Abstract
Glutamate-induced delayed calcium dysregulation (DCD) is a causal factor leading to neuronal death. The mechanism of DCD is not clear but Ca2+ influx via N-methyl-D-aspartate receptors (NMDAR) and/or the reverse plasmalemmal Na+/Ca2+ exchanger (NCXrev) could be involved in DCD. However, the extent to which NMDAR and NCXrev contribute to glutamate-induced DCD is uncertain. Here, we show that both NMDAR and NCXrev are critical for DCD in neurons exposed to excitotoxic glutamate. In rat cultured hippocampal neurons, 25μM glutamate produced DCD accompanied by sustained increase in cytosolic Na+ ([Na+]c) and plasma membrane depolarization. MK801 and memantine, noncompetitive NMDAR inhibitors, added shortly after glutamate, completely prevented DCD whereas AP-5, a competitive NMDAR inhibitor, failed to protect against DCD. None of the tested inhibitors lowered elevated [Na+]c or restored plasma membrane potential. In the experiments with NCX reversal by gramicidin, MK801 and memantine robustly inhibited NCXrev while AP-5 was much less efficacious. In electrophysiological patch-clamp experiments MK801 and memantine inhibited NCXrev-mediated ion currents whereas AP-5 failed. Thus, MK801 and memantine, in addition to NMDAR, inhibited NCXrev. Inhibition of NCXrev either with KB-R7943, or by collapsing Na+ gradient across the plasma membrane, or by inhibiting Na+/H+ exchanger with 5-(N-ethyl-N-isopropyl)amiloride (EIPA) and thus preventing the increase in [Na+]c failed to preclude DCD. However, NCXrev inhibition combined with NMDAR blockade by AP-5 completely prevented DCD. Overall, our data suggest that both NMDAR and NCXrev are essential for DCD in glutamate-exposed neurons and inhibition of individual mechanism is not sufficient to prevent calcium dysregulation.
Keywords: calcium dysregulation, NMDA receptor, Na+/Ca2+ exchanger, glutamate, neuron, excitotoxicity
INTRODUCTION
Glutamate excitotoxicity (Choi, 1992; Olney, 1969) is a critical factor in stroke, secondary brain injury following brain trauma, and various age-related neurodegenerative disorders (Greenamyre et al., 1999; Grunewald and Beal, 1999; Lipton, 2004; Mattson, 2003; Salinska et al., 2005). The mechanism of excitotoxicity is causally linked to a drastic elevation in cytosolic Ca2+ concentration ([Ca2+]c) (Kaku et al., 1993) that leads to cell injury and neuronal death (Berliocchi et al., 2005). Correspondingly, stabilizing low cytosolic Ca2+ by chelation with BAPTA1 increases the survival rate of neurons exposed to excitotoxic glutamate (Tymianski et al., 1993c; Tymianski et al., 1994). Thus, the sustained increase in [Ca2+]c, or “delayed calcium dysregulation” (DCD) (Tymianski et al., 1993a), represents an essential factor leading to neuronal death (Budd and Nicholls, 1996; Manev et al., 1989; Thayer and Miller, 1990; Tymianski et al., 1993b). However, despite extensive studies the precise molecular mechanisms leading to DCD remain poorly understood.
The NMDA-subtype of glutamate ionotropic receptors (NMDAR) is considered the major route for Ca2+ entry in neurons exposed to glutamate (Tymianski et al., 1993b). The augmented Ca2+ influx in glutamate-stimulated neurons is compensated, at least temporarily, by Ca2+ extrusion mediated by Na+/Ca2+ exchanger (NCX) (Carafoli et al., 2001). In neurons, NCX has the highest transport capacity for Ca2+, and therefore plays a key role in maintenance of calcium homeostasis (Carafoli et al., 2001). In the forward mode, NCX catalyzes an extrusion of 1 Ca2+ in the exchange for 3 Na+, and hence contributes to maintaining a low [Ca2+]c (Blaustein and Lederer, 1999). However, in neurons glutamate exposure causes collapse of the Na+ gradient and plasma membrane depolarization (Mayer and Westbrook, 1987; Tsuzuki et al., 1994; Yamaguchi and Ohmori, 1990) that result in a reversal of NCX (Kiedrowski et al., 1994). In this case, reverse operation of NCX (NCXrev) might contribute to sustained elevation in [Ca2+]c (Hoyt et al., 1998; Kiedrowski, 1999). KB-R7943, an inhibitor of NCXrev (Iwamoto et al., 1996), was used to demonstrate the contribution of NCXrev in NMDA-induced changes in [Ca2+]c (Czyz et al., 2002; Hoyt et al., 1998). However, KB-R7943 also inhibits NMDAR and mitochondrial Ca2+ accumulation (Brustovetsky et al., 2011; Sobolevsky and Khodorov, 1999) that confounds the results produced with this agent. Thus, the role of NCXrev in glutamate-induced DCD remains unclear.
