Abstract
Background
ABO incompatible (ABOi) kidney transplantation is an important modality to facilitate living donor transplant for incompatible pairs. To date, reports of the outcomes from this practice in the United States have been limited to single-center studies.
Methods
Using the Scientific Registry of Transplant Recipients, we identified 738 patients who underwent live-donor ABOi kidney transplantation between January 1, 1995 and March 31, 2010. These were compared with matched controls that underwent ABO compatible (ABOc) live-donor kidney transplantation. Subgroup analyses among ABOi recipients were performed according to donor blood type, recipient blood type, and transplant center ABOi volume.
Results
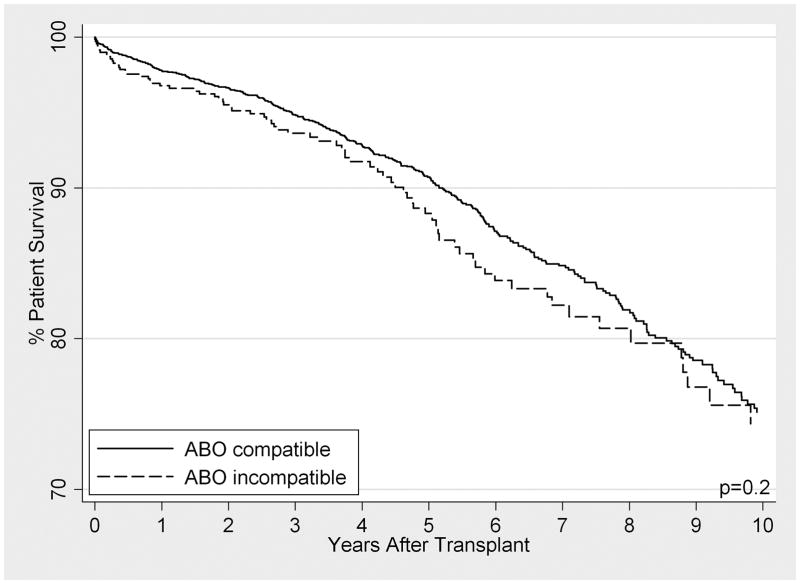
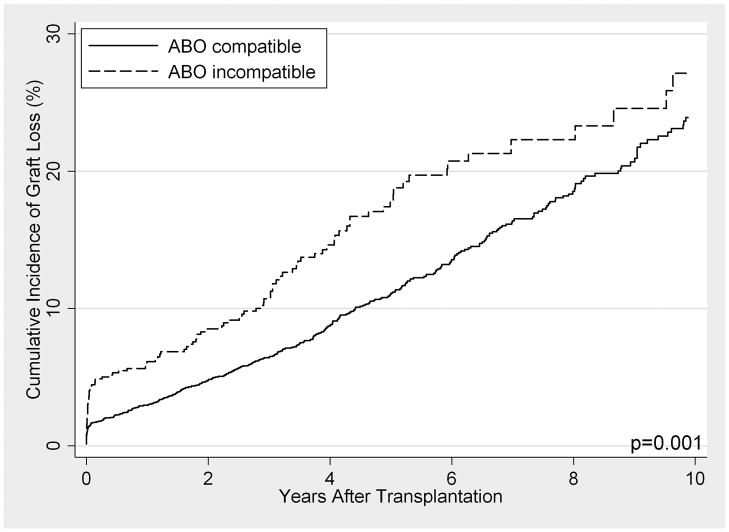
When compared to ABOc matched controls, long-term patient survival of ABOi recipients was not significantly different between the cohorts (p=0.2). However, graft loss was significantly higher, particularly in the first 14 days post-transplant (SHR 2.34, 95% CI 1.43–3.84, p=0.001), with little to no difference beyond day 14 (SHR 1.28, 95% CI 0.99–1.54, p=0.058). In subgroup analyses among ABOi recipients, no differences in survival were seen by donor blood type, recipient blood type, or transplant center ABOi volume.
Conclusions
These results support the use and dissemination of ABOi transplantation when a compatible live donor is not available, but caution that the highest period of risk is immediately post-transplant.
Keywords: ABOi, kidney, transplantation, outcome
INTRODUCTION
With a widening organ shortage in the US, patients with end-stage renal disease and healthy, willing, but incompatible living donors have increasingly pursued novel ways to achieve live donor kidney transplantation (LDKT) (1–3). While kidney paired donation can facilitate LDKT for some incompatible pairs, pairs with blood type-O recipients have low match rates (4–7). ABO incompatible (ABOi) transplantation is thus a critical modality for many of these recipients (8, 9). However, this practice has not yet reached widespread adoption, possibly due to concerns of higher rates of acute antibody mediated rejection, graft loss, or death in ABOi recipients compared to their ABO compatible (ABOc) counterparts (10, 11).
Much of the large-volume literature regarding ABOi transplantation has focused on the experience at Japanese centers, where this practice has been more widely adopted than in the US (12–21). However, these inferences may not generalize to other populations. In the US, research regarding ABOi outcomes has largely consisted of smaller, single-center studies. Although good outcomes have been reported (9, 22), such studies may be underpowered to detect subtle differences and to conduct subgroup analysis. Furthermore, comparison groups in most studies have been either absent or inadequately matched.
