick et al., 1993; Wang and Kadia, 2001; Gentner and Margoliash, 2003; Schnupp et al., 2006). However, it is not known whether cortical plasticity represents a general strategy for learning complex stimuli in mammals.
In this study, rats were trained to discriminate a target sequence from one or more distractor sequences (Fig. 1) to test the hypotheses that training 1) increases the response to the target stimulus relative to novel stimuli (as observed following operant training with simple stimuli) or 2) results in sequence specific facilitation (as observed after the sequence was paired with NB stimulation).
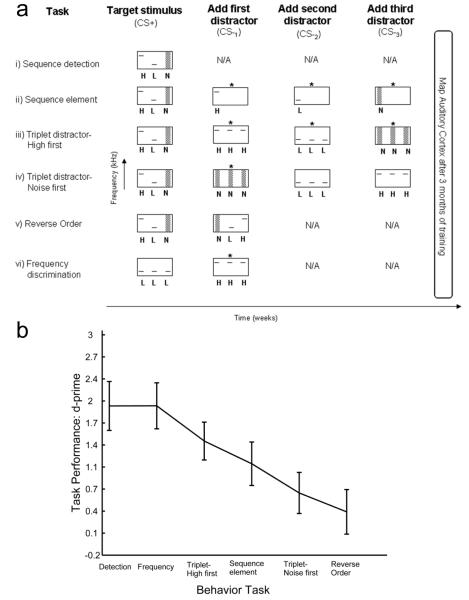
Figure 1.
Schematic of the go/no-go tasks and task performance. (a) Rats were required to lever press in response to the target sequence (CS+) and to withhold from pressing to 0, 1, or 3 distractor sounds (depending on the task). In all but the frequency discrimination task, the target sequence was a rapid High-Low-Noise (HLN) sequence (100 ms stimulus onset asynchrony). (i) Simple HLN detection task. No distractor sounds were presented. (ii) In the sequence element task, the distractors were the high tone, the low tone, and the noise burst elements presented individually. (iii & iv) In the two variants of the triplet distractor task, the three distractor sequences were the same (three high tones, three low tones and three noise bursts), but the order the distractors were added during training was reversed. (v) In the reverse order task, the distractor was the target sequence played in reverse (NLH). (vi) For the frequency discrimination task, rats had to discriminate a target low tone sequence (LLL) from a distractor high tone sequence (HHH). An asterisk (*) over a distractor sound indicates that rats were able to discriminate the sound from the target sound (d-prime above chance). After training, all rats were anesthetized and multi-unit responses were recorded from auditory cortex neurons. (b) Behavioral performance d-primes on the last day of training for each of the tasks (mean ± s.e.m).
Experimental Procedures
Rats were experimentally naïve young adults (200-250 g) at the start of training or sound exposure. They were food deprived for ~12-14 hrs prior to training or exposure. Water was provided ad libitum at all times and rats were maintained at 80-85% of their body weight. Each rat trained for two sessions a day for 1-1½ hours per session, 5 days a week. Rats were housed individually at the Animal Facility at the University of Texas at Dallas and were maintained on a reverse 12-hour light/dark cycle. Constant temperature and humidity were maintained in the rat colony room. Protocols and recording procedures conformed to the Ethical Treatment of Animals (NIH) and were approved by the Institutional Animal Care and Use Committee at the University of Texas at Dallas.
Behavioral training
Thirty-one rats trained on go/no-go operant conditioning tasks for a period of ~3 months (Fig. 1). Rats were trained in an operant cage (8” L × 8” W × 8” H) placed inside a sound-attenuated booth (19” L × 10.5” W × 20” H) in a closed room, and their behavior was monitored on a video monitor outside the room. A light source (house light) was affixed inside the booth and a second light source (cage light) was placed just above a lever inside the cage. Lever press triggered the delivery of a sugar pellet from a dispenser into a receptacle placed inside the cage. Sound stimuli were delivered via a calibrated speaker (Motorola 40-1221, Radio Shack) mounted outside the operant cage and ~10 cm away from the rat’s left ear. Acoustic stimuli were presented at 55 dB SPL. Lever presses and light status were monitored and recorded using custom programs (MATLAB, Mathworks) and Tucker-Davis Technologies hardware and software.
Training involved three stages: an initial shaping stage, detection stage and a discrimination stage. During the shaping stage, lever press triggered the presentation of the target sequence (along with activation of the cage light) followed by the delivery of a food reward. Rats were typically able to master the shaping task within a week. During the detection stage, rats were conditioned to press the lever within 3 s of the onset of the target (CS+) sequence. Pressing the lever at any other time resulted in a 6 s time out signaled by extinguishing the house light. The sequence was delivered every 10 s and was randomly interspersed with silent catch trials to quantify detection performance and achieve good stimulus control. Once rats mastered this task, they were required to discriminate the target sequence from one or more distractor (CS−) sequences. Target sounds, distractor sounds, and silent trials were randomly interleaved and presented every 7-10 seconds. Rats were rewarded for lever presses within 3 s of target sequence onset. There was no punishment for misses. Pressing the lever on a CS− trial resulted in a 6 s time out signaled by extinguishing the house light (false alarm). The house light was dim and extinguishing the light was a punishment to the rat because it signaled that no more sounds would be delivered and thus there was no chance to receive a food reward during the time out period. No reward was provided for correct rejection of distractors. If rats continued to press the lever during the CS− trial or 3 s after the onset of the CS+ trial, the start of the next trial was delayed. If the rat stopped performing the task (missed 5 consecutive target sounds without any lever presses) during a session, the house light was extinguished, a light over the lever was illuminated, and the start of the next trial was delayed until the rat initiated the trial by pressing the lever. Such a break in the task generally only occurred when the rats reached satiety and fell asleep or while the rats were drinking from the water bottle. Each session consisted of ~400-450 trials. All sounds were 25 ms long with 3 ms ramps with a stimulus onset asynchrony of 100 ms. The frequencies for the high (H) and low (L) tones were 12 kHz and 5 kHz, respectively. The noise burst (N) consisted of frozen white noise with a bandwidth of 1.2 – 30 kHz that was generated using SigGen software (Tucker-Davis Technologies).