In our previous study, we found that the NMDAR inhibitor, MK801, applied to neurons shortly after glutamate, completely prevented DCD (Brustovetsky et al., 2010). This finding supports the major role of NMDAR in DCD and apparently rejects the NCXrev hypothesis. Alternatively, this suggests that in addition to NMDAR MK801 also inhibits NCXrev. In the present study, we demonstrated for the first time that MK801 and memantine, non-competitive inhibitors of NMDAR (Chen et al., 1992; Huettner and Bean, 1988), inhibited NCXrev. In contrast, D-(−)-2-Amino-5-phosphonopentanoic acid (AP-5), a competitive NMDAR inhibitor (Clements and Westbrook, 1994), was much less efficacious. Neither NMDAR nor NCXrev inhibition alone was sufficient to preclude DCD in hippocampal neurons exposed to glutamate whereas combined inhibition of NMDAR and NCXrev was very effective in preventing DCD. Thus, our data suggest that both NMDAR and NCXrev are critical contributors to DCD in neurons exposed to glutamate and inhibition of both Ca2+ entry mechanisms is necessary to prevent DCD.
MATERIALS AND METHODS
Materials
Glutamate, glycine, EGTA, and gramicidin were purchased from Sigma (St. Louis, MO). Fura-2FF-AM and Fluo-4FF-AM were bought from Teflabs (Austin, TX) and Invitrogen (Carlsbad, CA), respectively. SBFI was purchased from Invitrogen as well. AP-5 and KB-R7943 were from Tocris (Ellisville, MO). KB-R7943 solutions were prepared daily and only used fresh on the day of the experiment. Sodium Green™ was obtained from Invitrogen. MK801 was purchased from Calbiochem (San Diego, CA), memantine was from Sigma (St.Lous, MO), 5-(N-ethyl-N-isopropyl)amiloride (EIPA) was from MP Biomedicals (Irvine, CA). Aninne-6plus was kindly provided by Dr. Michael Rubart (Indiana University School of Medicine, Indianapolis).
Cell culturing
Primary cultures of hippocampal neurons were prepared from postnatal day 1 rat pups according to Institutional Animal Care and Use Committee (IACUC) approved protocol and previously described procedures (Dubinsky, 1993). For fluorescence measurements, neurons were plated on glass-bottomed Petri dishes without preplated glia as previously described (Dubinsky, 1993). For all platings, 35 μg/ml uridine plus 15 μg/ml 5-fluoro-2′-deoxyuridine were added 24 hours after plating to inhibit proliferation of non-neuronal cells. Neuronal cultures were maintained in a 5% CO2 atmosphere at 37°C in Earl’s MEM supplemented with 10% NuSerum (BD Bioscience, Bedford, MA), 27 mM glucose and 26 mM NaHCO3 (Dubinsky et al., 1995).
Fluorescence imaging
In our experiments, rat cultured hippocampal neurons grown for 12–14 days in vitro (12–14 DIV) were used. The standard bath solution contained 139 mM NaCl, 3 mM KCl, 0.8 mM MgCl2, 1.8 mM CaCl2, 10 mM NaHEPES, pH 7.4, 5 mM glucose, and 65 mM sucrose. Throughout this study, sucrose was used to maintain osmolarity similar to that in the growth medium (340 mosm). Fluorescence imaging was performed with a Nikon Eclipse TE2000-U inverted microscope using a Nikon objectives Plan Fluor 20× 0.45 NA or Super Fluor 40× 1.3 NA and an EM-CCD Hamamatsu C9100–12 camera (Hamamatsu Photonic Systems, Bridgewater, NJ) controlled by Simple PCI software 6.1 (Compix Inc., Sewickley, PA). The excitation light was delivered by a Lambda-LS system (Sutter Instruments, Novato, CA). To minimize photobleaching and phototoxicity, the images were taken every 15 seconds during the time-course of the experiment.