In studies that compare ABOi subgroups, analysis has been primarily focused on transplants involving blood type A donors, comparing subtypes A1 and A2. Transplantation across the “minor” A2 barrier (A2→O, A2→B, or A2B→B) is thought to be associated with lower risk of adverse outcomes than those that cross the “major” A1 barrier (23, 24) because of decreased A antigen expression on the renal endothelial surface of A2 kidneys (23, 25, 26).
Another area of recent interest is ABOi outcomes by recipient blood type. Blood type O recipients have been shown to produce higher levels of anti-A/anti-B immunoglobulin-G (IgG) than blood type A or B recipients (27–31), and IgG is thought to be largely responsible for both acute antibody mediated rejection and chronic rejection (32–34).
Finally, it has been suggested that a transplant center’s size is associated with patient and allograft outcomes in general (35, 36). However, ABOi outcomes have not yet been studied in relation to transplant center volume.
The goals of this study were to describe the characteristics of ABOi donors and recipients in the United States, to quantify the growth of this practice over time, to compare outcomes between ABOi and matched ABOc recipients, and to explore ABOi outcomes in specific subgroups.
RESULTS
Study Population
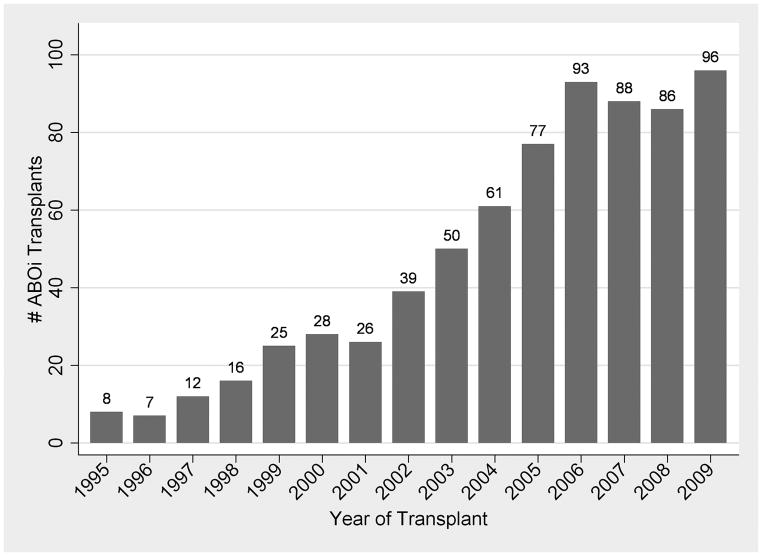
There were 738 ABOi and 77,455 ABOc transplants from 280 centers during the study period (Table 1). Median follow-up time was 5.0 years (range 0–15 years). Since 2006, ABOi transplants have grown to represent 1.5% of all living donor transplants in the US (Figure 1). Of the 738 ABOi transplant recipients, 513 (69.5%) were blood type O, 101 (13.7%) were A, and 124 (16.8%) were B (Table 2). Of ABOi pairs with blood type A donors, 46 (6.2%) were subtype A1 donors, 162 (22.0%) were subtype A2, and 306 were not sub-typed.
TABLE 1. Donor and recipient blood types of live donor kidney transplants in the United States between January 1, 1995–March 31, 2010.
| Donor Blood Type | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Recipient Blood Type | O | A | A1 | A2 | B | AB | A1B | A2B | Total |
| O | 34,675 | 216 (29.3%)a | 43 (5.8%) | 131 (17.8%) | 106 (14.4%) | 14 (1.9%) | 1 (0.1%) | 2 (0.3%) | 35,188 |
| A | 10,823 | 18,429 | 502 | 112 | 70 (9.5%) | 23 (3.1%) | 4 (0.5%) | 4 (0.5%) | 29,967 |
| B | 4,748 | 55 (7.5%) | 3 (0.4%) | 19 (2.6%) | 5,237 | 35 (4.7%) | 0 (0.0%) | 12 (1.6%) | 10,109 |
| AB | 617 | 984 | 26 | 8 | 679 | 595 | 17 | 3 | 2,929 |
| Total | 50,863 | 19,684 | 574 | 270 | 6,092 | 667 | 22 | 21 | 78,193 |
Denominator is total number of ABOi transplants (n = 738, dark-shaded boxes).
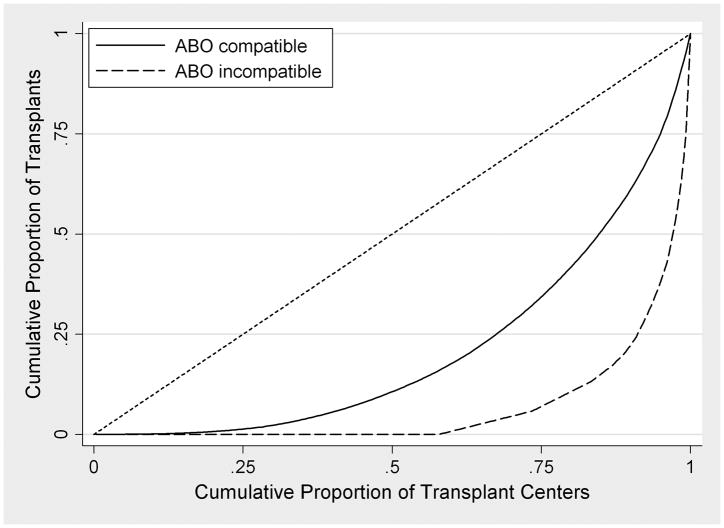
FIGURE 1. ABO Incompatible Live Donor Transplants in the United States, by Year and Transplant Center: (A) Number of Transplants; (B) National Center-Level Distribution comparing ABO Incompatible (Dashed Line) and ABO Compatible (Solid Line) Transplants.