In addition to the go/no-go tasks, four additional rats trained on an adaptive frequency discrimination task. The target stimulus was a train of 5.66 kHz tones (6 tone bursts, 55dB SPL presented at 5 Hz, each tone was 25 ms in duration), while the distractor stimuli ranged from 6.22 kHz to 8.2 kHz, or from 0.13 to 0.54 octaves away from the target stimulus (compared to the frequency discrimination task in which only a single distractor was presented). Four rats were exposed to sound sequences and the remaining rats (n=7) were age matched experimentally naïve controls. The exposure group heard the sound sequences: HLN, NNN, LLL and HHH. Sound delivery was uncorrelated with the delivery of food pellets. On average, exposure rats heard approximately the same number of sounds and received approximately the same number of pellets as the trained groups, and were exposed to the sequence sounds for a period equivalent to that of the trained groups.
Cortical mapping and stimulus presentation
Within 24 hours of the last training session, each rat was anesthetized with pentobarbital and action potentials were recorded from the right primary auditory cortex in response to tones and tone-noise sequences. The surgical procedures have been described previously (Engineer et al., 2004). Auditory stimuli were delivered from the left side of the rat via a calibrated speaker positioned 10 cm from the left ear in a shielded, double-walled sound attenuating chamber. Frequencies and intensities were calibrated using a B&K sound-level meter. Action potentials were recorded simultaneously from two Parylene-coated tungsten microelectrodes (FHC, 250 mm separation, 2 M). The neural signals were filtered (0.3-8 kHz) and amplified (10,000 times). Cortical responses were recorded at a depth of approximately 550 to 650 μm, corresponding to layers IV/V. Action potential waveforms were recorded whenever a set threshold was exceeded. A detailed map of auditory cortex was generated from 40-70 microelectrode penetrations during a typical mapping experiment. Frequency-intensity tuning curves were derived at each site by presenting a single repetition of 41 logarithmically spaced frequencies from 1 to 32 kHz at 16 intensities from 0 to 75 dB SPL (656 stimuli). All of the sounds used in each of the tasks (HLN, NLH, HHH, LLL, NNN, H, L, and N) were presented twenty times at every site. Eight additional sequence stimuli not presented during training were also presented at each recording site (16 sequence stimuli x 20 repetitions each = 320 total sequence stimuli). The High (H) and Low (L) frequencies were the same as that used during behavioral training. All sounds were separated by 1300 ms and randomly interleaved. The stimulus set took approximately 21 minutes per recording site.
Data Analysis
Behavioral performance was quantified using d-prime, a standard measure based on signal detection theory (Green and Swets, 1966). Paired t-tests were used to compare first and final day performance. Since no behavioral data was collected from the exposure group, the trivial “task” of eating randomly presented pellets was assigned a d-prime of 2.5, which is higher than the easiest task (detection) but not perfect (d’ = 3.0) performance (Recanzone et al., 1992a; Macmillan and Creelman, 2005). The results change little if other values are selected.
Tuning curve parameters were defined by an experienced blind observer using custom software that randomized the order of data from each recording site across all groups. The characteristic frequency (CF) is the frequency that evokes a reliable response at the lowest intensity (response threshold). The borders of A1 were defined based on continuous topography of CF and short response latency. Sites with high thresholds, long latencies, broad tuning, and discontinuities in CF topography were considered non-A1. Criteria for identifying non-A1 sites were subjective and were applied by well-trained, blind observers. Characteristic frequency, bandwidth (BW), response threshold, spontaneous rate, and latency measurements were recorded for each penetration. The receptive field (bandwidth) was characterized at each site and was defined as the range of frequencies that responded to a sound at 10, 20, 30 and 40 dB above neural threshold at an individual site. Post stimulus time histograms (PSTH) were created in response to all tones that were within each site’s receptive field and in response to each acoustic sequence. Peak latency was defined as the time from stimulus onset until the peak in the PSTH.
Neural responses to the stimuli presented during behavior training and several variations of the paired sequence were also included as part of the stimulus set. Twenty repetitions of each of the sequence elements presented alone and in the following combinations were randomly interleaved: HLN (50, 100, 200 ms), NLH (100 ms), LHN (100 ms), LN (100 ms), HN (200ms), LLL (100 ms), HHH (100 ms) and NNN (100 ms). Neural responses were evaluated by documenting the number of spikes to a sequence element in a 25 ms window when preceded by other elements of the sequence compared with the response to the same element in isolation. The suppression index was quantified as 100 times the logarithm base-two of the ratio of the number of spikes in response to a stimulus element in the context of a sequence and the number of spikes in response to the same element in isolation (Kilgard and Merzenich, 2002). A value of zero indicates no facilitation or suppression, while a value of −100 indicates 50% suppression. In all cases, error bars reflect standard error of the mean. For multiple comparisons, one way between groups ANOVA was performed followed by Tukey’s honestly significant difference (HSD) test with significance determined at P < 0.05 to determine specific differences between groups.