For calcium imaging experiments, neurons were loaded with either 2.6 μM Fura-2AM (in the experiments shown in Suppl. Fig. 1) or 2.6 μM Fura-2FF-AM (in all other calcium imaging experiments except experiments with EIPA or with SBFI/Fluo-4FF co-loading) for 60 minutes at 37°C to follow changes in [Ca2+]c. The excitation filters (340±5 and 380±7 nm) were controlled by a Lambda 10–2 optical filter changer (Sutter Instruments, Novato, CA). Fluorescence was recorded from individual neurons through a 505 nm dichroic mirror at 535±25 nm. The changes in [Ca2+]c were monitored by following Fura-2FF F340/F380 ratio. Here and in all other experiments, background was subtracted from fluorescence signals. Alternatively, the changes in [Ca2+]c were monitored simultaneously with changes in [Na+]c using a Ca2+-sensitive fluorescent dye Fluo-4FF-AM and a Na+-sensitive dye SBFI-AM. Neurons were loaded simultaneously with 2.5 μM Fluo-4FF-AM for 30 minutes and 9 μM SBFI-AM for 1 hour at 37°C. The excitation wavelengths were 340±5 and 380±7 nm for SBFI and 480±20 nm for Fluo-4FF. Fluorescence was recorded from individual neurons through a 505 nm dichroic mirror at 535±25 nm. The changes in [Na+]c were monitored by following SBFI F340/F380 ratio. The changes in [Ca2+]c were monitored by following Fluo-4FF F480 and normalized as F/F0. [Ca2+]c and [Na+]c were calculated using Grynkiewicz method (Grynkiewicz et al., 1985) assuming Kd for Fura-2FF is 5.5 μM, for Fluo-4FF is 9.7 μM, and for SBFI is 11.3 mM. The low affinity Ca2+-sensitive dyes Fura-2FF and Fluo-4FF were chosen to provide accurate Ca2+ concentration measurements in micromolar range, which is typically reached in neurons after prolonged glutamate exposure. However, Ca2+ measurements in nanomolar range with these dyes are less accurate and, therefore, the resting physiological [Ca2+]c in non-stimulated neurons should be considered estimates and taken cautiously.
In the experiments with EIPA, we used a Ca2+-sensitive dye Fluo-4FF-AM and Na+-sensitive fluorescent dye Sodium Green™ (Invitrogen) because EIPA interfered with Fura-2FF and SBFI measurements (Zhang and Melvin, 1996). Neurons were loaded with 2μM Sodium Green™ for 30 minutes at 37°C. The excitation wavelength was 480±20 nm and fluorescence was recorded from individual neurons through a 505 nm dichroic mirror at 535±25 nm. The changes in [Na+]c were monitored by following Sodium Green™ F480 and [Na+]c were calculated using Grynkiewicz method (Grynkiewicz et al., 1985) assuming Kd for Sodium Green™ is 21 mM. Since the assumptions made in using Kd’s, the calculated values should be considered estimates of [Ca2+]c and [Na+]c.
For plasma membrane potential measurements, we used potential-sensitive fluorescent dye Annine-6plus (Fromherz et al., 2008). Neurons were loaded with 4 μM Annine-6plus for 5 minutes at room temperature. The excitation wavelength was 480±20 nm; fluorescence was recorded from individual neurons through a long pass 570 nm filter. The changes in plasma membrane potential were monitored by following Annine-6plus F480 and expressed as F/F0.
Glutamate measurements
The glutamate concentrations were measured in the neuronal bath solutions following gramicidin application. Glutamate concentrations were quantified as previously described (Wang et al., 2010). Briefly, glutamate was determined using the Amplex Red glutamic acid/glutamate oxidase assay kit (Invitrogen) according to the manufacturer’s instructions. This assay utilizes the production of H2O2 that occurs during oxidation of glutamate to α-ketoglutarate by glutamate oxidase to fuel conversion of Amplex Red to the highly fluorescent resorufin by horseradish peroxidase. Samples were incubated with the Amplex Red enzyme mixture for 30 minutes before fluorescence was measured using an excitation of 530 nm and emission of 590 nm in a Victor3 V plate reader (Perkin Elmer, Shelton, CT).