In these Lorenz curves (B), perfect equality is represented by the diagonal reference line. Curves that are located farther to the right represent practices that are less-equally distributed amongst US transplant centers.
TABLE 2. Cohort Demographics: All ABO Compatible Pairs, Matched ABO Compatible Controls, and ABO Incompatible Pairs.
| ABOc (all) | ABOc (matched) | ABOi | |
|---|---|---|---|
| N | 77,455 | 3,679 | 738 |
| Recipient Characteristics | |||
| Age | 45.4 [13.7] | 46.8 [13.2] | 46.9 [13.6] |
| Female (%) | 40.8 | 41.6 | 42.1 |
| BMI | 26.7 [5.5] | 27.0 [5.6] | 26.9 [5.9] |
| College Educated (%) | 53.8 | 55.4 | 59.4 |
| Ethnicity (%) | |||
| Caucasian | 68.1 | 66.9 | 67.2 |
| African American | 14.6 | 15.6 | 18.2 |
| Hispanic | 12.3 | 12.7 | 10.3 |
| Private Insurance (%) | 58.1 | 59.4 | 59.4 |
| Diabetes (%) | 27.7 | 27.3 | 27.5 |
| Sensitizing Events (%) | |||
| Previous Transplant | 11.7 | 15.7 | 16.9 |
| Previous Transfusion | 19.7 | 12.6 | 10.9 |
| Previous Pregnancy | 25.6 | 27.5 | 26.3 |
| Peak %PRA | 10.2 [22.4] | 15.2 [28.3] | 15.9 [29.5] |
| Years of RRT | 2.3 [4.2] | 2.9 [5.1] | 3.0 [5.0] |
| Primary Diagnosis (%) | |||
| Diabetes | 23.1 | 22.6 | 22.6 |
| Hypertension/Large Vessel Disease | 18.2 | 17.8 | 15.9 |
| Polycystic Kidney Disease | 9.9 | 11.4 | 10.3 |
| Glomerulonephritis | 26.1 | 25.0 | 25.6 |
| Donor/Transplant Characteristics | |||
| Donor Age | 40.3 [11.1] | 40.3 [11.2] | 42.5 [11.4] |
| Female Donor (%) | 58.9 | 59.2 | 60.7 |
| Mean HLA Mismatches | 3.1 [1.7] | 3.2 [1.7] | 3.3 [1.7] |
| Crossmatch Positive (%) | 1.9 | 5.1 | 5.3 |
| Donor Relationship to Recipient (%) | |||
| Parent | 8.6 | 6.3 | 9.1 |
| Child | 18.5 | 19.2 | 15.3 |
| Sibling | 32.9 | 28.8 | 27.4 |
| Spouse/Life Partner | 12.8 | 13.3 | 21.4 |
| Other Related | 7.1 | 7.8 | 5.4 |
| Other Non-Related | 20.1 | 24.7 | 21.3 |
Age, BMI, %PRA, years of RRT, and HLA mismatches are presented as mean [standard deviation], and all other variables are presented as proportions. ABOc, ABO compatible; ABOi, ABO incompatible; BMI, body mass index; %PRA, percent panel reactive antibody; RRT, renal replacement therapy.
Cohort Demographics
Compared with ABOc recipients, ABOi recipients had higher peak %PRA (15.9 vs. 10.2%, p<0.001), more years of RRT (3.0 vs. 2.3 years, p<0.001), and were more likely to have had a previous transplant (16.9 vs. 11.7%, p<0.001), receive an organ from an unrelated donor (42.7 vs. 32.8%, p<0.001), and have a positive crossmatch with their donor (5.3 vs. 1.9%, p<0.001) (Table 1). They were also more likely to be college educated (59.4 vs. 53.8%, p=0.007), but less likely to have received a previous blood transfusion (10.9 vs. 19.7%, p<0.001) or have drug-treated hypertension (77.9 vs. 70.1%, p<0.001). ABOi recipients were older (46.9 vs. 45.4 years, p=0.003), as were their donors (42.5 vs. 40.3 years, p<0.001), though these differences were small and not likely to be of clinical significance. To account for any possible differences between ABOi and ABOc patients in general, a cohort of matched ABOc controls was selected for comparison of outcomes (Table 1).
Center Volume
Of 280 US centers performing ABOc transplants during the study period, 120 (42.9%) performed at least one ABOi transplant (Figure 1). Of these, 11 (9.2%) centers performed ≥15 ABOi transplants and were classified as high volume; these centers performed 423 (57.3%) of all ABOi transplants. Of remaining centers, 88 (73.3%) performed ≤5 ABOi transplants and 43 (35.8%) performed only one. ABOi recipients at high-volume centers had higher peak %PRA (18.4 vs. 12.6%, p=0.008), more years of RRT (3.9 vs. 2.4 years, p=0.009), and were less likely to receive a subtype A2 organ (18.2 vs. 27.0%, p=0.014).