Results
Behavioral performance
One group of rats was rewarded for pressing a lever in response to the HLN sequence in the absence of any distractors (Fig. 1a, Task i). The remaining five groups had to respond to a target sequence and withhold responding to non-target sequences. Three groups of rats were trained to discriminate the HLN sequence from three randomly interleaved distractor sequences (Fig. 1a, Task ii-iv; see Experimental Procedures for task description). The distractors were composed of the same sequence elements as the target. In the sequence element task (Fig. 1a, Task ii), the distractors were the individual high tone (H), the low tone (L), and the noise burst (N) stimuli. In the triplet distractor tasks (Fig. 1a, Tasks iii and iv), rats were trained to discriminate the HLN sequence from sequences of three high tones (HHH), three low tones (LLL), and three noise bursts (NNN). The HHH distractor was added first for one group (Fig. 1a, Task iii - high first) and the NNN was added first for another group (Fig. 1a, Task iv - noise first), but the target and the final set of distractors were identical in both groups. In the reverse order task (Fig. 1a, Task v), the distractor was identical to the target played in reverse (NLH). The sixth group of rats trained on a frequency discrimination task (LLL vs. HHH, Fig. 1a, Task vi).
Rats rapidly learned to respond to the target sequence (Figure 2, black lines). While rats were able to easily reject the distractor if it differed in frequency, they had more difficulty rejecting the distractors that were composed of the same elements as the target sequence. For the triplet distractor groups (Fig. 1a, Tasks iii & iv), the order of triplet distractor addition during training had a significant influence on behavioral performance. When HHH was the first distractor added, rats learned to ignore all three distractor sequences by the end of training. When NNN was the first distractor, rats learned to ignore this sequence, but did not learn to ignore the two tonal distractors added subsequently. Performance on the sequence element discrimination was not significantly different from the triplet distractor groups. Finally, the reverse order (NLH) distractor group was unable to acquire the task even after many weeks of training. Performance measures (d-prime) for all trained groups on the last day of training are shown in Figure 1b.
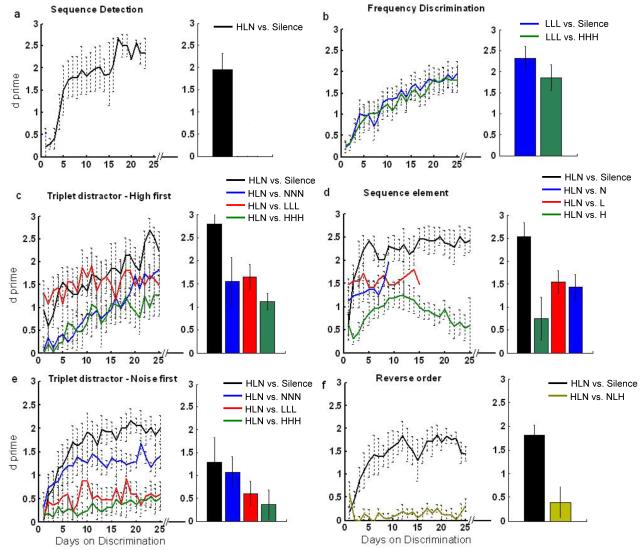
Figure 2.
Detection and discrimination performance. (a) All groups learned not to lever press during silent periods during the first few days of training and continued to correctly reject these catch trials up to the last day of training. The black line represents discrimination between the target sequence and silent catch trials. The bar graphs to the right of each panel represent the average performance on the last day of training. (b&c) The frequency discrimination and high first triplet distractor groups learned to correctly reject each of the distractors presented (p < 0.0001). (d) By the last day of training, the sequence element group was able to correctly reject the noise and the low tone (p < 0.0001), but not the high tone (p > 0.05). (e) The noise first triplet group learned to correctly reject the noise triplet (p < 0.0001), but not the two tone triplets (p > 0.05). (f) The reverse order group was unable to reject the reversed sequence (p > 0.05). Since additional distractors were added at different times during training depending on individual performance, the horizontal axis represents the number of days of training on each distractor. The time course for the sounds was shifted to better compare performance time course across the different sounds. Rats on each task trained for the same total length of time (~ 3 months). Error bars represent standard error of the mean for each group of trained rats. Statistical analyses were paired t-tests between first vs. last day of training.
Plasticity in primary auditory cortex
To test for target specific enhancement and sequence specific facilitation, we quantified multi-unit responses from A1 neurons in trained rats and compared them to responses from naïve rats and from a group of rats passively exposed to the sequences (see Experimental Procedures). We obtained responses from 57.3±16 (mean ± s.t.d) A1 sites from each barbiturate anesthetized rat (n=42 rats). Analysis of variance (ANOVA) revealed that discrimination training with complex sequences did not lead to target specific enhancement or stimulus-specific facilitation (frequency, combination, order, or interval specific) of cortical responses. The response to the target HLN sequence was enhanced in trained groups compared to naïve controls (HLN: F (5, 1694) = 11.49, MS=46.1, p < 0.000001). In tasks with LLL, HHH and NNN as distractors (High first and Noise first groups), enhanced responses to each of these sequences was observed compared to controls (Figure 3). After frequency discrimination training, responses to both the target (LLL) and distractor (HHH) were enhanced compared to controls (LLL: 2.2±0.1 vs. 1.8±0.1 spikes, p<0.01, HHH: 1.9±0.1 vs. 1.3±0.08, p <0.000001). For the reverse order group, responses to the target (HLN) and distractor (NLH) were enhanced compared to controls (HLN: 2.4±0.09 vs. 2.9±0.1 spikes, p<0.01, NLH: 2.4±0.09 vs. 2.8±0.1, p <0.001). We also quantified responses to novel sequences (sequences played during the acute recording sessions but not used in the training) in each of the trained rats. For all trained rats, except the Reverse order group, NLH was the novel sequence. For the reverse order group, HHH was used as the novel sequence. The response to NLH was enhanced in trained groups compared to controls (Figure 3, Column 5 (black trace); F (5, 1809) = 8.35, MS=34.8, p < 0.000001). The response to HHH was also enhanced after reverse order discrimination training compared to controls (Student’s t-test, p < 0.000001). Although no group exhibited plasticity that was specific to the target (or distractor) sequence, significant group differences were observed in neural responsiveness and frequency tuning.