Electrophysiological patch-clamp experiments
Whole-cell patch-clamp recordings were conducted as described previously (Brustovetsky et al., 2011). Briefly, patch-clamp recordings were performed at room temperature using a HEKA EPC-10 double amplifier. Data was acquired using the Pulse program (HEKA Electronic, Lambrecht/Pfalz, Germany). When examining NMDA- and gramicidin-induced currents, the composition of the electrode (“intracellular”) solution was as follows: 140 mM CsF, 10 mM NaCl, 1.1 mM EGTA, and 10 mM HEPES, pH 7.3 adjusted with CsOH. The external solution was the same as in the fluorescence imaging experiments, except Mg2+ was omitted. The composition of the electrode solution used for recording voltage ramp currents mediated by NCX was as follows: 20 mM KCl, 100 mM K-aspartate, 20 mM tetraethylammonium-Cl, 10 mM HEPES, 0.01 mM K-EGTA, 4.5 mM MgCl2, and 4 mM Na-ATP, pH 7.3 adjusted with KOH (Smith et al., 2006). The external solution used for recording currents was as follows: 129 mM NaCl, 10 mM CsCl (to block K+ channels), 3 mM KCl, 0.8 mM MgCl2, 1.8 mM CaCl2, 5 mM glucose, 10 mM Na-HEPES, pH 7.2, 65 mM sucrose, 0.01 mM nifedipine (to block voltage-gated Ca2+ channels), 0.02 mM ouabain (to inhibit Na+/K+-ATPase), 0.001 mM tetrodotoxin (to block Na+ channels). A perfusion Fast-Step system (Warner Instruments, LLC, Hamden, CT) was used to deliver drugs focally onto isolated hippocampal neurons in the whole-cell configuration. All drugs were diluted in the bath solution. The bath solution across the cells was perfused at approximately 1 ml/min using gravity flow.
Statistics
Statistical analysis consisted of unpaired t-test or one-way ANOVAs followed by Bonferroni’s post hoc test (GraphPad Prism™ 4.0, GraphPad Software Inc., San Diego, CA). Every experiment was performed using at least three separate neuronal platings. All data are mean ± standard error of the mean (s.e.m.) of at least 3 independent experiments.
RESULTS
Prolonged exposure of neurons to glutamate resulted in a sustained elevation in [Ca2+]c, also known as delayed calcium dysregulation (DCD) (Tymianski et al., 1993a) (Fig. 1A–C). In these experiments, changes in [Ca2+]c and cytosolic Na+ concentration ([Na+]c) were followed simultaneously using Ca2+-sensitive fluorescent dye Fluo-4FF and Na+-sensitive dye SBFI. Figures 1A and B show representative pseudocolored calcium images of cultured neurons loaded with Fluo-4FF prior to and after exposure to 25 μM glutamate plus 10 μM glycine, respectively. Figures 1C–F show averaged Fluo-4FF and SBFI signals recorded from individual neurons and converted into [Ca2+]c and [Na+]c. Neither nifedipine (5 μM), nor ω-connotoxin (1 μM), inhibitors of L- and N-types of voltage-gated Ca2+ channels (VGCC), respectively, affected glutamate-induced DCD (not shown). CNQX (10–100 μM), an inhibitor of AMPA/kainate subtype of ionotropic glutamate receptors, also had no effect on glutamate-induced DCD (Brustovetsky et al., 2011). These data indicate that neither VGCC nor AMPA/kainate receptors contribute significantly to DCD in cultured hippocampal neurons exposed to glutamate.
Figure 1. Glutamate-induced increases in [Ca2+]c and [Na+]c. MK801 and memantine but not AP-5 prevented sustained elevation in [Ca2+]c. None of the tested inhibitors influenced glutamate-induced [Na+]c increase.