Patient Survival
Patient survival was similar among ABOi recipients (96.8%, 93.7%, 88.3%, and 74.5% at 1, 3, 5, and 10 years) compared with ABOc matched controls (97.8%, 94.9%, 90.7%, and 75.1%) (HR 1.19 95% CI 0.93–1.51, p=0.2) (Figure 2). Among ABOi recipients, no statistically significant differences between A1 vs. A2 donors (HR 1.75 95% CI 0.52–5.93, p=0.4), O vs. non-O recipients (HR 1.12 95% CI 0.69–1.82, p=0.7), or high vs. low transplant center ABOi volume (HR 1.22 95% CI 0.78–1.91, p=0.4) were detected.
FIGURE 2. (A) Patient Survival and (B) Graft Loss After LDKT, Comparing ABO Incompatible Recipients (Dashed Line) with ABO Compatible Matched Controls (Solid Line).
ABOc controls were matched to ABOi recipients on presence of diabetes, crossmatch status, recipient age, year of transplant, recipient insurance type, peak percent panel reactive antigen, and years of renal replacement therapy.
Graft Loss
Cumulative incidence of graft loss (with death treated as a competing risk) was higher among ABOi recipients (5.9%, 10.4%, 17.4%, and 27.1% at 1, 3, 5, and 10 years) than ABOc matched controls (2.9%, 6.4%, 11.0%, and 23.9%) (p=0.001) (Figure 2). However, these differences were primarily driven by graft losses in the first 14 days post-transplant (SHR 2.34, 95% CI 1.43–3.84, p=0.001), with little to no difference beyond day 14 (SHR 1.28, 95% CI 0.99–1.54, p=0.058) (Table 3). Similar inferences were found over time, although the differences between ABOi and ABOc (both in the first 14 days and thereafter) were more pronounced in the early years (1995–2002) compared with the more recent era (2003–2010). The results were analogous when all crossmatch positive patients were dropped from analysis. Among ABOi recipients, no statistically significant differences between A1 vs. A2 donors (p=0.2), O vs. non-O recipients (p=0.9), or high vs. low transplant center ABOi volume (p=1.0) were detected in the overall allograft survival analysis, in the first 14 days, or subsequently (Table 3).
TABLE 3. Graft Loss, ABO Incompatible versusABO Compatible Transplants (Upper Rows) and Between Subgroups of ABO Incompatible Transplants (Lower Rows).
In these analyses, death is treated as a competing risk.
| Days 0–14 | Days >14 | Overall | ||||
|---|---|---|---|---|---|---|
| ABOi Versus ABOc (Matched Controls)a | SHR | p value | SHR | p value | SHR | p value |
| All | 1.432.343.84 | 0.001 | 0.991.281.64 | 0.058 | 1.141.421.78 | 0.002 |
| All, 1995–2002 | 1.343.458.89 | 0.01 | 1.151.973.37 | 0.01 | 1.402.243.57 | 0.001 |
| All, 2003–2010 | 1.142.053.66 | 0.015 | 0.921.311.88 | 0.1 | 1.081.471.99 | 0.012 |
| Only XM Negativeb | 1.592.624.32 | <0.001 | 0.971.251.62 | 0.090 | 1.141.431.80 | 0.002 |
| ABOi Subgroup Analysis | ||||||
| A2 Donorc | 0.130.693.53 | 0.7 | 0.753.1313.14 | 0.1 | 0.671.925.49 | 0.2 |
| O Recipientc | 0.601.624.35 | 0.3 | 0.540.871.40 | 0.6 | 0.650.991.52 | 1.0 |
| High-Volume Centerc | 0.300.681.55 | 0.4 | 0.651.011.57 | 1.0 | 0.630.931.36 | 0.7 |
SHR, Sub-Hazard Ratio (presented with 95% confidence intervals); ABOi, ABO incompatible; ABOc, ABO compatible; XM, crossmatch.
ABOc controls were matched on presence of diabetes, crossmatch status, recipient age, year of transplant, recipient insurance type, peak PRA, and years of renal replacement therapy.
Since some ABOi patients were also HLA incompatible (with a documented positive crossmatch), this sensitivity analysis represents only patients with pure ABO incompatibilities.
Reference group for ABOi pairs with A2 donors is ABOi pairs with A1 donors; reference group for ABOi pairs with O recipients is ABOi pairs with A or B recipients; reference group for high-volume centers is low volume centers (<15 ABOi transplants during study period).
DISCUSSION
In this national study, 738 ABOi transplants were identified as having been performed between 1995 and 2010 in the US. To determine whether blood type compatibility influenced allograft and patient survival, each ABOi recipient was matched with 5 ABOc patients in the SRTR database who were most closely matched on a number of important characteristics that can influence outcomes. Patient survival was comparable between ABOi and ABOc transplant recipients. Overall, ABOi recipients experienced a higher incidence of graft loss (27.1% at 10 years compared with 23.9%, p=0.001); however, this was largely driven by graft loss during the first 14 days (SHR 2.34, 95% CI 1.43–3.84, p=0.001). After this period, ABOi recipients had relatively comparable graft survival to their ABOc counterparts (SHR 1.28, 95% CI 0.99–1.54, p=0.058). Differences between ABOi and ABOc recipients seem to have lessened in recent years; while a hopeful interpretation would be that treatment paradigms have improved, the possibility of better patient selection and lower risk transplants (in ways not captured in the data) cannot be excluded. Amongst ABOi recipients, there were no significant differences in patient survival or graft loss by donor A subtype, recipient blood type, or transplant center ABOi volume.