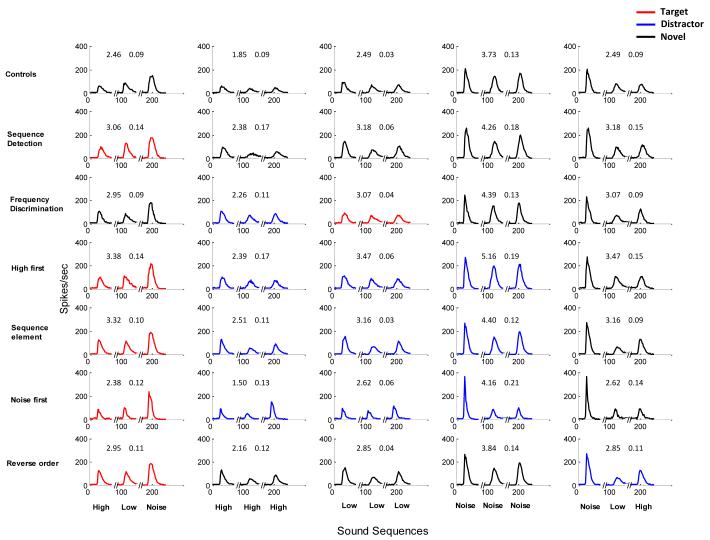
Figure 3.
A1 responses from trained and control rats show absence of target specific enhancement. Population PSTHs for control (Row 1) and trained (Rows 2-7) rats show absence of target specific enhancement. Each column represents the sequences HLN, HHH, LLL, NNN and NLH. HLN was the target (red) sequence in all groups except the frequency discrimination group. Distractor and novel sequences are shown in blue and black respectively. Response to the target sequence (HLN: Column 1, red) was enhanced for trained groups compared to controls. After frequency discrimination training, responses to both the target (LLL) and distractor (HHH) sequences increased compared to controls. In the reverse order discrimination task, response to both target (HLN) and distractor (NLH) increased compared to naïve controls. Rats that trained to discriminate the target HLN sequence from 3 distractor sequences (High first and Noise first groups) also had increased responses to both the target and each of the distractors. For all groups, except the Reverse order group, NLH was the novel sequence used in the analysis (Column 5, black). Responses to this novel sequence also increased significantly in all trained rats compared to controls. Numbers within each subplot represent mean spikes/sequence (± s.e.m).
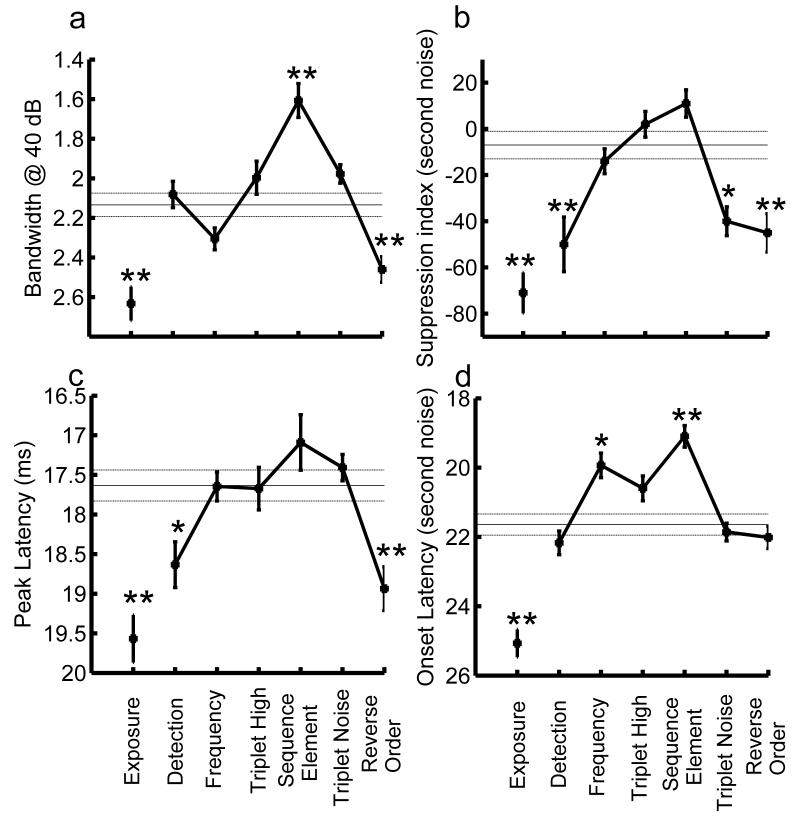
Sequence training generated plasticity in cortical receptive field size, paired-pulse depression, and response latency that appeared to be improved as an inverted-U function of task difficulty. Smaller receptive field size, greater suppression index, and faster responses are associated with improved sensory processing and are thus plotted as higher on the ordinate of Figure 4. An ANOVA followed by Tukey’s HSD post-hoc test showed that receptive field size increased by 15% after training on the difficult (reverse order) task (p<0.01) while it decreased by 23% after training on the sequence element task compared to controls (p<0.00001, Fig. 4a). Receptive field size was not significantly different from controls after training on the easy detection or frequency discrimination tasks (p > 0.05). Similarly, the degree of paired pulse depression (PPD) increased (increase in suppression index, see Experimental Procedures) in rats trained on the simple detection and difficult reverse order task (p<0.01). The intermediate sequence element task and the high triplet task (in which rats were able to discriminate the CS− sequences from the CS+ sequence) had less suppression compared to the simple detection and difficult reverse order discrimination tasks (p<0.01, Fig. 4b). Peak latency was increased by one ms in rats trained on the reverse order task compared to naïve controls (p<0.01, Fig. 4c). As seen previously, the intermediate sequence element task and the high first triplet task had shorter latencies compared to the reverse order group (p < 0.05). Finally, training on the intermediate sequence element task decreased the onset latency to the second of two noise bursts by 2 ms compared to controls (p < 0.00001, Fig. 4d). Compared to naïve controls, rats that were passively exposed to sound sequences (see Experimental Procedures) had larger receptive fields, more paired pulse depression, and longer latencies (p<0.00001, Fig. 4a-d). Training on any of the discrimination tasks except the most difficult (reverse order) task resulted in sharper receptive fields and faster response latencies compared to passive exposure controls (Fig. 4a-d). These plastic changes in A1 appear to follow an inverted U-shaped function with the greatest improvements after training on tasks of intermediate difficulty.
Figure 4.