In all experiments, neurons were treated with 25 μM glutamate (Glu, plus 10 μM glycine) and 1 μM MK801, or 50 μM memantine, or 200 μM AP-5. Here and in all other experiments, 0.2% DMSO was used as a vehicle. The inhibitors were added 90 seconds following glutamate application. In A and B, pseudocolored images of cultured neurons taken prior to and after exposure to 25 μM glutamate plus 10 μM glycine, respectively. In C–F, simultaneous measurements of [Ca2+]c and [Na+]c in hippocampal neurons loaded with a Ca2+-sensitive fluorescent dye Fluo-4FF and a Na+-sensitive dye SBFI. The time scale shown in panel F is applicable to all traces in C–E. [Ca2+]c and [Na+]c were calculated using Grynkiewicz method (Grynkiewicz et al., 1985). Here and in other Figures, the traces show mean±s.e.m. from individual experiments (n=18–25 neurons per experiment). In G and H, statistical analyses of glutamate-induced [Ca2+]c and [Na+]c changes over time in dependence on the presence of different inhibitors. Data are mean ± s.e.m., *p<0.01 compared to vehicle, n=3.
Conversely, DCD was completely prevented by MK801 (1 μM) or memantine (50 μM) applied either prior to glutamate (not shown) or 90 seconds after glutamate (Fig. 1D,E). Because we were interested in the mechanisms of DCD, in most of our experiments inhibitors were applied shortly after glutamate just before onset of DCD. The strong inhibition of DCD with MK801 or memantine suggested that Ca2+ influx via NMDAR plays a major role in DCD consistent with the previous reports (Tymianski et al., 1993b). Surprisingly, AP-5 (20–200 μM) failed to prevent DCD (Fig. 1F). Figure 1G shows a statistical analysis of the calcium imaging experiments. Here and in other Figures, glutamate-induced changes in [Ca2+]c over time were quantified by calculating the area under the curve (AUC) as it has been done previously (Chang et al., 2006). All tested NMDAR antagonists completely inhibited NMDA-induced increases in [Ca2+]c with IC50=0.2±0.04 μM for MK801, 3.6±0.05 μM for memantine, and 2.2±0.03 μM for AP-5, respectively (Suppl. Fig. 1), and inhibited NMDA-induced ion currents measured in whole-cell voltage-clamp experiments (Suppl. Fig. 2) suggesting complete inhibition of NMDAR. MK801 (1 μM), memantine (10 μM), and AP-5 (20 μM) also completely prevented DCD induced by 30 μM NMDA (plus 10 μM glycine) (Suppl. Fig. 3), indicating that all tested NMDAR inhibitors were effective in inhibiting NMDAR. However, despite inhibition of NMDAR, AP-5, in contrast to MK801 and memantine, failed to prevent glutamate-induced DCD (Fig 1F).
In addition to DCD, glutamate produced sustained elevation in [Na+]c (Fig. 1C) and plasma membrane depolarization (Fig. 2B). Figure 2A shows changes in Annine-6plus fluorescence following step-wise plasma membrane depolarizations with increasing concentrations of KCl. MK801, memantine, and AP-5 neither attenuated the glutamate-induced increases in [Na+]c (Fig. 1D–F) nor prevented plasma membrane depolarizations (Fig. 2C–E). The changes in [Na+]c were assessed similar to changes in [Ca2+]c (Fig. 1G) by calculating AUC (Fig. 1H). The change in plasma membrane potential was evaluated by calculating the percentage of glutamate-induced decline in membrane potential compared to maximal depolarization produced by complete replacement of NaCl for KCl in the bath solution (Fig. 2F). In contrast to glutamate-induced alterations in [Na+]c and plasma membrane potential, MK801 (1 μM), memantine (10 μM), and AP-5 (20 μM) significantly lowered [Na+]c elevated after NMDA application (Suppl. Fig. 4) and completely prevented plasma membrane depolarization induced by 30 μM NMDA (plus 10 μM glycine) (Suppl. Fig. 5), confirming the effectiveness of the NMDAR inhibitors.
Figure 2. KCl- and glutamate-induced plasma membrane depolarizations in cultured hippocampal neurons. Neither MK801, nor memantine, nor AP-5 prevented membrane depolarization.
In A–E, the changes in plasma membrane potential were monitored by following Annine-6plus F480 and expressed as F/F0. Panel A shows changes in Annine-6plus fluorescence following step-wise plasma membrane depolarizations with increasing concentrations of KCl. In B–E, where indicated, neurons were treated with 25 μM glutamate (Glu, plus 10 μM glycine) and 200 μM AP-5, or 1 μM MK801, or 50 μM memantine. At the end of the experiments, NaCl in the bath solution was replaced by 142 mM KCl to completely depolarize plasma membrane. The time scale shown in panel E is applicable to all traces in A–D. In F, statistical analysis of glutamate-induced plasma membrane depolarizations over time expressed as percentage of plasma membrane potential under resting conditions. Data are mean ± s.e.m., *p<0.01 compared to vehicle, n=3.