Our finding that ABOi transplant recipients are at a higher risk of graft loss during the early postoperative period is consistent with some smaller single-center studies (10, 11). In ABOi transplantation, the acceptable titer of circulating anti-A or anti-B isohemagglutinins at the time of transplant has varied between 8–32 depending upon the center’s criteria (9, 22, 37–40). Furthermore, rapid increases in isohemagglutinin titers in the immediate post-operative period have been shown to increase the risk of allograft injury and antibody mediated graft loss (22). These findings underscore the importance of careful ABOi recipient monitoring in the immediate post-operative period, when early detection and treatment of antibody-mediated rejection has been shown to prolong graft survival (41).
Among ABOi recipients, our finding of similar outcomes between donor A1 and A2 subtypes is consistent with a previous study by Gloor et al. (22). Likewise, we were unable to detect higher graft loss between blood type O and non-O recipients. In a study by Toki et al. (31), overall graft loss rates did not differ between recipient blood groups, but O recipients were significantly more likely to experience graft loss within the first six months after transplantation (14% vs. 3%, p=0.011). However, results from high volume single centers the US and Sweden showed excellent results for ABOi transplantation across all donor and recipient blood groups (9, 42).
Our analysis of outcomes by transplant center ABOi volume has several important implications. First, it illustrates that despite excellent outcomes, a minority of transplant centers in the US are performing ABOi transplants. While ABOi transplantation has grown to represent 1.5% of all living donor transplants since 2006, the practice has been clustered in a handful of centers and has not expanded appreciably since then. Second, comparable short- and long-term outcomes between low and high volume centers suggest that there may not be a large center-level effect on ABOi outcomes. To test this null hypothesis even further, a sensitivity analysis of centers at the extremes of ABOi volume (centers with ≤5 total ABOi transplants vs. those with ≥40 total ABOi transplants) was conducted, and the inferences remained unchanged. This suggests a minimal “learning curve” for ABOi transplantation and encourages its dissemination to other centers possessing the necessary facilities and personnel (43). Furthermore, our group has shown that successful ABOi transplantation can be accomplished using a plasmapheresis-based protocol without anti-CD20 antibody or splenectomy (9, 39, 44).
This study has important limitations to note. All cases were identified retrospectively. As the SRTR contains no information on initial isohemagglutinin titers, pre-transplant desensitization treatments, or incidence of antibody mediated rejection, we are unable to draw any inferences regarding these factors. The matched-control study design is a useful tool to identify a comparable control group with similar characteristics as the study group (45); however, residual confounding may be present because of characteristics not measured in the SRTR database. Finally, our subgroup analyses was limited to ABOi transplants only, making it less powered to detect differences than the overall ABOi/ABOc analysis.
This is the largest study of ABOi transplants at US centers and demonstrates excellent long-term patient and graft survival for ABOi recipients, though short-term graft survival is not yet equivalent to ABOc recipients. Combined with the finding that outcomes are not associated with transplant center ABOi volume, this encourages the continued adoption of this modality throughout the US.
MATERIALS AND METHODS
Study population
Using data organized by the Scientific Registry of Transplant Recipients (SRTR), 78,220 adult live donor renal transplants at 280 centers in the United States between January 1, 1995 and March 31, 2010 were identified. Donor and recipient blood types were captured for all but 27 transplants which were excluded from analysis. Based on reported blood types, a study population of 738 ABOi live donor transplant recipients at 120 centers was identified. Using radius matching (47), five ABOc matched controls were identified for each ABOi recipient based on the following variables: presence of diabetes, crossmatch status, recipient age, year of transplant, recipient insurance type, peak percent panel reactive antigen (%PRA), and years of renal replacement therapy (RRT).
Subgroup Analysis
ABOi subgroup analysis was performed according to donor blood type A subtype (A1 vs. A2, excluding those with unclassified subtype), recipient blood type (O vs. non-O), and transplant center volume (high ≥15 vs. low <15).
Outcomes
Death and graft-loss were ascertained by center-report and augmented by linkage to the Social Security Death Master File (SSDMF) and the Centers for Medicare/Medicaid Services End Stage Renal Disease (CMS-ESRD) database. Subjects who resumed maintenance dialysis or underwent re-transplantation were classified as having graft loss. Recipients who did not experience death or graft loss by the date of last follow-up (or end-of-study) were censored.
Survival analysis
Characteristics of the study and control groups were compared using χ2-test for categorical variables and two-sided Student’s t-test for continuous variables. Non-parametric competing risks models were used to estimate the cumulative incidence of graft loss; since death with a functioning graft precludes subsequent graft loss, methods were chosen such that death was considered a competing risk, as previously described (48). Pepe and Mori tests were used to compare outcomes between groups (49). To quantify the magnitude of the change in hazard in the context of a competing risk model, the method of Fine and Gray was utilized to semi-parametrically model the sub-hazard for the competing event of interest (i.e. graft loss) (50). A time-varying coefficient was incorporated in this model to account for the changing sub-hazard ratio over time (51). Because graft loss does not preclude risk of death, traditional Kaplan-Meier functions with log-rank tests and Cox proportional hazards models were used to study mortality.
