Abstract
The efficient delivery of plasmids encoding antigenic determinants into dendritic cells (DCs) that control immune response is a promising strategy for rapid development of new vaccines. In this study, we prepared a series of targeted cationic lipoplex based on two synthetic lipid components, mannose-poly(ethylene glycol, MW3000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine (Mannose-PEG3000-DSPE) and O-(2R-1,2-di-O-(1'Z,9'Z-octadecadienyl)-glycerol)-3-N-(bis-2-aminoethyl)-carbamate (BCAT), that were formulated with 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) for evaluation as non-viral vectors for transgene expression in DCs. First, we optimized the N:P ratio for maximum transfection and then screened the effects of mannose targeting for further enhancement of transfection levels. Our results indicate that efficient delivery of gWIZ GFP plasmid into DCs was observed for mannose compositions of ~10%, whereas low transfection efficiencies were observed with non-targeted formulations. Mannose-targeted lipofectamine complexes also showed high GFP expression levels in DCs relative to non-targeted lipofectamine controls. The best transfection performance was observed using 10 mol % Mannose-PEG3000-DSPE, 60 mol% BCAT, and 30 mol % DOPE, indicating that the most efficient delivery into DCs occurs via synergistic interaction between mannose targeting and acid-labile, fusogenic BCAT:DOPE formulations. Our data suggest that mannose-PEG3000-DSPE:BCAT:DOPE formulations may be effective gene delivery vehicles for the development of DC-based vaccines.
Introduction
Dendritic cells (DCs) are potent professional antigen presenting cells in the immune system that play a central role in adaptive immunity against a variety of pathogens and tumors.1–4 The DC system can induce and prevent disease, as well as generate resistance to infections by controlling T-cell recognition and responsiveness. DCs capture and process antigens for T-cell presentation to elicit antigen-specific immune responses.
After internalization by DCs via endocytosis, antigenic proteins are fragmented into ~20 amino acid peptides. Introduction of these peptides into the major histocompatibility complex (MHC) class II system results in the activation of CD4+ T lymphocytes that subsequently produce humoral immunity. In contrast, proteasomal degradation of antigenic proteins in the cytosol of DCs, followed by introduction into the MHC class I system of DCs leads to the activation of CD8+ cytotoxic T lymphocytes (CTLs) for the induction of cellular immunity.
DCs have a unique receptor-mediated endocytic system that delivers antigens to the processing compartment via nonspecific recognition uptake receptors like C-type lectin.5, 6 A variety of C-type lectins and lectin-like receptors on the surface of DCs are able to recognize specific carbohydrate structures and interact with carbohydrates. Among these lectins, the mannose receptor (MR, CD206) is one of the most important receptors of DCs for endocytosis. MRs bind glycoproteins bearing terminal mannose, fucose and N-acetylglucosamine moieties on the surface of pathogens, such that their internalization leads to their enhanced presentation for T-cell recognition.7, 8 It has been reported that mannosylated peptides, protein antigens, and polyethylenimine-DNA conjugates are more effective and have enhanced potency for transfecting DCs relative to non-mannosylated controls.9–11 Given the antigen internalization, processing, and presentation ability of DCs, this specialized cell type is a promising target for the rapid and safe development of potent vaccines.
Several types of biodegradable nanoparticles including poly(lactic-co-glycolic acid) (PLGA), chitosan, liposomes, and virus-like particles, have been investigated as non-viral vectors for transfecting DCs for vaccine applications.4, 12–17 Recently, Powell and coworkers reported a layer-by-layer assembly process for nanoparticle assembly from artificial biofilms composed of oppositely charged polypeptides and target-designed peptides on CaCO3 cores that showed efficient cellular uptake and potent cross-presentation to CD8+ T-cells and more efficient presentation to CD4+ T-cells by DCs in vitro.18 Hirosue and coworkers have also shown that poly(propylene sulfide) (PPS) nanoparticles consisting of a cross-linked rubbery core of PPS surrounded by a hydrophilic corona of poly(ethylene glycol) achieved the successful delivery of disulfide conjugated peptide antigens both on MHC I and MHC II molecules, translating to TCR transgenic T cell activation both in vitro and in vivo. These PPS nanoparticles appeared to be taken up by DCs via several pathways including macropinocytosis and clathrin-mediated endocytosis.19, 20
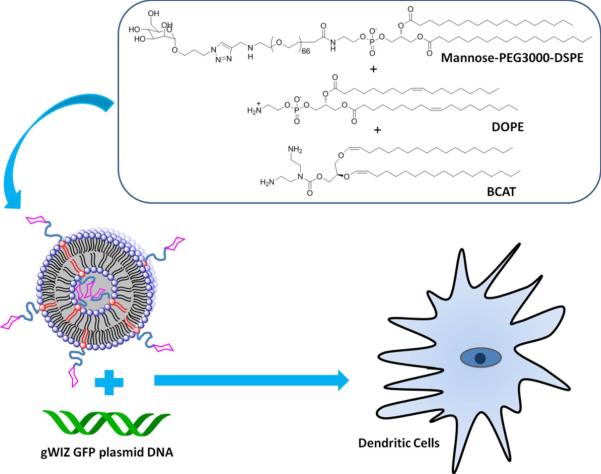
An attractive strategy for efficient gene delivery into DCs is the use membrane-based biocompatible nanocarriers such as liposomes because of their low toxicity, high capacity for multivalent interactions, and their ability to be readily optimized with respect to lipid composition, all of which can help promote DC internalization. In this study, we have synthesized Mannose-PEG3000-DSPE as a target ligand to incorporate into dispersions of the acid-labile cationic lipid, O-(2R-1,2-di-O-(1'Z,9'Z-octadecadienyl)-glycerol)-3-N-(bis-2-aminoethyl)-carbamate (BCAT)21 that was prepared by an improved synthetic method. We then investigated the effect of mannose density and DOPE on the delivery of gWIZ green fluorescent protein (GFP) into DCs using these novel materials (Figure 1).
Figure 1.
Conceptual diagram of gWIZ GFP pDNA delivery to DCs via lipoplex composed of Mannose-PEG3000-DSPE, BCAT, and DOPE.
EXPERIMENTAL SECTION
General method
All chemical reagents were obtained from commercial suppliers and used without further purification unless otherwise noted. All reactions were carried out under a blanket of N2 gas. Reaction progress was monitored by thin-layer chromatography (TLC) analysis. TLC spots were visualized either by UV light (254nm) exposure or by staining (e.g., ninhydrin staining with 100 mL n-BuOH, 3 mL AcOH, 0.3 g ninhydrin or by I2 in silica gel). THF was distilled from sodium benzophenone. Et3N and CH2Cl2 were distilled from CaH2. Flash column chromatography was carried out using 230–400 mesh silica gel and analytical grade solvents. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded with a Varian INOVA (300MHz) spectrometer. Chemical shifts are reported in ppm relative to the residual solvent peaks as internal standard. Peak multiplicities in 1H NMR spectra are abbreviated as s (singlet), d (doublet), t (triplet), dd (doublet of doublet), m (multiplet), br (broad), q (quartet). Mass spectrometry was performed by the MCMP Mass Spectrometry Service of Purdue University.