Mean receptive field size, paired pulse depression and latency, for A1 recordings from each group compared to naïve controls (horizontal solid and dashed lines, mean ± s.e.m) (a) Frequency bandwidth: Training on the reverse order task increased bandwidth at 40 db above threshold while training on the sequence element task decreased bandwidth compared to controls. (b) Paired pulse suppression: Responses to the second of two noise bursts separated by 100 ms were strongly suppressed in rats trained on the easy task (detection) and the two most difficult tasks. (c) The time to peak response to tones was significantly lengthened compared to controls in rats trained on the easy (detection) and difficult (reverse order) task. (d) Onset latencies in response to the second element of two noise bursts (separated by 100 ms) were shorter for the sequence element task but not significantly different from controls for the easy and difficult tasks. The exposure group had significantly greater frequency bandwidth, paired pulse depression and longer latencies compared to naïve and trained rats. Symbols denote significant differences (ANOVA with post-hoc Tukey, * p < 0.05, ** p < 0.01) compared to naïve rats. Error bars represent standard error of the mean (s.e.m). The y-axes in panels a, c and d are reversed so that improvements in frequency selectivity and response latency are represented at the top of the figure. For clarity, the groups are ordered along the x axis by task difficulty.
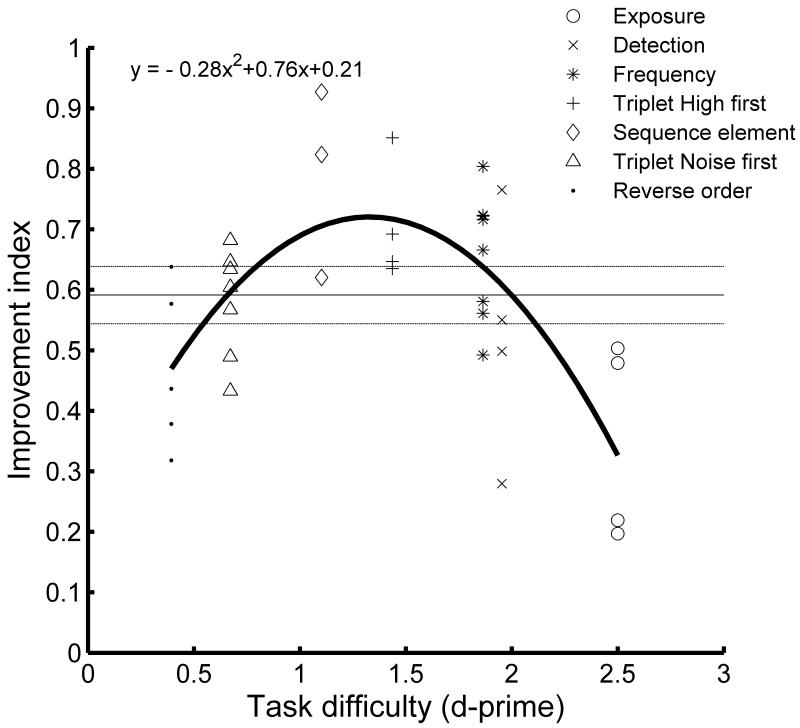
To formalize the relationship between cortical refinement and task difficulty, we developed a single measure of relative cortical precision that includes each of the measures discussed above (receptive field size, response latency and response suppression). The mean value of each parameter was linearly scaled from 0 to 1 for each rat. A value of 1 denotes the rat with the shortest latency, sharpest tuning, or least suppression of all 43 rats tested. A value of 0 indicates that rat had the longest latency, broadest tuning, or most suppression. The improvement index was simply the average of these measures for each rat. Rats trained on the intermediate difficulty tasks had significantly higher improvement indices than the sound exposure group. Rats trained on the two hardest tasks (noise first triplet and reverse order discrimination) and the easiest tasks (sequence detection) were not significantly different. The relationship between cortical refinement (improvement index) and task difficulty (average d-prime) was best fit by a second order polynomial regression curve (Fig. 5, F (2, 32) =14.2, MSE = 0.01, p < 0.0001). F-values were lower when more degrees of freedom (3-6) were used. We obtained very similar results using a Gaussian fit (R2 of 0.48) compared to the second order polynomial curve (R2 of 0.47). Each of the individual parameters in Figure 4 was also well fit by an inverted U function (F> 3.5). To directly test our hypothesis that intermediate task difficulty stimulates maximal cortical plasticity on both simple and complex tasks, we trained rats on an adaptive frequency discrimination task (See Experimental Procedures). For this task, d-prime performance for the most difficult distractor was an average of 0.25±0.04, while performance on the easiest distractor was 2.36±0.07. The average d-prime between the target stimulus and all distractors was 1.21 ± 0.06, indicating that the discrimination task was intermediate in difficulty. Interestingly, this was the only group in which training significantly increased the percent of A1 neurons that responded to the target tone (5.6 kHz) compared to controls (46.8% vs. 23.5%, p<0.001, Student’s t-test).