Statistical analysis
P-values <0.05 were considered to be statistically significant. Confidence intervals are reported as per the method of Louis and Zeger (52–54). All analysis and graphs were generated through STATA version 11.2/SE for Linux (STATA Corp., College Station, TX) and the stcompet package.
Acknowledgments
Supported by a grant (RC1 DK086731) from the National Institute of Diabetes and Digestive and Kidney Diseases and by a grant from the Charles T. Bauer Foundation (to Drs. Montgomery, Warren, and Segev). This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. It was approved by the Institutional Review Board at the Johns Hopkins University School of Medicine.
Abbreviations
- ABOi
ABO incompatible
- ABOc
ABO compatible
- LDKT
live donor kidney transplantation
- IgG
immunoglobulin-G
- SRTR
Scientific Registry of Transplant Recipients
- %PRA
percent panel reactive antigen
- RRT
renal replacement therapy
Footnotes
Authors’ contributions:
John R. Montgomery (1): research design, writing, and data analysis
Jonathan C. Berger, MD, MHS (1): research design, writing, and data analysis
Daniel S. Warren, PhD (1): writing
Nathan James, MS (2): data analysis
Robert A. Montgomery, MD, DPhil (1): writing
Dorry L. Segev, MD, PhD (1,2): research design, writing, and data analysis
No conflicts of interest to disclose.
References
- 1.Gentry SE, Montgomery RA, Segev DL. Kidney Paired Donation: Fundamentals, Limitations, and Expansions. Am J Kidney Dis. 2011;57(1):144–151. doi: 10.1053/j.ajkd.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery RA. Renal transplantation across HLA and ABO antibody barriers: integrating paired donation into desensitization protocols. Am J Transplant. 2010;10(3):449–457. doi: 10.1111/j.1600-6143.2009.03001.x. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery RA, Simpkins CE, Segev DL. New options for patients with donor incompatibilities. Transplantation. 2006;82(2):164–165. doi: 10.1097/01.tp.0000226105.42713.37. [DOI] [PubMed] [Google Scholar]
- 4.Gentry S, Montgomery R, Segev D. Blood group O recipients and compatible kidney paired donation. Am J Transplant. 2007;7:392–392. doi: 10.1111/j.1600-6143.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 5.Segev DL, Kucirka LM, Gentry SE, Montgomery RA. Utilization and outcomes of kidney paired donation in the United States. Transplantation. 2008;86(4):502–510. doi: 10.1097/TP.0b013e3181812f85. [DOI] [PubMed] [Google Scholar]
- 6.Terasaki PI, Gjertson DW, Cecka JM. Paired kidney exchange is not a solution to ABO incompatibility. Transplantation. 1998;65(2):291. doi: 10.1097/00007890-199801270-00030. [DOI] [PubMed] [Google Scholar]
- 7.Gentry SE, Segev DL, Montgomery RA. A comparison of populations served by kidney paired donation and list paired donation. Am J Transplant. 2005;5(8):1914–1921. doi: 10.1111/j.1600-6143.2005.00964.x. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery RA. ABO incompatible transplantation: to B or not to B. Am J Transplant. 2004;4(7):1011–1012. doi: 10.1111/j.1600-6143.2004.00496.x. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery RA, Locke JE, King KE, Segev DL, Warren DS, Kraus ES, et al. ABO Incompatible Renal Transplantation: A Paradigm Ready for Broad Implementation. Transplantation. 2009;87(8):1246–1255. doi: 10.1097/TP.0b013e31819f2024. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin WE, Mims MM, Kaufman JJ. Human renal transplantation. III. Technical problems encountered in six cases of kidney homotransplantation. J Urol. 1963;89:349–356. doi: 10.1016/S0022-5347(17)64556-7. [DOI] [PubMed] [Google Scholar]
- 11.Kawase T, Abe M, Ishii T, Murakami T, Utumi K, Tojimbara T, et al. Short-term results in ABO-incompatible living related kidney transplantation. Transplant Proc. 2002;34(7):2773. doi: 10.1016/s0041-1345(02)03405-x. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, Saito K, Takahara S, Okuyama A, Tanabe K, Toma H, et al. Excellent long-term outcome of ABO-incompatible living donor kidney transplantation in Japan. Am J Transplant. 2004;4(7):1089–1096. doi: 10.1111/j.1600-6143.2004.00464.x. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Takahara S, Uchida K, Yoshimura N, Toma H, Oshima S, et al. Successful results after 5 years of tacrolimus therapy in ABO-incompatible kidney transplantation in Japan. Transplant Proc. 2005;37(4):1800–1803. doi: 10.1016/j.transproceed.2005.02.100. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Saito K. Present status of ABO-incompatible kidney transplantation in Japan. Xenotransplantation. 2006;13(2):118–122. doi: 10.1111/j.1399-3089.2006.00278.x. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, Saito K, Tanabe K, Takahara S, Uchida K, Aikawal A, et al. Breaking ABO barrier in kidney transplantation: Excellent outcome of ABO-incompatible kidney transplantation in Japan. Am J Transplant. 2008;8:182–182. [Google Scholar]