Synthesis of Mannose-PEG3000-DSPE
1,2,3,4,6 Penta-O-acetyl-α,β-D-Mannopyranoside (2)
Compound 2 was prepared according to the method reported by Timmons et al.22
3'-Bromopropyl-2,3,4,6-tetra-O-acetyl-α-D-mannopyranoside (3)
Compound 3 was prepared as described by Hayes et al.23
3'-Azidopropyl-2,3,4,6-tetra-O-acetyl-α-D-mannopyranoside (4)
Compound 4 was prepared as described by Hayes et al.23
3'-Azidopropyl-O-α-D-mannopyranoside (5)
Compound 5 was prepared according to the method reported by Ladmiral et al.24
H2N-PEG3000-DSPE (8)
DSPE (147 mg, 187 μmol) was added to Boc-HN-PEG-NHS (400 mg, 133 μmol) in CHCl3 (5 mL). After stirring at 50 °C for 15 min, the temperature was lowered to 22 °C and the mixture stirred for another 14 h. The solvent was evaporated and the residue dissolved in MeCN (10 mL). After storage at 4 °C for 5 h, the solution was centrifuged to sediment the insoluble (unreacted) DSPE and the supernatant evaporated to give Compound 7 (460 mg, 95%). This intermediate (460 mg, 126 μmol) was diluted in CH2Cl2 (14 mL) before addition of CF3CO2H (7 mL). The reaction mixture was stirred at 22 °C for 15 h and the mixture concentrated under reduced pressure to give compound 8 (438 mg, 98%). 1H NMR (300 MHz, CDCl3) δ 4.21 (m, 4H), 3.96 (m, 2H), 3.72 (s, 270H), 3.47 (m, 4H), 3.24 (m, 4H), 2.37 (m, 4H), 1.62 (m, 4H), 1.28 (s, 56H), 0.88 (t, J = 6.45 Hz, 6H).
Compound 9
Propargyl bromide (38 μL, 424 μmol) was added to H2N-PEG-DSPE (150 mg, 42.5 μmol) in CHCl3 (5 mL). After stirring at 22 °C for 15 h, the solvent was evaporated under reduced pressure. Diethyl ether was added to the residue, the sample stored at 4 °C for 4h, and then centrifuged to separate the unreacted propargyl bromide. The residue (solid) was dialyzed against de-ionized water for 2 d using a MWCO2000 membrane and then lyophilized to give Compound 9 (150 mg, 98%). 1H NMR (300 MHz, CDCl3) δ 4.18 (m, 4H), 3.94 (m, 2H), 3.73 (s, 270H), 3.48 (m, 4H), 3.42 (d, J = 2.4 Hz, 2H), 3.23 (m, 4H), 2.38 (m, 4H), 2.26 (t, J = 2.4 Hz, 1H), 1.59 (m, 4H), 1.54 (s, 1H), 1.26 (s, 56H), 0.89 (t, J = 6.3 Hz, 6H).
Mannose-PEG3000-DSPE
3'-Azidopropyl-O-α-D-mannopyranoside (100 mg, 38 μmol), Compound 9 (150 mg, 42 μmol), and 0.1 M CuSO4·5H2O (50μl, 4.2 μmol) were dissolved in water (4 mL) and t-BuOH (4 mL). Sodium ascorbate (0.1M, 100 μL, 8.3 μmol) was added and the reaction mixture stirred at 22 °C for 2 d. Then, the mixture was dialyzed (MWCO2000 membrane) against deionised water for 2 d before lyophilizing to give Mannose-PEG3000-DSPE (110 mg, 75%) as a white solid. 1H NMR (300 MHz, CDCl3) δ 7.02 (s, 1H), 4.87 (m, 1H), 4.21 (m, 4H), 3.40–3.96 (m, 282H), 3.21 (m, 4H), 2.39 (m, 4H), 1.80 – 1.98 (m, 2H), 1.62 (m, 4H), 1.28 (s, 56H), 0.88 (t, J = 6.9 Hz, 6H).
Synthesis of BCAT
1-Allyl-3-t-butyl-dimethylsilyl-rac-glycerol (12)
Compound 12 was prepared as described by Van den Bossche et al.25
1,2-Diallyl-3-t-butyl-dimethylsilyl-rac-glycerol (13)
Compound 13 was prepared as described by Van den Bossche et al.25
1,2-O-Di-1'-(Z)-octadecenyl-3-tert-butyl-dimethylsilyl-rac-glycerol (14)
sec-BuLi (5 mL, 7 mmol) was slowly added to compound 13 (1 g, 3.5 mmol) in THF (50 mL) at −78 °C. After the resulting mixture was stirred for 30 min at −78 °C, anhydrous BaI2 (3 g, 7 mmol) in THF (50 mL) (prepared by drying 4 g of commercially available BaI2·hydrate) at −78 °C was added and the mixture stirred for an additional 1 h. Pentadecyl iodide (2.3 g, 7.7 mmol) in ether (100 mL) was added dropwise and the mixture stirred for an additional 30 min at −78 °C. The reaction was then warmed to − 45 °C for 20 min and then to 0 °C for 10 min. It was quenched by the addition of hexane (30 mL) and water (50 mL) at 0 °C. The water phase was extracted with hexanes (3 × 100 mL), the combined organic phases were collected and dried over K2CO3 before solvent evaporation to yield a crude mixture that was purified using silica gel chromatography with a stepwise gradient of 9:1 hexanes:CH2Cl2 to 4:1 hexanes:CH2Cl2. The crude residue was purified by silica gel flash chromatography using 4:1 hexanes:CH2Cl2 as eluent to give Compound 14 (1.5 g, 59%) as a clear oil. 1H NMR (300 MHz, CDCl3): 6.08 (d, J = 6 Hz, 1H), 5.98 (d, J = 6 Hz, 1H), 4.48 (quart, J= 7 Hz, 2H), 3.93–3.78 (m, 5H), 2.11 (q, J = 7 Hz, 2H), 1.33 (m, 54H), 0.97–0.86 (m, 15H), 0.09 (s, 6H); 13C NMR (75 MHz, CDCl3): 145.2, 144.3, 107.6, 107.3, 81.1, 71.3, 62.5, 32.0, 29.8, 29.7, 29.6, 29.4, 25.9, 24.0, 22.7, 18.3,14.2, – 5.45; HRMS (ESI): (M+H)+ m:z calc'd for C45H90O3Si = 706.6659, found 707.0822.
1,2-O-Di-1'-(Z)-octadecenyl-rac-glycerol (15)
TBAF (6 mL of 1M, 6 mmol) was added to a solution of Compound 14 (1.2 g, 1.7 mmol) and imidazole (1 g, 14 mmol) in THF (50 mL) at 0 °C. The reaction mixture was stirred at 22 °C for 1 h. After water and Et2O (75 mL) were added, the aqueous phase was extracted with EtOAc (3 × 100 mL). The combined organic phases were dried over K2CO3, filtered and evaporated. The crude product was purified with silica gel flash chromatography using 3:2 Hexanes:Et2O as an eluent to give Compound 15 (0.95 g, 94%) as a clear oil. 1H (300 MHz, CDCl3): 6.08 (d, J = 6 Hz, 1H), 5.97 (d, J = 6 Hz, 1H), 4.44 (m, 2H), 3.89–3.79 (m, 5H), 2.11 (q, J = 7 Hz, 2H), 2.09 (t, J = 6 Hz), 1.32 (s, 56H), 0.94 (t, J = 7 Hz, 6H); 13C (75 MHz, CDCl3): 145.2, 144.3, 107.6, 107.3, 81.1, 71.3, 62.5, 32.0, 29.8, 29.6, 29.5, 29.4, 25.9, 24.0, 22.8, 18.3, 14.2; HRMS (ESI): (M+H)+ m:z calc'd for C39H76O3 = 592.5794, found 592.6182.