The increase in [Na+]c and plasma membrane depolarization are the major pre-requisites for the reversal of NCX, which in the reverse mode brings Ca2+ into the cell in exchange for cytosolic Na+ (Hoyt et al., 1998; Kiedrowski et al., 1994). Keeping this in mind, complete prevention of the glutamate-induced DCD by MK801 and memantine suggested that either (i) NCXrev was not involved in glutamate-induced DCD, or (ii) MK801 and memantine, in addition to NMDAR, also inhibited NCXrev. In the following experiments we tested whether MK801 and memantine inhibited NCXrev. The reversal of NCX was triggered by treating neurons with gramicidin, a monovalent cation ionophore that increases plasma membrane permeability for Na+ and K+ but not for Ca2+ (Myers and Haydon, 1972). Gramicidin (5 μM) induced Na+ influx into the cytosol and depolarized neurons (Fig. 3A,B), providing conditions for NCX reversal and hence leading to the increase in [Ca2+]c due to NCXrev operation (Fig. 3C) (Brustovetsky et al., 2011; Newell et al., 2007). Gramicidin applied with perfusion (1 ml/min) also produced an increase in [Ca2+]c (Fig. 3D). MK801 and memantine very effectively inhibited gramicidin-induced increase in [Ca2+]c (Fig. 3E,F) with IC50=0.5±0.08 μM and 17.1±0.24 μM, respectively (Suppl. Fig. 6). AP-5, on the other hand, was potent (IC50=2.8±0.7 μM) but much less efficacious at blocking gramicidin-induced increases in [Ca2+]c (Fig. 3G).
Figure 3. Gramicidin reversed Na+/Ca2+ exchanger (NCX) leading to the increase in [Ca2+]c. MK801 and memantine but not AP-5 inhibited the reverse NCX.
In A and B, gramicidin (5 μM) produced an increase in [Na+]c and plasma membrane depolarization, respectively. The changes in [Na+]c were monitored by following SBFI F340/F380 ratio. The changes in plasma membrane potential were monitored by following Annine-6plus F/F0. The changes in [Ca2+]c were monitored by following Fura-2FF F340/F380 ratio. [Ca2+]c and [Na+]c were calculated using Grynkiewicz method (Grynkiewicz et al., 1985). In A–G, where indicated, neurons were treated with 5 μM gramicidin (Gram) and 1 μM MK801, or 50 μM memantine, or 200 μM AP-5. In D, neurons were perfused with the bath solution at 1 ml/min rate. In all experiments, bath solution was supplemented with 5 μM nifedipine, a blocker of voltage-gated Ca2+ channels, and 1 mM ouabain, an inhibitor of the Na+/K+-ATPase. The time scale shown in panel G is applicable to all traces in C–F. In H, statistical analysis of glutamate-induced [Ca2+]c changes over time in dependence on the presence of different inhibitors. Data are mean ± s.e.m., *p<0.01 compared to vehicle, n=3.
In addition to NCX reversal, the collapse of Na+ gradient across the plasma membrane could trigger reversal of Na+-glutamate co-transporter and a release of endogenous glutamate. Indeed, we found 0.97±0.07 μM glutamate (mean ± s.e.m., n=6) in the bath solution following 5 minutes exposure of neurons to 5 μM gramicidin compared to 0.18±0.07 μM glutamate (n=11) in the bath solution with untreated neurons. This glutamate concentration failed to induce sustained elevation in [Ca2+]c. However, gramicidin-induced collapse of Na+ gradient most likely inhibited NCX operation in the forward mode and, thus, hindered Ca2+ extrusion from the cell. If forward mode of NCX was turned off by replacing Na+ in the bath solution by equimolar N-methyl-D-glucamine (NMDG), 1μM glutamate produced sustained elevation in [Ca2+]c (Suppl. Fig. 7). Therefore, in the experiments with gramicidin we used a glutamate-pyruvate transaminase (GPT from pig heart, Roche, Indianapolis, IN) to eliminate glutamate from the bath solution. This enzyme catalyzes glutamate-pyruvate transamination reaction producing alanine and α-ketoglutarate and thus protecting neurons from glutamate excitotoxicity (Matthews et al., 2000; Matthews et al., 2003). The GPT (25 μg/ml plus 2 mM pyruvate) applied together with gramicidin reduced glutamate concentration in the bath solution to 0.14±0.08 μM (n=3). In parallel calcium imaging experiments, GPT plus pyruvate somewhat slowed down gramicidin-induced elevation in [Ca2+]c but failed to prevent it (Suppl. Fig. 8). Presumably, gramicidin-induced increase in [Ca2+]c involved Ca2+ influx via both NMDAR stimulated by the released glutamate and NCXrev. Consequently, the partial inhibition of gramicidin-induced [Ca2+]c increase by AP-5 could be due to inhibition of NMDAR (Fig. 3F). Overall, our data suggest that both MK801 and memantine, in addition to inhibiting NMDAR, strongly inhibit NCXrev whereas AP-5 inhibits NMDAR but not NCXrev.
