Abstract
The mature fullerene cone-shaped capsid of the human immunodeficiency virus 1 is composed of about 1,500 copies of the capsid protein (CA). The CA is 231 residues long, and consists of two distinct structural domains, the N-terminal domain and the C-terminal domain (CTD), joined by a flexible linker. The wild type CA exhibits monomer–dimer equilibrium in solution through the CTD–CTD dimerization. This CTD–CTD interaction, together with other intermolecular interdomain interactions, plays significant roles during the assembly of the mature capsid. In addition, CA–CA interactions also play a role in the assembly of the immature virion. The CA also interacts with some host cell proteins within the viral replication cycle. Thus, the capsid protein has been of significant interest as a target for designing inhibitors of assembly of immature virions and mature capsids and inhibitors of its interactions with host cell proteins. However, the equilibrium exhibited by the wild-type CA protein between the monomeric and dimeric states, along with the inherent flexibility from the interdomain linker, have hindered attempts at structural determination by solution NMR and X-ray crystallography methods. In this study, we have utilized a CA protein with W184A and M185A mutations that abolish the dimerization of CA protein as well as its infectivity, but preserve most of the remaining properties of the wild type CA. We have determined the detailed solution structure of the monomeric W184A/M185A-CA protein using 3D-NMR spectroscopy. Here, we present the detailed sequence-specific NMR assignments for this protein.
Keywords: HIV-1, Capsid protein, Monomeric mutant, 3D-NMR, Assignments
Biological context
The mature capsid of HIV-1 is a fullerene cone formed by hexamers of the capsid protein (CA) with twelve pentamers of CA closing both ends of the cone (Ganser-Pornillos et al. 2007). HIV-1 CA is 231 residues long, with two distinct domains, the N-terminal domain (NTD) and the C-terminal domain (CTD) joined by a highly flexible linker. The capsid surface consists of NTD domains in hexagonal and pentagonal rings stabilized by NTD–NTD interactions, with each ring linked to neighboring hexamers through intermolecular CTD–CTD dimerization (Gamble et al. 1997). Additional inter-domain interactions further stabilize the fullerene lattice (Pornillos et al. 2009; Byeon et al. 2009). CA also plays a significant role, as part of the Gag polyprotein, in the assembly of the immature virus particle (Wright et al. 2007). HIV-1 CA also interacts with host cell proteins such as cyclophilin A and lysyl tRNA synthetase in its replication cycle. Thus, there has been a significant interest in CA as a target to develop inhibitors of the early and late stage events within the viral replication cycle (Adamson and Freed 2010).
Methods and experiments
Plasmid WISP98-85 (from Dr. Peter Prevelige, Jr) encoding W184A/M185A HIV-1 CA was used. Its coding sequence was amplified with PCR to introduce NdeI/SalI cutting sites at 5′ and 3′ ends, respectively, and ligated with a NdeI and XhoI cut pET20B (EMD). This construct, pET20bHIV1CA, which is expected to translate a HIV-1CA with a start codon Met and a C-terminal His Tag, was transformed into E. coli Rosetta2 (EMD). Auto-induction medium P5052N25, in which the ammonium chloride concentration was reduced to 25 mM from 50 mM in 15N-HIV1CA. The reduction of ammonium chloride did not affect the yield of capsid protein production in E. coli. For 15N/13C labeling of HIV1CA, bacteria were grown in medium P040, with 25 mM 15N NH4Cl and 0.4% 13C glucose and all the inorganic salts in P5052N.
Overexpression was induced with 0.8 mM ITPG at OD600 nm 1.2–1.8 for 8 h at 37°C. E. coli adapted in 100% D2O were transferred to P040 containing 75% D2O for 2H/15N/13C labeling of HIV-1 CA. Protein was induced as above, for 12 h. Cells were harvested by centrifugation at 6,000×g for 10 min, and lysed using sonication. The lysate was centrifuged at 20,000×g for 30 min, and the supernatant retained.
The pellet was subjected to ammonium sulfate precipitation, and the pelleted material from the 65% fraction was retained. After ammonium sulfate precipitation, the pellet was solubilized in buffer containing 50 mM NaCl and 50 mM Tris (pH 8.0), and dialyzed in the same buffer. A Q-column (GE) was used for absorption of impurities from dialysate. Fractions of flow-through with purest HIV-1 CA content were pooled. His-tagged HIV-1 CA lysate was immobilized on colbalt-agarose beads (BD), and eluted using manufacturer’s recommendations.