O-(1,2-O-Di-1'-(Z)-octadecenyl-rac-glycerol)-N-(bis-2-phthalamidylethyl)-carbamate (17)
4-Nitrophenyl chloroformate (548 mg, 2.52 mmol) was added to Compound 15 (320 mg, 0.539 mmol), and DIEA (2.0 mL, 11 mmol) in CH2Cl2 (20 mL) and the reaction mixture stirred at 22 °C for 4 h. The reaction mixture was extracted with NaHCO3 (50 mL) and washed with saturated NaCl solution (50 mL). The CH2Cl2 layer was dried over K2CO3, evaporated, and the resulting oil dissolved in DMF (20 mL). 1,5-Dipthalamidyldiethylenetriamine (612 mg, 1.68 mmol) was added to this solution and the reaction mixture stirred at 80 °C for 2 d. The solution was evaporated and the crude mixture purified via silica gel flash chromatography using 3:2 hexanes:Et2O as an eluent to give Compound 17 (0.25 g, 47%) as a white solid. 1H (300 MHz, CDCl3): 7.72 (m, 4H), 7.68 (m, 4H), 6.12 (d, J = 6 Hz, 2H), 4.30 (quart, J= 6, 2H), 3.83–3.51 (m, 10H), 3.44 (m, 4H), 2.01 (m, 4H), 1.17 (m, 56H), 0.94 (m, 6H); 13C (75 MHz, CDCl3): 168.2, 155.8, 145.0, 143.9, 132.0, 123.4, 108.3, 107.5, 78.1, 71.3, 64.4, 46.3, 45.5, 35.8, 31.9, 29.7, 29.5, 29.4, 23.9, 22.7, 14.1: HRMS (ESI): (M+H)+ m:z calc'd for C39H76O3 = 981, 6809 found 982.0293.
O-(1,2-O-Di-1'-(Z)-octadecenyl-rac-glycerol)-N-(bis-2-aminoethyl)-carbamate (18)
Hydrazine hydrate (50 μl, 1.4 mmol) was added to Compound 17 (240 mg, 0.24 mmol) in MeOH (60 mL) and the reaction mixture stirred at 22 °C for 2 d. The solution was evaporated, yielding an off white precipitate. Dichloromethane (50 mL) was added, the precipitate side product removed via filtration, and the filtrate evaporated and then dried in vacuo overnight. The crude product was purified with silica gel flash chromatography using 89:10:1 CH2Cl2:MeOH:TEA as an eluent to give Compound 18 (0.13 g, 79%) as a white solid. 1H (300 MHz, CDCl3): 6.06 (d, J = 6 Hz, 1H), 5.99 (d, J = 6 Hz, 1H), 4.45 (m, 2H), 4.32–4.21 (m, 2H), 3.93 (q, J = 5 Hz, 1H), 3.89 (d, J = 6 Hz, 2H), 3.45 (bs, 4H), 2.92 (bs, 4H), 2.17 (m, 4H), 1.42–1.37 (m, 48H), 0.99 (t, J = 7 Hz, 6H); 13C (75 MHz, C6D6): 155.7, 145.1, 144.4, 107.5, 107.4, 78.9, 71.3, 64.1, 32.0, 29.9, 29.7, 29.5, 29.4, 24.19, 22.79, 14.04; HRMS (ESI): (M+H)+ m:z calc'd for C44H87N3O4 = 721, 6697 found 721.7954.
Liposome preparations
Lipids (DOPE and BCAT) and Mannose-PEG3000-DSPE were dissolved in benzene, the solvent evaporated under a stream of N2 to produce a thin film, and the traces of organic solvent removed under vacuum overnight. HEPES-buffered saline (HBS, 20 mM HEPES, 150 mM NaCl, pH 7.8) then was added to the lipid film to produce a final concentration of 5 mM. The hydrated lipid was then subjected to ten freeze-thaw-vortex cycles before extruding the suspension ten times at room temperature through 100 nm pore size track-etch membranes.
Dynamic light scattering and zeta potential measurements
The size and zeta potentials of the different liposome formulations were determined using a Nano ZS-90 analyzer (Malvern Instruments, England). The measurements were made by diluting 50 μL of the liposome dispersion with 1 mL of double de-ionized H2O before making the measurements at 25 °C and calculating the zeta potential using the manufacturer's supplied software. Experiments were run in triplicate.
Gel electrophoresis retardation assay
The binding avidity of pDNA with the various liposome formulations was determined by 1% agarose gel electrophoresis using TAE buffer (242 g Tris, 57.1 mL glacial acetic acid, and 0.5 mM EDTA, pH 8.0) containing 0.5 μg/mL ethidium bromide. Approximately 100 ng of pDNA lipoplex were loaded onto the gel at different N:P ratios. A gel loading dye (New England BioLabs, MA) then was added to each well and electrophoresis carried out at a constant voltage of 80 V for 50 min. The pDNA bands were then visualized at 365 nm using a gel transilluminator.
Isolation and culture of porcine dendritic cells (DCs)
Heparinized pig blood was collected from healthy pigs at a local abattoir. Peripheral blood mononuclear cells (PBMC) were isolated from the buffy-coat fraction of pig blood by using Histopaque-1077 (Sigma-Aldrich, St. Louis, MO) through density-gradient centrifugation (400 g for 30 min). SWC3-positive monocytes were enriched from total PBMC by immunomagnetic labeling of cells using the anti-SWC3 porcine pan-myeloid cell marker (CD172a, AbD serotec, Raleigh, NC) and goat-anti-mouse IgG microbeads (Miltenyi Biotec, Auburn, CA). DCs were generated from sorted monocytes for 7 d at 39 °C26 and 5% CO2 in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, HyClone, Logan, UT), 1× MEM Non-essential Amino Acid Solution (Sigma-Aldrich, St. Louis, MO), 0.11 mg/mL sodium pyruvate (Sigma-Aldrich, St. Louis, MO), 2 mM L-glutamine (Sigma-Aldrich, St. Louis, MO), 10 μg/mL Gentamicin (Invitrogen, Carlsbad, CA), 25 ng/mL recombinant pig IL-4 (Cell Sciences, Canton, MA), and 2× recombinant pig GM-CSF (as a gift from Dr. Harm HogenEsch, Purdue University, IN).27 At days 2 and 5, half of the old medium was replaced with fresh medium.
gWIZ GFP plasmid transfection of DCs
DCs were seeded in 24-well plates at a density of 1×106 cells:well in 1 mL medium without Gentamicin one night before plasmid transfection. One microgram of gWIZ GFP plasmid (Genlantis, San Diego, CA) and 2.5 μL liposomes (1 μg/μL) were mixed with 50 μL of medium of OPTI-MEM (Invitrogen, Carlsbad, CA), respectively. After being incubated at room temperature for 5 min, they were combined for another 30 min at 22 °C. After 0.5 mL cell culture medium was removed carefully from each well, the transfection mixture was added drop-wise to cells in each well. After 6 h incubation at 39 °C and 5% CO2 in a humidified incubator, 1 mL fresh cell culture medium was added to each well. Eighteen hours later, the cells were prepared for flow cytometric analysis.