In electrophysiological whole-cell, voltage-clamp experiments, repetitive voltage ramp protocols produced ion currents mediated predominantly by the NCXrev (Convery and Hancox, 1999; Watanabe and Kimura, 2000). These currents were strongly attenuated by 5 mM Ni2+ (Fig. 4B,C) and by 10 μM KB-R7943, both are NCXrev inhibitors (Iwamoto et al., 1996; Kimura et al., 1987). These currents were also inhibited by 10 μM MK801 and by 50 μM memantine, but not by 50 μM AP-5 (Fig. 4D,E) consistent with calcium imaging experiments with NCX reversal induced by gramicidin (Fig. 3C–F). Thus, electrophysiological data confirmed that MK801 and memantine inhibited NCXrev, whereas AP-5 was practically without effect.
Figure 4. The effects of Ni2+, MK801, memantine, and AP-5 on NCXrev-mediated ion currents recorded with cultured rat hippocampal neurons.
In A, the ascending voltage ramp protocol employed in these experiments and associated current obtained with and without 10 μM KB-R7943. In B–F, the effects of 5 mM Ni2+ (C), 10 μM MK801 (D), 50 μM AP-5 (E) and 50 μM memantine (F) on NCXrev-mediated ion currents. The effects of the inhibitors were calculated as percentage from the peak current without inhibitor taken as 100%. The time scale shown in panel F is applicable to all traces in B–E. For further details, see Materials and Methods.
In the following experiments, we evaluated the role of NCXrev in glutamate-induced DCD. Despite numerous off-target effects, KB-R7943 remains one of the most efficacious and widely used NCXrev inhibitors. Previously, we found that KB-R7943 inhibited NCXrev in hippocampal neurons with IC50 = 5.7±2.1 μM (Brustovetsky et al., 2011). In our experiments with glutamate-treated neurons, KB-R7943 (10 μM) failed to prevent DCD (Fig. 5A,B). KB-R7943 also inhibits NMDAR with IC50=13.4±3.6 μM (Brustovetsky et al., 2011), which is much higher than IC50 for MK801 (0.2±0.04 μM) and memantine (3.6±0.05 μM). Therefore, 10 μM KB-R7943 could not inhibit NMDAR completely. The NMDAR inhibition with 200 μM AP-5 also failed to prevent DCD (Fig. 5C). However, the combined application of 10 μM KB-R7943 and 20 μM AP-5 completely prevented DCD (Fig. 5D). Thus, inhibition of only one of Ca2+ entry mechanisms, either NMDAR with AP-5 or NCXrev with KB-R7943, was not sufficient to protect neurons against DCD.
Figure 5. Glutamate-induced sustained elevation in [Ca2+]c is inhibited by a combination of KB-R7943 and AP-5 but not by individual inhibitors applied separately.
In A–D, cultured hippocampal neurons were exposed to 25 μM glutamate (Glu, plus 10 μM glycine). Where indicated, 10 μM KB-R7943 or 200 μM AP-5, or a combination of 10 μM KB-R7943 and 20 μM AP-5 were applied. The time scale shown in panel C is applicable to all traces in A, B, and D. In E, statistical analysis of glutamate-induced [Ca2+]c changes over time in dependence on the presence of different inhibitors. Data are mean ± s.e.m., *p<0.01 compared to vehicle, n=3.