Figure 5.
Relationship between cortical responses and task difficulty. For each rat, the mean values of the response properties shown in figure 4a-d were linearly scaled from 0 to 1 such that a value of 1 denotes the rat with the shortest latency, sharpest tuning, or least suppression whereas a value of 0 was given to the rat with the longest latency, broadest tuning, or most suppression. The improvement index is the average of these four values. The relationship between the improvement index and task performance was best fit by a second order polynomial regression curve which was highly significant (F (2, 35) =14.2, MSE = 0.01, p < 0.0001). Each dot represents a single rat. The horizontal black line and dotted lines are the mean index and standard error of the mean for naïve rats.
Unlike training with simple stimuli, long term training with complex stimuli that were spectrally and temporally distributed did not specifically enhance cortical responses to the target. While this study was not designed to evaluate whether other cortical fields might exhibit stimulus-specific plasticity, the current results indicate that training with complex stimuli does not result in sequence specific plasticity in A1, even though such plasticity was observed in A1 after NB stimulation was repeatedly paired with HLN (Kilgard and Merzenich, 2002). However, our results reveal a form of generalized refinement of cortical responses that is induced by tasks that are challenging, but doable. Specifically, we observed improved response latency, greater frequency selectivity and reduced suppression in rats trained on intermediate difficulty tasks compared to rats trained on easy and difficult tasks. These results indicate that long-term sequence training results in auditory cortex plasticity that is task-specific but not stimulus-specific.
Discussion
Our results show that optimal plasticity was obtained in primary auditory cortex when the task was neither too easy nor too difficulty. The observation that task difficulty has a significant effect on plasticity may explain discrepancies in earlier studies of cortical map plasticity evoked by training with simple stimuli. Several weeks of frequency discrimination training increased the A1 response to the trained tones in monkeys (Recanzone et al., 1993), but not in cats (Brown et al., 2004). While species specific differences in cortical plasticity mechanisms are possible, the result could also be explained by the greater difficulty of the task used in the monkey study. Training rats on a difficult frequency discrimination task generates map plasticity (Polley et al., 2006) that was not seen when we trained rats on an easy frequency discrimination task (Fig.1, Task vi). These results support our hypothesis that task difficulty influences cortical plasticity. Collectively, these results extend earlier studies by showing that task difficulty regulates plasticity after training with both simple and complex stimuli.
Previous studies have shown that discrimination learning can be modulated by task difficulty (Ahissar and Hochstein, 1997; Amitay et al., 2006; Atiani et al., 2009; Bieszczad and Weinberger, 2010a). Both arousal and attention are modulated by task difficulty and are known to regulate plasticity in primary and non-primary sensory cortex (Spitzer et al., 1988; Ahissar et al., 1992; Irvine et al., 2000; Boudreau et al., 2006). An inverted U-shaped function relates neuromodulator release and task performance (Aston-Jones et al., 1999). While an easy operant task results in little acetylcholine release during performance, a difficult sustained attention task increases acetylcholine release during weeks of daily training (Orsetti et al., 1996; Arnold et al., 2002). The level of arousal is also related to task performance by an inverted U-shaped function (Yerkes and Dodson, 1908). Our results could be explained by the optimal neuromodulatory release and/or cortical signal-to-noise ratio produced by challenging tasks, compared to trivial or impossible tasks.
Plasticity after behavior training can be modulated by several different factors, including task difficulty and the motivation level of the subjects trained to perform the task. For example, Weinberger and colleagues have observed that rats performing a simple tone detection task can develop expanded representation of the conditioned tone if they are water deprived to a weight of 85%, but not if they insufficiently water deprived (90-95%, (Rutkowski and Weinberger, 2005)) or are excessively water deprived (70%, (Bieszczad and Weinberger, 2010b)). These findings also support the hypothesis that plasticity after learning develops under conditions of optimal neuromodulator release.
In the current study all animals trained for approximately three months. It is possible that longer periods of training would have led to improved performance on the trained discrimination task (Recanzone et al., 1993), although longer periods of training do not always result in greater plasticity (Reed et al., 2011). However, because in the current study both the duration of training and motivation level (ie, body weight caused by food deprivation) were similar across all groups, we infer that differences in task difficulty were responsible for the differences in plasticity that we observed.