Fluorescent staining and flow cytometric analysis
DCs were removed from each well with cell scrapers and washed with flow buffer (0.5% BSA and 0.1% NaN3 in PBS). DCs were stained for cell surface marker using anti-SWC3 monoclonal antibody28, 29 and R-Phycoerythrin labeled goat-anti-mouse IgG (KPL, Gaithersburg, MD). Cells were fixed with 0.5 mL of 2% paraformaldehyde before analysis with a Cytomics FC500 flow cytometer (Beckman Coulter, Fullerton, CA). A total of 1 × 104 cells were evaluated and the data analyzed with WinMDI 2.9 software (Joe Trotter, Scripps Institute, La Jolla, CA).
Fluorescence microscopy experiments
Cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, HyClone, Logan, UT), 1× MEM Non-essential Amino Acid Solution (Sigma-Aldrich, St. Louis, MO), 0.11 mg/mL sodium pyruvate (Sigma-Aldrich, St. Louis, MO), and 2 mM L-glutamine (Sigma-Aldrich, St. Louis, MO). The cells were seeded in 24-well plates at a concentration of 2 × 105 cells/well in 1 mL of complete cell culture medium 12 h before transfection. One microgram of gWIZ GFP plasmid (Genlantis, San Diego, CA) and a varying amount of liposome formulation (1 μg/μl) needed to achieve the desired N:P ratio were mixed with 50 μl of OPTI MEM (Invitrogen, Carlsbad, CA) for 5 min at 25 °C, respectively, followed by combining the mixtures and incubating for another 30 min at 25 °C. After the cells were rinsed once with OPTI MEM, the solution containing the pDNA lipoplex was added onto the cells. After 6 h incubation at 37 °C and 5% CO2 in a humidified incubator, 1 mL fresh cell culture medium was added to each well. After 18 h, the cells were observed with Nikon Eclipse TE2000-S (Nikon instruments, Melville, NY).
Cytotoxicity studies
The cytotoxicity of the cationic liposomes in comparison with Lipofectamine was evaluated using the MTS assay in 3D4:31 cells. The cells were cultured in complete RPMI medium supplemented with 10% FBS at 37 °C, 5% CO2, and 95% relative humidity. The cells were seeded in a 96-well microtiter plate (Nunc, Wiesbaden, Germany) at densities of 7500 cells/well. After 24 h, the culture media was replaced with serum-supplemented culture media containing the lipids (1mg/mL), and the cells were incubated for 24 h. Then, 30 μL of the MTS reagent was added to each well. After incubation for 2 h, the absorbance was measured using a microplate reader (Spectra Plus, TECAN) at a wavelength of 490 nm. The relative cell viability (%) related to control cells cultured in media without lipids was calculated with [A]test:[A]control × 100%, where [A]test is the absorbance of the wells with polymers and [A]control is the absorbance of the control wells. All experiments were conducted for three samples and averaged.
RESULTS AND DISCUSSION
Synthesis of Mannose-PEG3000-DSPE
Mannose-PEG3000-DSPE was prepared via the synthetic pathway shown in Scheme 1. D-Mannose was globally protected with acetic anhydride to produce acylated Compound 2.22, 30 The azide intermediate, Compound 4, was prepared from Compound 2 by sequential treatment with 3-bromo-propan-1-ol under Lewis acid-mediated conditions, followed by treatment with NaN3 in DMF at 60 °C for 6 h.23, 24 Intermediate 5 was prepared by de-O-acetylation of Compound 4 with sodium methoxide in methanol.
Scheme 1.
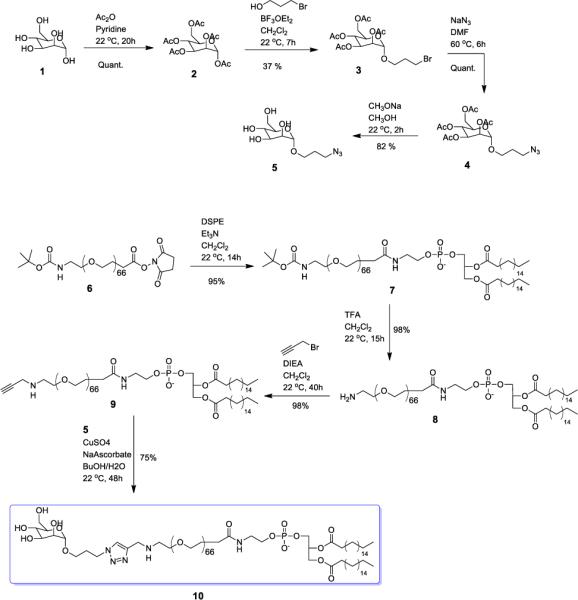
Synthesis route for Mannose-PEG3000-DSPE.
Boc-NH-PEG3000-NHS was treated with the high phase transition temperature lipid DSPE under basic conditions to give Boc-NH-PEG3000-DSPE, followed by the deprotection of the amine group with trifluoroacetic acid.31 Nucleophilic substitution of NH2-PEG3000-DSPE with propargyl bromide under basic conditions produced Compound 9 in 98% yield. The final target lipid, Mannose-PEG3000-DSPE, was obtained via copper-catalyzed click coupling of Compound 9 with Compound 5,32, 33 to afford a construct with strong membrane-association properties that will be prevented from phase separating in the BCAT:DOPE phase due to the large PEG3000 substituent.
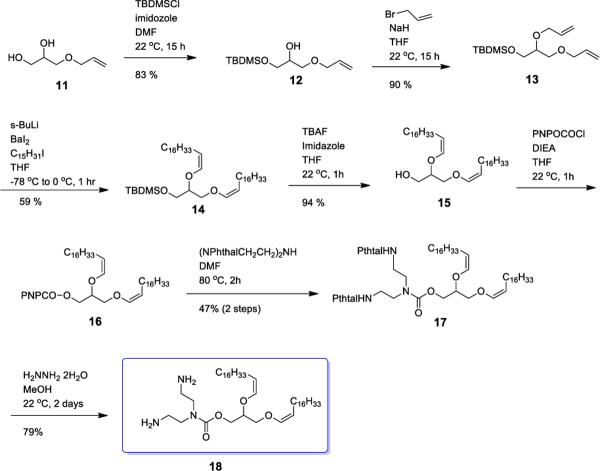
Synthesis of BCAT
Cationic liposomes are a promising non-viral vehicle for gene delivery because their positive surface charge helps the formation of complexes such as lipoplexes with plasmid DNA and enhances their cellular binding affinity due to electrostatic interaction with the anionic plasma membrane.34–36 Previously, we prepared a diplasmologen derivative containing a diethylenetriamine (BCAT) headgroup mounted on a glyceryl backbone bearing acid-labile vinyl ether fatty acid linkages. This natural product derivative improved transfection efficiency by facilitating endosomal escape via degradation of the cationic lipid carrier within the acidic endolysosomal compartment.37 The transfection efficiency of BCAT was shown to be three orders of magnitude higher, while also displaying lower cytotoxicity, than the commercial standard DOTMA:Chol formulation. Thus, the vinyl ether and carbamate linkages are promising bioresponsive motifs for transfection reagent development.
An improved synthesis of BCAT, using a barium-mediated alkylation transformation, is shown in Scheme 2. The primary alcohol of allyl glycerol was selectively protected with TBDMS, followed by alkylation of secondary alcohol in the presence of NaH to give Compound 13. BaI2-mediated bisalkylation of Compound 13 produced Compound 14 in 59% yield. Removal of the TBDMS group was achieved in the presence of TBAF and imidazole in THF before activation of Compound 15 with 4-nitrophenyl chloroformate. The coupling reaction of Compound 16 with diphthalamidyldiethylenetriamine in DMF at 80 °C for 2 d produced Compound 17. The target BCAT was obtained by treatment of 17 with hydrazine for 2 d.