Purified HIV-1 CA was dialyzed against buffer containing 25 mM deuterated sodium acetate (pH 5.5), 25 mM sodium chloride, 1 mM deuterated EDTA, 10 mM deuterated DTT, 0.02% sodium azide, and 0.1 mM AEBSF. After concentration with Centricon ultrafiltration devices, samples were made 10% D2O or lyophilized and resolubilized in 100% D2O.
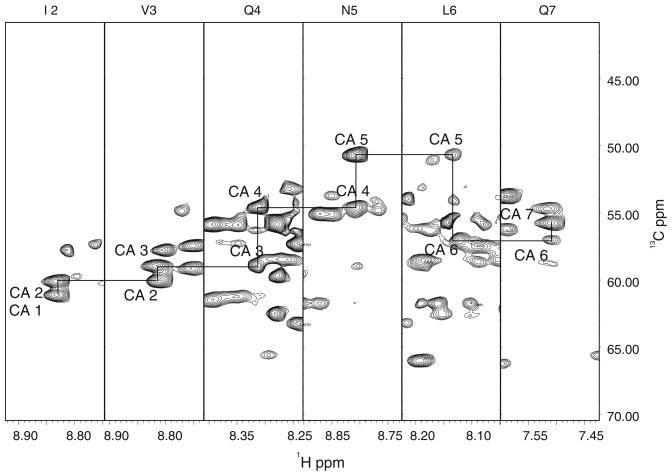
2D- and 3D-NMR measurements were performed at 30°C on a Bruker AVANCE 600 MHz NMR system equipped with a TCI-CryoProbe. Data were processed using NMRPipe (Delaglio et al. 1995). A squared sinebell with a 60° shift function was used in all dimensions, with no linear prediction. The processed spectra were analyzed using the program XEASY (Bartels et al. 1995). 1HN, 15N, 13Cα and 13Cβ resonances were assigned using sequential connectivities in the TROSY based HNCACB, CBCA (CO)NH, and HNCA spectra (Fig. 1). Side chain resonances were assigned using the spectra from the HCCH-TOCSY, HCCH-COSY, 15N NOESY-HSQC (125 ms), and 13C NOESY-HSQC (175 and 250 ms) experiments. Aromatic resonances were assigned using the spectra from the 15N NOESY-HSQC and 13C NOESY-HSQC experiments. 13C carbonyl resonances were assigned using the TROSY-HNCO experiment. Exchangeable amide protons were identified using a 15N SOFAST-HMQC experiment (Table 1).
Fig. 1.
Strip plot from the HNCA spectrum of the double mutant HIV1-CA showing sequential 13Cα connectivities for residues Ile 2–Gln 7. Connectivities between adjacent Cα nuclei are shown with solid lines
Table 1.
Summary of 2D/3D-NMR measurements
| Sample | Experiment | Scans | F3 | F2 | F1 |
|---|---|---|---|---|---|
| 1H/15N HIV1-CA DM, 10% D2O | TROSY-15N HSQC | 16 | 2,048 | 200 | |
| 15N NOESY-HSQC, 125 ms mixing time | 24 | 2,048 | 40 | 128 | |
| 1H/15N HIV1-CA DM, 100% D2O | SOFAST15N HMQC | 8 | 2,048 | 128 | |
| 1H/13C/15N HIV1-CA DM, 10% D2O | TROSY-HNCA | 16 | 2,048 | 40 | 100 |
| TROSY-HNCACB | 32 | 2,048 | 40 | 128 | |
| TROSY-CBCA(CO)NH | 32 | 2,048 | 40 | 128 | |
| TROSY-HNCO | 16 | 2,048 | 40 | 100 | |
| 1H/13C/15N HIV1-CA DM, 100% D2O | 13C HSQC | 16 | 2,048 | 256 | |
| 13C NOESY-HSQC, 175 mixing time | 24 | 2,048 | 64 | 128 | |
| 13C NOESY-HSQC, 250 mixing time | 24 | 2,048 | 64 | 128 | |
| HCCH COSY | 24 | 2,048 | 64 | 128 | |
| HCCH TOCSY | 24 | 2,048 | 64 | 128 | |
| 2H/13C/15N HIV1-CA DM, 10% D2O | TROSY-HNCACB | 32 | 2,048 | 40 | 128 |
| 2H/13C/15N HIV1-CA DM, 100% D2O | HCCH TOCSY | 32 | 2,048 | 64 | 128 |
The list of 3D-NMR and 2D-NMR experiments (at 600 MHz) that were utilized in the sequence-specific assignment of backbone and sidechain nuclei, as well as identification of structural constraints. Sample conditions were: protein concentration 1.2 mM, 303 K, 25 mM perdeuterated acetate (pH 5.5), 25 mM NaCl, 0.02% azide, and 0.1 mM AEBSF, 10 mM DTT. 15N-, 15N/13C- and 15N/13C/2H (70%)-labeled proteins were utilized as appropriate. DM refers to double mutant (W184A/M185A-CA)
This monomeric mutant CA assignments are consistent with previously published assignments of the isolated NTD and CTD domains (Tang et al. 2002; Wong et al. 2008), and solid state studies of the full-length protein (Chen and Tycko 2010; Han et al. 2010), and opens up new opportunities for full-length structure based design of antiviral inhibitors. The solution structure consists of a NTD domain with seven helices joined by a highly flexible five-residue linker to the CTD domain with five helices (including a short 310 helix). The first and last residues of the interdomain linker are part of the unwound helices of the two domains on either side. The middle three residues of the linker do not display any sequential NOEs that would be indicative of a bent structure, and their chemical shifts are characterized by random coil values.