Neural responses in primary auditory cortex during behavior are affected by the difficulty of a discrimination task. Atiani and colleagues demonstrated that detection of tones embedded in noise leads to enhanced responses to the target and suppression of the distracting background noise. These effects were enhanced when the tone was more difficult to detect and when animals were more aroused (Atiani et al., 2009). We have demonstrated that task difficulty altered the degree of plastic changes after behavior training. These findings suggest that conditions that encourage the brain to optimize responses during behavior lead to optimal long-term cortical plasticity.
Cortical responses recorded in this study were likely influenced by the use of anesthesia since auditory cortex has long been known to be affected by pharmacologic agents. Our earlier study on anesthesia effects in rat primary auditory cortex indicated that stimuli presented at rates from 15-30 Hz were particularly reduced by general ketamine anesthesia (Rennaker et al., 2007). As noted in the Experimental Procedures, the functional data reported in this study were recorded from rats anesthetized with pentobarbital. All rats in this study received the same anaesthetic, and the effects of anesthesia were held constant across groups. However, we and others have found that temporal response characteristics recorded under anesthesia have been well correlated with auditory perceptual behavior (Engineer et al., 2008; Shetake et al., 2011). We have recently shown that the spatiotemporal activity patterns in A1 predict behavioral performance using both awake and anesthetized preparations (Engineer et al., 2008). The speech stimuli used in the experiment were both spectrally and temporally complex, and this complexity was preserved in the anesthetized response patterns. While we acknowledge that the results from this study may not have fully detailed how training on the various sequence tasks reshaped primary auditory cortex, it represents part of an ongoing attempt to fully document some of the principles of sensory reorganization in the auditory modality. It will be important to confirm our hypothesis by recording responses while rats are awake and behaving.
An inverted U-shaped function may explain clinical observations that rehabilitation with easy tasks does not generate optimal recovery. For example, training on an unskilled motor task generates less plasticity than training on a skilled motor task (Kleim et al., 1998; Plautz et al., 2000). This suggests that stroke rehabilitation focused on relearning skilled tasks would generate more plasticity than simply performing a trivial task. Neurological rehabilitation will likely benefit from greater knowledge of the relationship between task difficulty and cortical plasticity (Merzenich et al., 1996; Nudo and Friel, 1999). Additional behavioral, pharmacological, and imaging studies are needed to develop novel therapies that more effectively stimulate plasticity to aid recovery from brain injury.
Tone sequence discrimination does not generate sequence response enhancement
Neural responses improved for intermediate but not easy or difficult tasks
An inverted- U function relates neural plasticity and task difficulty
Acknowledgements
We thank Allison Tessmer, Cathy Hauptstueck, Rafael Carrasco, Cherri Whang, Claudia Perez, Pei-Lan Kan and Chris Heydrick for help with behavior training. We thank Srikantan Nagarajan and Henry Mahncke for their constructive criticism of the manuscript. This work was supported by research grants from the NIH/NIDCD (R03-DC04534 and R15-DC006624).
Abbreviations
- NB
nucleus basalis
- A1
primary auditory cortex
- PSTH
post stimulus time histogram
- PPD
paired pulse depression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahissar E, Vaadia E, Ahissar M, Bergman H, Arieli A, Abeles M. Dependence of cortical plasticity on correlated activity of single neurons and on behavioral context. Science. 1992;257:1412–1415. doi: 10.1126/science.1529342. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- Amitay S, Irwin A, Moore DR. Discrimination learning induced by training with identical stimuli. Nat Neurosci. 2006;9:1446–1448. doi: 10.1038/nn1787. [DOI] [PubMed] [Google Scholar]
- Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience. 2002;114:451–460. doi: 10.1016/s0306-4522(02)00292-0. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Atiani S, Elhilali M, David SV, Fritz JB, Shamma SA. Task difficulty and performance induce diverse adaptive patterns in gain and shape of primary auditory cortical receptive fields. Neuron. 2009;61:467–480. doi: 10.1016/j.neuron.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci U S A. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Sekuler R. A specific and enduring improvement in visual motion discrimination. Science. 1982;218:697–698. doi: 10.1126/science.7134968. [DOI] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci. 2004;7:974–981. doi: 10.1038/nn1293. [DOI] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM. Representational gain in cortical area underlies increase of memory strength. Proc Natl Acad Sci U S A. 2010a;107:3793–3798. doi: 10.1073/pnas.1000159107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM. Learning strategy trumps motivational level in determining learning-induced auditory cortical plasticity. Neurobiol Learn Mem. 2010b;93:229–239. doi: 10.1016/j.nlm.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Heiser MA, Caywood M, Merzenich MM. Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron. 2006;52:371–381. doi: 10.1016/j.neuron.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau CE, Williford TH, Maunsell JH. Effects of task difficulty and target likelihood in area V4 of macaque monkeys. J Neurophysiol. 2006;96:2377–2387. doi: 10.1152/jn.01072.2005. [DOI] [PubMed] [Google Scholar]