Scheme 2.
Synthesis route for BCAT.
Preparation of mannose-targeted liposome formulations
A series of liposomes containing different concentrations of mannosylated lipid were prepared for evaluating the effect of liposomal mannose density on transfection efficiency of DCs. A second series of liposomes containing different BCAT and DOPE ratios were prepared for examining effect of DOPE on the transfection efficiency of DCs. Both sets of liposome preparations were produced as described in the Experimental Methods section. The diameter of the liposomes was measured by a dynamic light scattering and found to range between 90 nm and 130 nm.
Lipoplex zeta potentials
The zeta potentials of the pDNA lipoplexes generated for this study are shown in Tables 1 and 2. The zeta potentials of the lipoplex were found to increase with increasing N:P ratio up to a N:P of 10; statistically significant increases in the observed ζ were not observed above N:P = 10. The observed zeta potentials for mannosylated lipoplex were found to decrease as the Mannose-PEG3000-DSPE lipid content increased. We infer from these observations that the surface-grafted PEG-mannose layer serves to screen the excess positive charge of the lipoplex under these conditions.
Table 1.
Zeta potentials for 10 mol% Mannose-PEG3000-DSPE in 1:1 BCAT:DOPE liposomes after complexation with gWIZ GFP plasmid.
| N:P ratio | ζ (mV) |
|---|---|
| 0.1 | −27.1 ± 0.6 |
| 0.2 | −21.2 ± 1.2 |
| 1 | −2.6 ± 0.5 |
| 5 | 12.0 ± 2.9 |
| 10 | 18.1 ± 3.2 |
| 20 | 21.0 ± 1.8 |
Table 2.
Zeta potentials for 1:1 BCAT:DOPE liposomes with varying Mannose-PEG3000-DSPE content after complexation with gWIZ GFP plasmid at a 5:1 N:P ratio.
| Mannose Content (mol%) in 1:1 BCAT:DOPE Liposomes | ζ (mV) |
|---|---|
| 0% | 23.2 ± 3.4 |
| 5% | 18.2 ± 1.3 |
| 10% | 12.0 ± 2.9 |
| 20% | 9.4 ± 1.6 |
| 40% | 2.1 ± 0.4 |
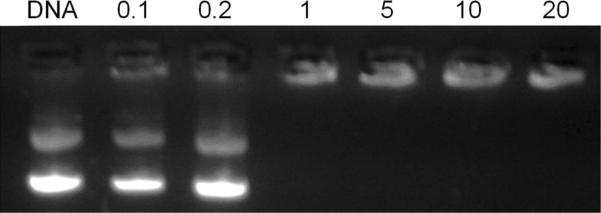
Gel retardation assay
Gel retardation assays were conducted to evaluate the complexation capacity of the 1:1 BCAT:DOPE liposomes with gWIZ GFP pDNA. Using a fixed quantity of DNA (100 ng/μL), a gradual quenching of the EtBr fluorescence from the DNA band was observed as the liposome content increased from an N:P ratio of 0.1 – 20. Complete retardation of the pDNA mobility was achieved at N:P ratios ≥ 1 (Figure 2).
Figure 2.
Gel retardation assay of 10 mol% Mannose-PEG3000-DSPE in 1:1 BCAT:DOPE:gWIZ GFP plasmid lipoplexes at different N:P ratios.
In Vitro Cell Transfection
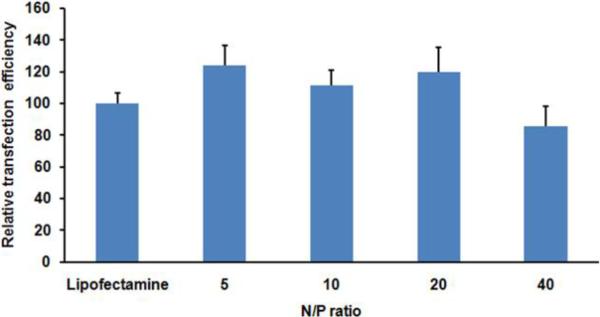
Transfection efficiency of the mannose-targeted liposomes was compared with Lipofectamine as a positive control38, 39 in dendritic cells using the gWIZ GFP plasmid. The transfection performance of Lipofectamine was set as 100 % in all experiments performed.
Initial experiments focused on optimizing the N:P ratios for transfection. Cationic liposomes containing 10 mol % mannosylated lipid in 1:1 BCAT:DOPE were mixed with gWIZ GFP plasmid at different N:P ratios (5:1 – 40:1) for the formation of the lipoplexes and were evaluated for their capacity toward gene delivery in dendritic cells after 18 h of transfection. We found that most of these liposomes displayed similar or slightly higher transfection efficiencies relative to Lipofectamine, except the 40:1 N:P ratio formulation that was slightly less efficient than Lipofectamine (Figure 3). We attribute the modest increases in transfection efficiency of these cationic liposomes to the combined effects of mannose targeting and the acid-labile vinyl ether linkages of BCAT that was previously observed to promote the gene transfection efficiency due to enhanced endosomal escape, promoted by the hydrolyzed fatty acid fragments of BCAT.37
Figure 3.
Transfection efficiency of dendritic cells treated with gWIZ GFP pDNA:BCAT:DOPE lipoplex containing 10 mol% Mannose-PEG3000-DSPE. Lipofectamine was used as a positive control. The ratio of GFP fluorescence-positive cells is reported relative to Lipofectamine as 100%.
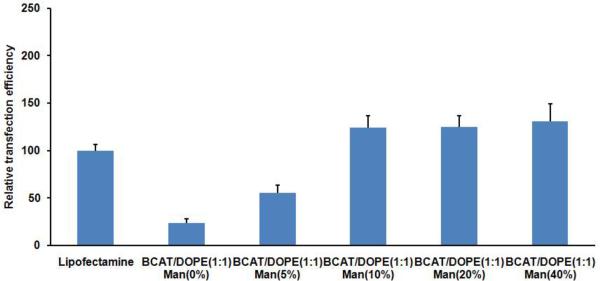
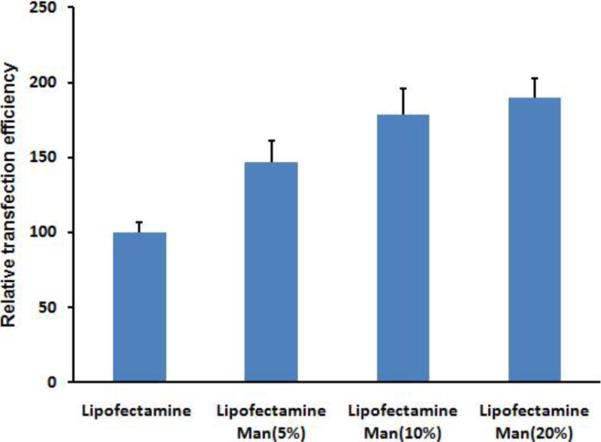
To examine the effects of mannose targeting of liposomes to dendritic cells, transfection experiments were next performed with different concentration of Mannose-PEG3000-DSPE in the liposome formulations at a 5:1 N:P ratio. After incubation of dendritic cells with liposomes of varying mannose content at 39 °C, the resulting cell-associated fluorescence detected by flow cytometry showed that increased mannose surface density produced increases in the observed transfection levels (Figure 4). For formulations containing 10 % Mannose-PEG3000-DSPE in BCAT:DOPE, there was a significant increase in transfection efficiency relative to non-mannosylated liposomes (Figure 5), however, increased Mannose-PEG3000-DSPE content did not provide further improvements in transfection efficiency. Transfection studies of Lipofectamine containing Mannose-PEG3000-DSPE showed that transgene expression efficiency also increased with increasing density of mannosylated lipid (Figure 6).