Assignments and data deposition
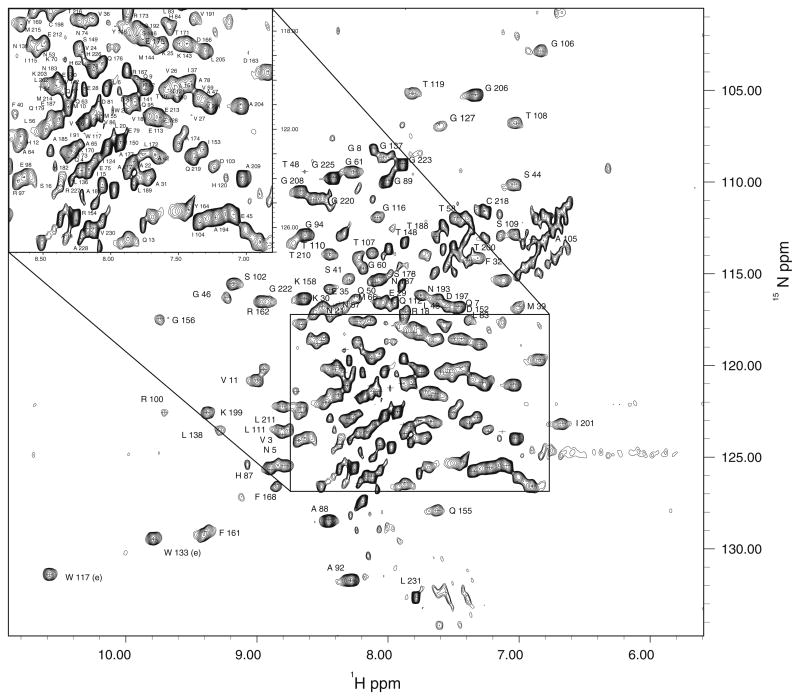
Backbone 1H and15N assignments were made for all non-proline residues of the protein (the residues in the C-terminal His-tag sequence were not assigned). All assignments are present in the HSQC experiment (Fig. 2). 13Cα and 13Cβ assignments were made for all residues except for P122 and R143. Excluding aromatic residues, 13Cγ, 13Cδ, and 13Cε resonance assignments were determined for 156/177, 85/90, and 13/19 nuclei, respectively. Carbonyl 13C resonances were determined for all residues except S16, S33, I37, T48, M55, M68, H84, G89, A92, E98, R100, N121, P122, I124, R132, S146, G156, E159, N195, G206, G223, L231, 17 of which precede proline residues.
Fig. 2.
Assigned 1H/15N gHSQC spectrum for the full length monomeric mutant HIV1-CA protein. All non-proline residues are labeled. The inset at upper left shows the assignment in the crowded region (box) in the full spectrum standard P5052N to save 15N label, was used to produce
1Hα and 1Hβ assignments were made for all residues except for P49. Excluding aromatic residues, 1Hγ, 1Hδ, and 1Hε assignments were made for 236/268, 103/127, and 25/28 nuclei, respectively.
The 1H, 13C, and 15N chemical shifts have been deposited at the BioMagResBank (http://www.bmrb.wisc.edu) under accession code 17738.