- 16.Uchida J, Machida Y, Iwai T, Kuwabara N, Iguchi T, Naganuma T, et al. Clinical Outcome of ABO-Incompatible Living Unrelated Donor Kidney Transplantation. Urol Int. 2011;86(3):307–314. doi: 10.1159/000324103. [DOI] [PubMed] [Google Scholar]
- 17.Tanabe K, Ishikawa N, Tokumoto T, Takahashi K, Kanematsu A, Kitani R, et al. Long-term results of living kidney transplantation under tacrolimus immunosuppression: a single-center experience. Transplant Proc. 1998;30(4):1224–1226. doi: 10.1016/s0041-1345(98)00219-x. [DOI] [PubMed] [Google Scholar]
- 18.Jeon BJ, Kim IG, Seong YK, Han BH. Analysis of the Results of ABO-Incompatible Kidney Transplantation: In Comparison with ABO-Compatible Kidney Transplantation. Korean J Urol. 2010;51(12):863–869. doi: 10.4111/kju.2010.51.12.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanabe K, Takahashi K, Sonda K, Tokumoto T, Ishikawa N, Kawai T, et al. Long-term results of ABO-incompatible living kidney transplantation: a single-center experience. Transplantation. 1998;65(2):224–228. doi: 10.1097/00007890-199801270-00014. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa N, Yagisawa T, Sakuma Y, Fujiwara T, Nukui A, Yashi M, et al. Transplantation of ABO-incompatible and living unrelated donor-recipient combinations. Transplant Proc. 2008;40(7):2292–2293. doi: 10.1016/j.transproceed.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Tanabe K, Tokumoto T, Ishida H, Toma H, Nakajima I, Fuchinoue S, et al. ABO-incompatible renal transplantation at Tokyo Women’s Medical University. Clin Transpl. 2003:175–181. [PubMed] [Google Scholar]
- 22.Gloor JM, Lager DJ, Moore SB, Pineda AA, Fidler ME, Larson TS, et al. ABO-incompatible kidney transplantation using both A2 and non-A2 living donors. Transplantation. 2003;75(7):971–977. doi: 10.1097/01.TP.0000058226.39732.32. [DOI] [PubMed] [Google Scholar]
- 23.Breimer ME, Molne J, Norden G, Rydberg L, Thiel G, Svalander CT. Blood group A and B antigen expression in human kidneys correlated to A1/A2/B, Lewis, and secretor status. Transplantation. 2006;82(4):479–485. doi: 10.1097/01.tp.0000231697.15817.51. [DOI] [PubMed] [Google Scholar]
- 24.Alkhunaizi AM, de Mattos AM, Barry JM, Bennett WM, Norman DJ. Renal transplantation across the ABO barrier using A2 kidneys. Transplantation. 1999;67(10):1319–1324. doi: 10.1097/00007890-199905270-00005. [DOI] [PubMed] [Google Scholar]
- 25.Breimer ME, Samuelsson BE. The specific distribution of glycolipid-based blood group A antigens in human kidney related to A1/A2, Lewis, and secretor status of single individuals. A possible molecular explanation for the successful transplantation of A2 kidneys into O recipients. Transplantation. 1986;42(1):88–91. [PubMed] [Google Scholar]
- 26.Clausen H, Levery SB, Nudelman E, Tsuchiya S, Hakomori S. Repetitive A epitope (type 3 chain A) defined by blood group A1-specific monoclonal antibody TH-1: chemical basis of qualitative A1 and A2 distinction. Proc Natl Acad Sci U S A. 1985;82(4):1199–1203. doi: 10.1073/pnas.82.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan NS, Fleetwood P, Higgins RM, Hathaway M, Zehnder D, Mitchell D, et al. Application of flow cytometry to monitor antibody levels in ABO incompatible kidney transplantation. Transplantation. 2008;86(3):474–477. doi: 10.1097/TP.0b013e31817c4c4c. [DOI] [PubMed] [Google Scholar]
- 28.Contreras M, Armitage SE, Hewitt PE. Response to immunization with A and B human glycoproteins for the procurement of blood grouping reagents. Vox Sang. 1984;47(3):224–235. doi: 10.1111/j.1423-0410.1984.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 29.Kay LA, Locke D. Distribution of immunoglobulin G subclasses in anti-A and anti-B sera. J Clin Pathol. 1986;39(6):684–687. doi: 10.1136/jcp.39.6.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stussi G, Huggel K, Lutz HU, Schanz U, Rieben R, Seebach JD. Isotype-specific detection of ABO blood group antibodies using a novel flow cytometric method. Br J Haematol. 2005;130(6):954–963. doi: 10.1111/j.1365-2141.2005.05705.x. [DOI] [PubMed] [Google Scholar]
- 31.Toki D, Ishida H, Horita S, Yamaguchi Y, Tanabe K. Blood group O recipients associated with early graft deterioration in living ABO-incompatible kidney transplantation. Transplantation. 2009;88(10):1186–1193. doi: 10.1097/TP.0b013e3181ba07ec. [DOI] [PubMed] [Google Scholar]
- 32.Scornik JC, Salomon DR, Lim PB, Howard RJ, Pfaff WW. Posttransplant antidonor antibodies and graft rejection. Evaluation by two-color flow cytometry. Transplantation. 1989;47(2):287–290. doi: 10.1097/00007890-198902000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Nelson PW, Helling TS, Shield CF, Beck M, Bryan CF. Current experience with renal transplantation across the ABO barrier. Am J Surg. 1992;164(5):541–544. doi: 10.1016/s0002-9610(05)81197-3. discussion 544–545. [DOI] [PubMed] [Google Scholar]