Figure 4.
Effect of liposomal Mannose-PEG3000-DSPE content in 1:1 BCAT:DOPE:gWIZ GFP pDNA lipoplex on DC transfection efficiency.
Figure 5.
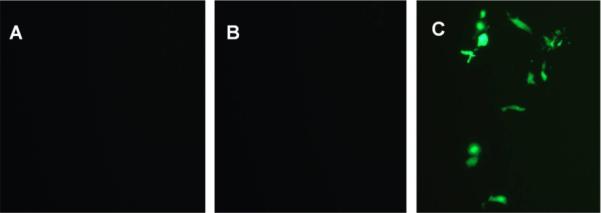
Fluorescence images of dendritic cells treated with (A) gWIZ GFP pDNA; (B) 1:1 BCAT:DOPE:gWIZ GFP pDNA lipoplex without Mannose-PEG3000-DSPE; and (C) 1:1 BCAT:DOPE:gWIZ GFP pDNA lipoplex with 10 mol% Mannose-PEG3000-DSPE.
Figure 6.
Effect of Mannose-PEG3000-DSPE content on Lipofectamine:gWIZ GFP pDNA lipoplex on DC transfection efficiency.
Taken together, these results suggest that targeted mannosylated formulations enhance interactions with the mannose receptors at the surface of dendritic cells, thereby promoting their internalization and expression of transgene. Mannose loading studies, however, revealed that the mannose concentration effect on transfection was saturated at 10 mol% Mannose-PEG3000-DSPE in the lipid formulation. We infer from these findings that the transfection complexes may not be capable of accommodating more than approximately 10 mol% of Mannose-PEG3000-DSPE in the BCAT:DOPE formulation due to steric demands of the PEG3000 moiety on the surface of the lipoplex.
Next, we explored the effect of 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) on DC transfection efficiency. It is well known that fusogenic liposomes are produced when DOPE is blended with other lipids.40–43 DOPE contributes to the formation of a stable lamellar phase with many different phospholipids, however, under acidic conditions, the bilayer is destabilized by DOPE via formation of an inverted hexagonal II phase, leading to the release of entrapped aqueous contents and fusion with proximal bilayer membranes.44–46 In addition, it is well known that the transfection efficiency of lipoplex can be boosted by incorporation of DOPE as a helper lipid in the formulation.47–49
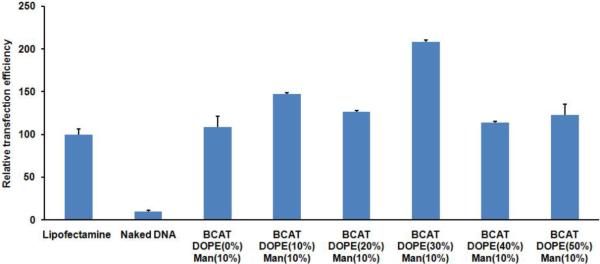
A series of 10 mol% Mannose-PEG3000-DSPE liposome compositions containing different BCAT:DOPE ratios (100:0, 90:10, 80:20, 70:30, 60:40, and 50:50) was formulated for transfection of gWIZ GFP plasmid in DCs using 10% Mannose-PEG3000-DSPE target ligand at a 5:1 N:P ratio (Figure 7). Among these targeted formulations, BCAT liposomes containing 30 mol % DOPE (ζ = 18.3 ± 2.7) showed a two-fold higher transfection efficiency compared to mannose-targeted Lipofectamine complexes. Further increases in DOPE molar ratio appeared to have little effect on transfection levels relative to 10% and 20% DOPE loadings.
Figure 7.
Effect of DOPE on transfection efficiency of DCs with gWIZ GFP plasmid.
Our data show that effective dendritic cell transfections are greatly dependent on the formulation ratio of BCAT to DOPE, with the 30% DOPE composition displaying the most promising results for the efficient delivery of transgenes to DC. These results are consistent with previous observations that cationic liposomes containing significant DOPE loadings display similarly high levels of transfection.47,50–53
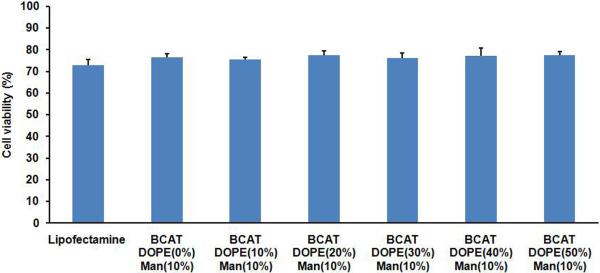
In Vitro Cytotoxicity
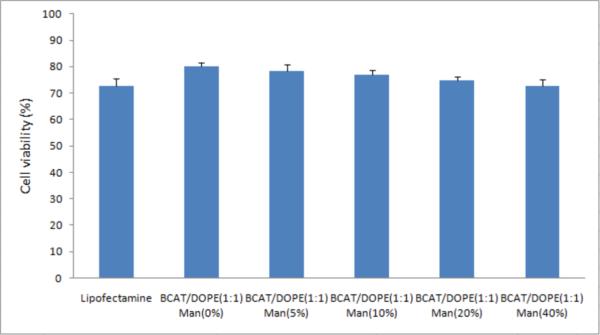
A standard MTS assay was used to examine the cytotoxicity of mannose-targeted BCAT:DOPE liposomes and Lipofectamine. After a 24 h exposure of cells to targeted formulations, the viabilities were found to be approximately 75% for both BCAT:DOPE and Lipofectamine. Increasing mannose density produced a modest 2–8% decrease in cell viability for both BCAT:DOPE and Lipofectamine (Figure 8), presumably due to enhanced uptake and internalization of the cationic lipids in these formulations.12 In contrast, cell viability was not affected by the density of DOPE in the formulation (Figure 9), even though DOPE was proven to significantly influence the transfection efficiency in DCs. This finding suggests that cationic liposomes containing 10 % of Mannose-PEG3000-DSPE in 6:3 BCAT:DOPE may be a good candidate for efficient DC transfection.
Figure 8.
BCAT:DOPE (1:1) cytotoxicity as a function of Mannose-PEG3000-DSPE density, evaluated by MTS assay. Cell viability was determined based on the observed viability normalized relative to untreated control cells as 100%.
Figure 9.
Cytotoxicity of 10% Mannose-PEG3000-DSPE liposomes at various BCAT:DOPE ratios, evaluated by the MTS assay. Cell viability was determined by normalizing the untreated control cells to 100%.