Acknowledgments
Support of this work by the NIAID Grants 1R21AI081591, 3R21AI081591-02S1, the NCI Grant 1P30 CA13148 that supported the NMR Facility, and the NCRR grant 1S10RR02 1064-01A1 for the 600 MHz CryoProbe are gratefully acknowledged. Some pilot 3D-NMR studies on a non-His-tag double mutant CA were initially performed at the University of Georgia’s 900 MHz NMR Facility funded by the NIGMS grant GM66340. The authors thank Prof. Peter Prevelige, Jr for the clone of the non-His-tag double mutant CA, and Dr. Clemens Anklin of Bruker Biospin Inc for the SOFAST-HMQC pulse sequence.
Contributor Information
Ronald Shin, Email: shinr@uab.edu, UAB High-Field NMR Facility, Comprehensive Cancer Center, University of Alabama at Birmingham, Birmingham, AL 35294, USA.
Ywh-Min Tzou, Department of Biochemistry and Molecular Genetics, University of Alabama at Birmingham, Birmingham, AL 35294, USA.
Hing C. Wong, Department of Biochemistry and Molecular Genetics, University of Alabama at Birmingham, Birmingham, AL 35294, USA
N. Rama Krishna, Email: nrk@uab.edu, UAB High-Field NMR Facility, Comprehensive Cancer Center, University of Alabama at Birmingham, Birmingham, AL 35294, USA. Department of Biochemistry and Molecular Genetics, University of Alabama at Birmingham, Birmingham, AL 35294, USA.
References
- Adamson CS, Freed EO. Novel approaches to inhibiting HIV-1 replication. Antiviral Res. 2010;85:119–141. doi: 10.1016/j.antiviral.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels C, Xia T, Billeter M, Guentert P, Wüthrich K. The program XEASY for computer-supported NMR spectral analysis of biological macromolecules. J Biomol NMR. 1995;6:1–10. doi: 10.1007/BF00417486. [DOI] [PubMed] [Google Scholar]
- Byeon I, Meng X, Jung J, Zhao G, Yang R, Ahn J, Shi J, Concel J, Aiken C, Zhang P, Gronenborn A. Structural convergence between Cryo-EM and NMR reveals intersubunit interactions critical for HIV-1 capsid function. Cell. 2009;139:780–790. doi: 10.1016/j.cell.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Tycko R. Structural and dynamical characterization of tubular HIV-1 capsid protein assemblies by solid state nuclear magnetic resonance and electron microscopy. Protein Sci. 2010;4:716–730. doi: 10.1002/pro.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Bax A. NMRPipe—a multidimensional spectral processing system based On UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Gamble TR, Yoo S, Vajdos F, von Schwedler U, Worthylake D, Wang H, McCutcheon J, Sundquist W, Hill C. Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science. 1997;278:849–853. doi: 10.1126/science.278.5339.849. [DOI] [PubMed] [Google Scholar]
- Ganser-Pornillos BK, Cheng A, Yeager M. Structure of full-length HIV-1 CA: a model for the mature capsid lattice. Cell. 2007;131:70–79. doi: 10.1016/j.cell.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Han Y, Ahn J, Concel J, Byeon I, Gronenborn A, Yang J, Polenova T. Solid-state NMR studies of HIV-1 capsid protein assemblies. J Am Chem Soc. 2010;6:1976–1987. doi: 10.1021/ja908687k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornillos O, Ganser-Pornillos BK, Kelly BN, Hua Y, Whitby FG, Stout CD, Sundquist WI, Hill CP, Yeager M. X-ray structures of the hexameric building block of the HIV capsid. Cell. 2009;137:1282–1292. doi: 10.1016/j.cell.2009.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Ndassa Y, Summers MF. Structure of the N-terminal 283-Residue Fragment of the HIV-1 Gag Polyprotein. Nat Struct Biol. 2002;9(7):537–543. doi: 10.1038/nsb806. [DOI] [PubMed] [Google Scholar]
- Wong HC, Shin R, Krishna NR. Solution structure of a double mutant of the carboxy-terminal dimerization domain of the HIV-1 capsid protein. Biochemistry. 2008;8:2289–2297. doi: 10.1021/bi7022128. [DOI] [PubMed] [Google Scholar]
- Wright ER, Schooler JB, Ding HJ, Kieffer C, Fillmore C, Sundquist WI, Jensen GJ. Electron cryotomography of immature HIV-1 virions reveals the structure of the CA and SP1 Gag shells. EMBO J. 2007;26:2218–2226. doi: 10.1038/sj.emboj.7601664. [DOI] [PMC free article] [PubMed] [Google Scholar]