- Brown M, Irvine DR, Park VN. Perceptual learning on an auditory frequency discrimination task by cats: association with changes in primary auditory cortex. Cereb Cortex. 2004;14:952–965. doi: 10.1093/cercor/bhh056. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. Pharmacological modulation of perceptual learning and associated cortical reorganization. Science. 2003;301:91–94. doi: 10.1126/science.1085423. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- Engineer CT, Perez CA, Chen YH, Carraway RS, Reed AC, Shetake JA, Jakkamsetti V, Chang KQ, Kilgard MP. Cortical activity patterns predict speech discrimination ability. Nat Neurosci. 2008;11:603–608. doi: 10.1038/nn.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer ND, Percaccio CR, Pandya PK, Moucha R, Rathbun DL, Kilgard MP. Environmental enrichment improves response strength, threshold, selectivity, and latency of auditory cortex neurons. J Neurophysiol. 2004;92:73–82. doi: 10.1152/jn.00059.2004. [DOI] [PubMed] [Google Scholar]
- Fahle M. Specificity of learning curvature, orientation, and vernier discriminations. Vision Res. 1997;37:1885–1895. doi: 10.1016/s0042-6989(96)00308-2. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Kanwal JS, Butman JA, Suga N. Combination-sensitive neurons in the primary auditory cortex of the mustached bat. J Neurosci. 1993;13:931–940. doi: 10.1523/JNEUROSCI.13-03-00931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel MY, Sawtell NB, Diogo AC, Yoon B, Neve RL, Bear MF. Instructive effect of visual experience in mouse visual cortex. Neuron. 2006;51:339–349. doi: 10.1016/j.neuron.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Margoliash D. Neuronal populations and single cells representing learned auditory objects. Nature. 2003;424:669–674. doi: 10.1038/nature01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose GM. Learning in mammalian sensory cortex. Curr Opin Neurobiol. 2004;14:513–518. doi: 10.1016/j.conb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. Wiley; 1966. [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP. Increases in cortical acetylcholine release during sustained attention performance in rats. Brain Res Cogn Brain Res. 2000;9:313–325. doi: 10.1016/s0926-6410(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Irvine DR, Martin RL, Klimkeit E, Smith R. Specificity of perceptual learning in a frequency discrimination task. J Acoust Soc Am. 2000;108:2964–2968. doi: 10.1121/1.1323465. [DOI] [PubMed] [Google Scholar]
- Karmarkar UR, Buonomano DV. Temporal specificity of perceptual learning in an auditory discrimination task. Learn Mem. 2003;10:141–147. doi: 10.1101/lm.55503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc Natl Acad Sci U S A. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998a;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998b;1:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Order-sensitive plasticity in adult primary auditory cortex. Proc Natl Acad Sci U S A. 2002;99:3205–3209. doi: 10.1073/pnas.261705198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- Lotze M, Braun C, Birbaumer N, Anders S, Cohen LG. Motor learning elicited by voluntary drive. Brain. 2003;126:866–872. doi: 10.1093/brain/awg079. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: A user’s guide. 2nd Edition Lawrence Erlbaum Associates Publishers; Mahwah, NJ: 2005. [Google Scholar]
- Merzenich MM, Jenkins WM, Johnston P, Schreiner C, Miller SL, Tallal P. Temporal processing deficits of language-learning impaired children ameliorated by training. Science. 1996;271:77–81. doi: 10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Friel KM. Cortical plasticity after stroke: implications for rehabilitation. Rev Neurol (Paris) 1999;155:713–717. [PubMed] [Google Scholar]
- Orsetti M, Casamenti F, Pepeu G. Enhanced acetylcholine release in the hippocampus and cortex during acquisition of an operant behavior. Brain Res. 1996;724:89–96. doi: 10.1016/0006-8993(96)00292-2. [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000;74:27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Jenkins WM, Hradek GT, Merzenich MM. Progressive improvement in discriminative abilities in adult owl monkeys performing a tactile frequency discrimination task. J Neurophysiol. 1992a;67:1015–1030. doi: 10.1152/jn.1992.67.5.1015. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992b;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V, Kilgard MP. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70:121–131. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Rennaker RL, Carey HL, Anderson SE, Sloan AM, Kilgard MP. Anesthesia suppresses nonsynchronous responses to repetitive broadband stimuli. Neuroscience. 2007;145:357–369. doi: 10.1016/j.neuroscience.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci U S A. 2005;102:13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnupp JW, Hall TM, Kokelaar RF, Ahmed B. Plasticity of temporal pattern codes for vocalization stimuli in primary auditory cortex. J Neurosci. 2006;26:4785–4795. doi: 10.1523/JNEUROSCI.4330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoups A, Vogels R, Qian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- Shetake JA, Wolf JT, Cheung RJ, Engineer CT, Ram SK, Kilgard MP. Cortical activity patterns predict robust speech discrimination ability in noise. Eur J Neurosci. 2011 doi: 10.1111/j.1460-9568.2011.07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer H, Desimone R, Moran J. Increased attention enhances both behavioral and neuronal performance. Science. 1988;240:338–340. doi: 10.1126/science.3353728. [DOI] [PubMed] [Google Scholar]
- van Wassenhove V, Nagarajan SS. Auditory cortical plasticity in learning to discriminate modulation rate. J Neurosci. 2007;27:2663–2672. doi: 10.1523/JNEUROSCI.4844-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kadia SC. Differential representation of species-specific primate vocalizations in the auditory cortices of marmoset and cat. J Neurophysiol. 2001;86:2616–2620. doi: 10.1152/jn.2001.86.5.2616. [DOI] [PubMed] [Google Scholar]
- Wright BA, Buonomano DV, Mahncke HW, Merzenich MM. Learning and generalization of auditory temporal-interval discrimination in humans. J Neurosci. 1997;17:3956–3963. doi: 10.1523/JNEUROSCI.17-10-03956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. The Journal of Comparative Neurology and Psychology. 1908;18:459–482. [Google Scholar]
- Zohary E, Celebrini S, Britten KH, Newsome WT. Neuronal plasticity that underlies improvement in perceptual performance. Science. 1994;263:1289–1292. doi: 10.1126/science.8122114. [DOI] [PubMed] [Google Scholar]