CONCLUSION
Various strategies, including targeted liposomes, have been used for non-viral gene delivery in dendritic cells to increase transfection efficiency. Our approach has focused on revealing a synergistic effect of mannose-based targeting, degradable cationic lipids, and fusogenic helper lipid in a transfection formulation that has not been described thus far. This work reports a novel synthetic method for the synthesis of Mannose-PEG3000-DSPE and BCAT, and the formulation of targeted mannosylated vesicles composed of mannosylated lipids, BCAT, and DOPE. We found that the transfection efficiency of BCAT:DOPE-based liposomes was dependent on the mannose density in the liposome up to 10% Mannose-PEG3000-DSPE. Our experiments also show that transfection efficiency is significantly enhanced by incorporating 30 mol% DOPE into BCAT formulations containing 10 mol% Mannose-PEG3000-DSPE. These findings suggest that Mannose-PEG3000-DSPE targeted BCAT:DOPE formulations may be effective and low toxicity non-viral vehicles for delivery of transgenes to dendritic cells for vaccine development.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by grants from the National Institutes of Health (GM 087016) and the Showalter Foundation.
REFERENCES
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 3.Mellman I, Steinman RM. Dendritic cells: Specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 4.Yuba E, Kojima C, Harada A, Tana, Watarai S, Kono K. pH-Sensitive fusogenic polymer-modified liposomes as a carrier of antigenic proteins for activation of cellular immunity. Biomaterials. 2010;31:943–951. doi: 10.1016/j.biomaterials.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 6.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu. Rev.Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 7.Stahl PD, Rodman JS, Miller MJ, Schlesinger PH. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc. Nat'l. Acad. Sci. USA. 1978;75:1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor PR, Gordon S, Martinez-Pomares L. The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005;26:104–110. doi: 10.1016/j.it.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Tan MCAA, Mommaas AM, Drijfhout JW, Jordens R, Onderwater JJM, Verwoerd D, Mulder AA, vanderHeiden AN, Scheidegger D, Oomen LCJM, Ottenhoff THM, Tulp A, Neefjes JJ, Koning F. Mannose receptor-mediated uptake of antigens strongly enhances HLA class II-restricted antigen presentation by cultured dendritic cells. Eur. J. Immunol. 1997;27:2426–2435. doi: 10.1002/eji.1830270942. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami S, Sato A, Nishikawa M, Yamashita F, Hashida M. Mannose receptor-mediated gene transfer into macrophages using novel mannosylated cationic liposomes. Gene Ther. 2000;7:292–299. doi: 10.1038/sj.gt.3301089. [DOI] [PubMed] [Google Scholar]
- 11.Hattori Y, Kawakami S, Lu Y, Nakamura K, Yamashita F, Hashida M. Enhanced DNA vaccine potency by mannosylated lipoplex after intraperitoneal administration. J. Gene Med. 2006;8:824–834. doi: 10.1002/jgm.910. [DOI] [PubMed] [Google Scholar]
- 12.Espuelas S, Thumann C, Heurtault B, Schuber F, Frisch B. Influence of ligand valency on the targeting of immature human dendritic cells by mannosylated liposomes. Bioconj. Chem. 2008;19:2385–2393. doi: 10.1021/bc8002524. [DOI] [PubMed] [Google Scholar]
- 13.Zhang ZP, Tongchusak S, Mizukami Y, Kang YJ, Ioji T, Touma M, Reinhold B, Keskin DB, Reinherz EL, Sasada T. Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials. 2011;32:3666–3678. doi: 10.1016/j.biomaterials.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 14.Molavi L, Mahmud A, Hamdy S, Hung RW, Lai R, Samuel J, Lavasanifar A. Development of a poly(D,L-lactic-co-glycolic acid) nanoparticle formulation of STAT3 inhibitor JSI-124: Implication for cancer immunotherapy. Mol. Pharmaceut. 2010;7:364–374. doi: 10.1021/mp900145g. [DOI] [PubMed] [Google Scholar]
- 15.Bal SM, Slutter B, van Riet E, Kruithof AC, Ding Z, Kersten GFA, Jiskoot W, Bouwstra JA. Efficient induction of immune responses through intradermal vaccination with N-trimethyl chitosan containing antigen formulations. J. Control. Rel. 2010;142:374–383. doi: 10.1016/j.jconrel.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Bal SM, Hortensius S, Ding Z, Jiskoot W, Bouwstra JA. Co-encapsulation of antigen and Toll-like receptor ligand in cationic liposomes affects the quality of the immune response in mice after intradermal vaccination. Vaccine. 2011;29:1045–1052. doi: 10.1016/j.vaccine.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 17.Plummer EM, Manchester M. Viral nanoparticles and virus-like particles: Platforms for contemporary vaccine design. WIREs Nanomed. Nanobiotechnol. 2011;3:174–196. doi: 10.1002/wnan.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powell TJ, Palath N, DeRome ME, Tang J, Jacobs A, Boyd JG. Synthetic nanoparticle vaccines produced by layer-by-layer assembly of artificial biofilms induce potent protective T-cell and antibody responses in vivo. Vaccine. 2011;29:558–569. doi: 10.1016/j.vaccine.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Rel. 2006;112:26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Hirosue S, Kourtis IC, van der Vlies AJ, Hubbell JA, Swartz MA. Antigen delivery to dendritic cells by poly(propylene sulfide) nanoparticles with disulfide conjugated peptides: Cross-presentation and T cell activation. Vaccine. 2010;28:7897–7906. doi: 10.1016/j.vaccine.2010.09.077. [DOI] [PubMed] [Google Scholar]
- 21.Boomer JA, Thompson DH, Sullivan SM. Formation of plasmid-based transfection complexes with an acid-labile cationic lipid: characterization of in vitro and in vivo gene transfer. Pharm. Res. 2002;19:1292–1301. doi: 10.1023/a:1020342523694. [DOI] [PubMed] [Google Scholar]
- 22.Timmons SC, Jakeman DL. Stereoselective chemical synthesis of sugar nucleotides via direct displacement of acylated glycosyl bromides. Org. Lett. 2007;9:1227–1230. doi: 10.1021/ol063068d. [DOI] [PubMed] [Google Scholar]
- 23.Hayes W, Osborn HMI, Osborne SD, Rastall RA, Romagnoli B. One-pot synthesis of multivalent arrays of mannose mono- and disaccharides. Tetrahedron. 2003;59:7983–7996. [Google Scholar]
- 24.Ladmiral V, Mantovani G, Clarkson GJ, Cauet S, Irwin JL, Haddleton DM. Synthesis of neoglycopolymers by a combination of “click” chemistry and living radical polymerization. J.Am. Chem. Soc. 2006;128:4823–4830. doi: 10.1021/ja058364k. [DOI] [PubMed] [Google Scholar]
- 25.Van den Bossche J, Shin J, Thompson DH. Improved plasmalogen synthesis using organobarium intermediates. J. Org. Chem. 2007;72:5005–5007. doi: 10.1021/jo0705059. [DOI] [PubMed] [Google Scholar]