- 34.Bryan CF, Nelson PW, Aeder MI, Beck ML, Helling TS, Hughes TM, et al. Current experience with renal transplantation across the ABO blood group barrier. Transplant Proc. 1992;24(6):2527–2529. [PubMed] [Google Scholar]
- 35.Gjertson DW. Center and other factor effects in recipients of living-donor kidney transplants. Clin Transpl. 2001:209–221. [PubMed] [Google Scholar]
- 36.Schurman SJ, Stablein DM, Perlman SA, Warady BA. Center volume effects in pediatric renal transplantation. A report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol. 1999;13(5):373–378. doi: 10.1007/s004670050626. [DOI] [PubMed] [Google Scholar]
- 37.Warren DS, Zachary AA, Sonnenday CJ, King KE, Cooper M, Ratner LE, et al. Successful renal transplantation across simultaneous ABO incompatible and positive crossmatch barriers. Am J Transplant. 2004;4(4):561–568. doi: 10.1111/j.1600-6143.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- 38.Sonnenday CJ, Warren DS, Cooper M, Samaniego M, Haas M, King KE, et al. Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. Am J Transplant. 2004;4(8):1315–1322. doi: 10.1111/j.1600-6143.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 39.Segev DL, Simpkins CE, Warren DS, King KE, Shirey RS, Maley WR, et al. ABO incompatible high-titer renal transplantation without splenectomy or anti-CD20 treatment. Am J Transplant. 2005;5(10):2570–2575. doi: 10.1111/j.1600-6143.2005.01031.x. [DOI] [PubMed] [Google Scholar]
- 40.Tobian AA, Shirey RS, Montgomery RA, Cai W, Haas M, Ness PM, et al. ABO antibody titer and risk of antibody-mediated rejection in ABO-incompatible renal transplantation. Am J Transplant. 2010;10(5):1247–1253. doi: 10.1111/j.1600-6143.2010.03103.x. [DOI] [PubMed] [Google Scholar]
- 41.Locke JE, Zachary AA, Haas M, Melancon JK, Warren DS, Simpkins CE, et al. The utility of splenectomy as rescue treatment for severe acute antibody mediated rejection. Am J Transplant. 2007;7(4):842–846. doi: 10.1111/j.1600-6143.2006.01709.x. [DOI] [PubMed] [Google Scholar]
- 42.Genberg H, Kumlien G, Wennberg L, Berg U, Tyden G. ABO-incompatible kidney transplantation using antigen-specific immunoadsorption and rituximab: a 3-year follow-up. Transplantation. 2008;85(12):1745–1754. doi: 10.1097/TP.0b013e3181726849. [DOI] [PubMed] [Google Scholar]
- 43.Jordan SC. Transplantation: ABO-incompatible renal transplants: time for increased use? Nat Rev Nephrol. 2009;5(9):491–492. doi: 10.1038/nrneph.2009.131. [DOI] [PubMed] [Google Scholar]
- 44.Montgomery RA, Locke JE. ABO-incompatible transplantation: less may be more. Transplantation. 2007;84(12 Suppl):S8–9. doi: 10.1097/01.tp.0000296032.12974.bb. [DOI] [PubMed] [Google Scholar]
- 45.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, Warren DS, Simpkins CE, Dagher NN, Singer AL, Zachary AA, Segev DL. Desensitization of HLA-Incompatible Kidney Recipients and Survival. N Engl J Med. 2011 doi: 10.1056/NEJMoa1012376. (In Press) [DOI] [PubMed] [Google Scholar]
- 46.Schold J, Srinivas TR, Sehgal AR, Meier-Kriesche HU. Half of kidney transplant candidates who are older than 60 years now placed on the waiting list will die before receiving a deceased-donor transplant. Clin J Am Soc Nephrol. 2009;4(7):1239–1245. doi: 10.2215/CJN.01280209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segev DL, Gentry SE, Warren DS, Reeb B, Montgomery RA. Kidney paired donation and optimizing the use of live donor organs. JAMA. 2005;293(15):1883–1890. doi: 10.1001/jama.293.15.1883. [DOI] [PubMed] [Google Scholar]
- 48.Berger JC, Hoque M, Garonzik-Wang J, Hall EC, Muzaale A, Massie A, et al. Live Kidney Donation over Age 70: National Donor and Recipient Outcomes. Am J Transplant. 2011;11:57–57. [Google Scholar]
- 49.Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993;12(8):737–751. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 50.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 51.Beyersmann J, Schumacher M. Time-dependent covariates in the proportional subdistribution hazards model for competing risks. Biostatistics. 2008;9(4):765–776. doi: 10.1093/biostatistics/kxn009. [DOI] [PubMed] [Google Scholar]
- 52.Massie AB, Desai NM, Montgomery RA, Singer AL, Segev DL. Improving distribution efficiency of hard-to-place deceased donor kidneys: Predicting probability of discard or delay. Am J Transplant. 10(7):1613–1620. doi: 10.1111/j.1600-6143.2010.03163.x. [DOI] [PubMed] [Google Scholar]
- 53.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;10(1):1–2. doi: 10.1093/biostatistics/kxn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Massie A, Zeger SL, Montgomery RA, Segev DL. The Effects of DonorNet 2007 on Kidney Distribution Equity and Efficiency. Am J Transplant. 2009;9(7):1550–1557. doi: 10.1111/j.1600-6143.2009.02670.x. [DOI] [PubMed] [Google Scholar]