- 26.Natale VAI, McCullough KC. Macrophage culture: influence of species-specific incubation temperature. J. Immunol. Methods. 1998;214:165–174. doi: 10.1016/s0022-1759(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 27.Carrasco CP, Rigden RC, Schaffner R, Gerber H, Neuhaus V, Inumaru S, Takamatsu H, Bertoni G, McCullough KC, Summerfield A. Porcine dendritic cells generated in vitro: morphological, phenotypic and functional properties. Immunology. 2001;104:175–184. doi: 10.1046/j.0019-2805.2001.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dominguez J, Ezquerra A, Alonso F, McCullough K, Summerfield A, Bianchi A, Zwart RJ, Kim YB, Blecha F, Eicher S, Murtaugh M, Pampusch M, Burger K. Porcine myelomonocytic markers: summary of the second international swine CD workshop. Vet.Immunol. Immunop. 1998;60:329–341. doi: 10.1016/s0165-2427(97)00109-8. [DOI] [PubMed] [Google Scholar]
- 29.Ezquerra A, Revilla C, Alvarez B, Perez C, Alonso F, Dominguez J. Porcine myelomonocytic markers and cell populations. Dev. Comp. Immunol. 2009;33:284–298. doi: 10.1016/j.dci.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Dowlut M, Hall DG, Hindsgaul O. Investigation of nonspecific effects of different dyes in the screening of labeled carbohydrates against immobilized proteins. J. Org. Chem. 2005;70:9809–9813. doi: 10.1021/jo051503w. [DOI] [PubMed] [Google Scholar]
- 31.Zalipsky S, Brandeis E, Newman MS, Woodle MC. Long circulating, cationic liposomes containing amino-PEG-phosphatidylethanolamine. FEBS Lett. 1994;353:71–74. doi: 10.1016/0014-5793(94)01013-7. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-Megia E, Correa J, Riguera R. “Clickable” PEG-dendritic block copolymers. Biomacromolecules. 2006;7:3104–3111. doi: 10.1021/bm060580d. [DOI] [PubMed] [Google Scholar]
- 33.Liu XM, Thakur A, Wang D. Efficient synthesis of linear multifunctional poly(ethylene glycol) by copper(I)-catalyzed huisgen 1,3-dipolar cycloaddition. Biomacromolecules. 2007;8:2653–2658. doi: 10.1021/bm070430i. [DOI] [PubMed] [Google Scholar]
- 34.Byk G, Dubertret C, Escriou V, Frederic M, Jaslin G, Rangara R, Pitard B, Crouzet J, Wils P, Schwartz B, Scherman D. Synthesis, activity, and structure-activity relationship studies of novel cationic lipids for DNA transfer. J. Med. Chem. 1998;41:224–235. doi: 10.1021/jm9704964. [DOI] [PubMed] [Google Scholar]
- 35.Cooper RG, Etheridge CJ, Stewart L, Marshall J, Rudginsky S, Cheng SH, Miller AD. Polyamine analogues of 3 beta-[N-(N',N '-dimethylaminoethane)carbomoyl]cholesterol (DC Chol) as agents for gene delivery. Chem. Eur. J. 1998;4:137–151. [Google Scholar]
- 36.Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, Border R, Ramsey P, Martin M, Felgner PL. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J. Biol. Chem. 1994;269:2550–2561. [PubMed] [Google Scholar]
- 37.Boomer JA, Thompson DH, Sullivan SM. Formation of plasmid-based transfection complexes with an acid-labile cationic lipid: characterization of in vitro and in vivo gene transfer. Pharmaceut Res. 2002;19:1292–1301. doi: 10.1023/a:1020342523694. [DOI] [PubMed] [Google Scholar]
- 38.Morales-Sanfrutos J, Megia-Fernandez A, Hernandez-Mateo F, Giron-Gonzalez MD, Salto-Gonzalez R, Santoyo-Gonzalez F. Alkyl sulfonyl derivatized PAMAM-G2 dendrimers as nonviral gene delivery vectors with improved transfection efficiencies. Org. Biomol. Chem. 2011;9:851–864. doi: 10.1039/c0ob00355g. [DOI] [PubMed] [Google Scholar]
- 39.Li C, Tian H, Rong N, Liu K, Liu F, Zhu YJ, Qiao RZ, Jiang YY. Chitosan grafted with macrocyclic polyamines on C-2 and C-6 positions as nonviral gene vectors: Preparation, characterization, and in vitro transfection studies. Biomacromolecules. 2011;12:298–305. doi: 10.1021/bm100819z. [DOI] [PubMed] [Google Scholar]
- 40.Holland JW, Cullis PR, Madden TD. Poly(ethylene glycol)-lipid conjugates promote bilayer formation in mixtures of non-bilayer-forming lipids. Biochemistry. 1996;35:2610–2617. doi: 10.1021/bi951999j. [DOI] [PubMed] [Google Scholar]
- 41.Johnsson M, Edwards K. Phase behavior and aggregate structure in mixtures of dioleoylphosphatidylethanolamine and poly(ethylene glycol)-lipids. Biophys. J. 2001;80:313–323. doi: 10.1016/S0006-3495(01)76016-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evjen TJ, Nilssen EA, Barnert S, Schubert R, Brandl M, Fossheim SL. Ultrasound-mediated destabilization and drug release from liposomes comprising dioleoylphosphatidylethanolamine. Eur. J. Pharm. Sci. 2011;42:380–386. doi: 10.1016/j.ejps.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Evjen TJ, Nilssen EA, Fowler RA, Rognvaldsson S, Brandl M, Fossheim SL. Lipid membrane composition influences drug release from dioleoylphosphatidylethanolamine-based liposomes on exposure to ultrasound. Int. J. Pharm. 2011;406:114–116. doi: 10.1016/j.ijpharm.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 44.Cullis PR, de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim. Biophys. Acta. 1979;559:399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez M, Aranda FJ, Teruel JA, Ortiz A. New pH-sensitive liposomes containing phosphatidylethanolamine and a bacterial dirhamnolipid. Chem. Phys. Lipids. 2011;164:16–23. doi: 10.1016/j.chemphyslip.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Bergstrand N, Arfvidsson MC, Kim J-M, Thompson DH, Edwards K. Interactions between pH-sensitive liposomes and model membranes. Biophys. Chem. 2003;104:361–379. doi: 10.1016/s0301-4622(03)00011-5. [DOI] [PubMed] [Google Scholar]
- 47.Bellavance MA, Poirier MB, Fortin D. Uptake and intracellular release kinetics of liposome formulations in glioma cells. Int. J. Pharm. 2010;395:251–259. doi: 10.1016/j.ijpharm.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 48.Vaidya B, Nayak MK, Dash D, Agrawal GP, Vyas SP. Development and characterization of site specific target sensitive liposomes for the delivery of thrombolytic agents. Int. J. Pharm. 2011;403:254–261. doi: 10.1016/j.ijpharm.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 49.Van den Bossche J, Al-Jamal WT, Yilmazer A, Bizzarri E, Tian BW, Kostarelos K. Intracellular trafficking and gene expression of pH-sensitive, artificially enveloped adenoviruses in vitro and in vivo. Biomaterials. 2011;32:3085–3093. doi: 10.1016/j.biomaterials.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 50.Bailey AL, Cullis PR. Membrane fusion with cationic liposomes: Effects of target membrane lipid composition. Biochemistry. 1997;36:1628–1634. doi: 10.1021/bi961173x. [DOI] [PubMed] [Google Scholar]
- 51.Hart SL. Lipid carriers for gene therapy. Curr. Drug Deliv. 2005;2:423–428. doi: 10.2174/156720105774370230. [DOI] [PubMed] [Google Scholar]
- 52.Medina-Kauwe LK, Xie J, Hamm-Alvarez S. Intracellular trafficking of nonviral vectors. Gene Ther. 2005;12:1734–1751. doi: 10.1038/sj.gt.3302592. [DOI] [PubMed] [Google Scholar]
- 53.Hoekstra D, Rejman J, Wasungu L, Shi F, Zuhorn I. Gene delivery by cationic lipids: in and out of an endosome. Biochem. Soc. Trans. 2007;35:68–71. doi: 10.1042/BST0350068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.