KB-R7943 has off-target effects that might confound the results obtained with this drug (Brustovetsky et al., 2011). Therefore, we looked for alternative approaches to turn off NCXrev. To achieve this, we replaced Na+ by K+ in the bath solution and released cytosolic Na+ by applying 5 μM gramicidin thus completely eliminating the Na+ gradient across the plasma membrane. This stopped NCX operation in both forward and reverse modes. Under these conditions glutamate produced instantaneous calcium dysregulation apparently due to a failure of NCX to extrude Ca2+ from the cytosol (Fig. 6A). Importantly, now AP-5 (20 μM) readily inhibited glutamate-induced calcium dysregulation (Fig. 6B). This observation confirms that both NMDAR and NCXrev have to be inhibited to prevent glutamate-induced calcium dysregulation and supports our hypothesis that both NMDAR and NCXrev contribute to DCD.
Figure 6. Inhibition of forward and reverse Na+/Ca2+ exchanger by eliminating Na+ gradient across the plasma membrane permits complete inhibition of glutamate-induced calcium dysregulation by AP-5.
In A and B, where indicated, NaCl in the bath solution was replaced by 142 mM KCl (−Na+/+K+). Then, neurons were treated with 5 μM gramicidin (Gram) to release cytosolic Na+. After that, neurons were exposed to 25 μM glutamate (Glu, plus 10 μM glycine) without (A) or with 20 μM AP-5 (B). In these experiments, bath solution was supplemented with 5 μM nifedipine and 1 mM ouabain. The time scale shown in panel B is applicable to the trace in A.
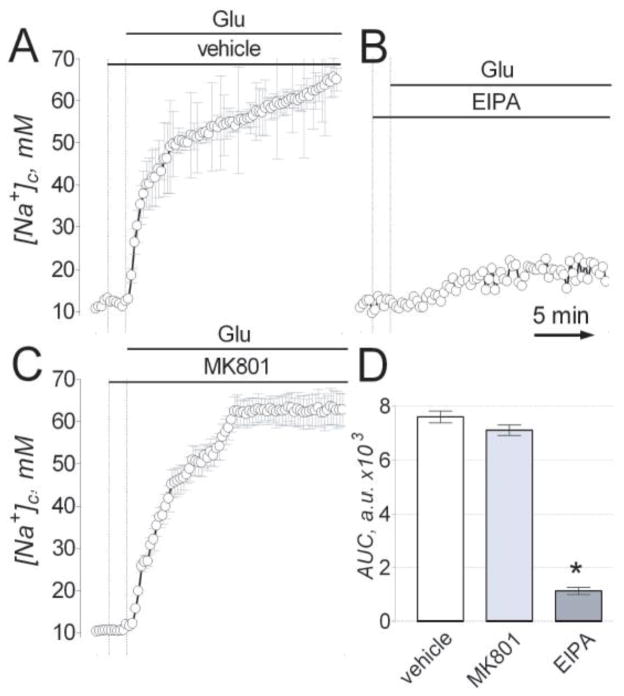
Our experiments with the Na+-sensitive fluorescent dye SBFI demonstrated that glutamate produced an increase in [Na+]c (Fig. 1). None of the tested NMDAR inhibitors prevented or attenuated this [Na+]c increase suggesting an alternative route for Na+ influx into cells. Recently, it has been shown that Na+/H+ exchanger (NHE) plays a central role in [Na+]c increase in neurons following oxygen/glucose deprivation (Luo et al., 2005). In our experiments, 5-(N-ethyl-N-isopropyl)amiloride (EIPA, 10 μM), an inhibitor of NHE (Vigne et al., 1983), significantly attenuated glutamate-induced increase in [Na+]c (Fig. 7A,B,D). The glutamate-induced changes in [Na+]c over time were quantitatively assessed by calculating the AUC (Fig. 7D) as it has been done previously for [Ca2+]c changes (Chang et al., 2006). In these experiments we used Na+-sensitive fluorescent dye Sodium Green™ because EIPA interferes with SBFI measurements (Zhang and Melvin, 1996). Similar to experiments with SBFI (Fig. 1B), MK801 failed to prevent or diminish glutamate-induced increase in [Na+]c (Fig. 7C).
Figure 7. EIPA but not MK801 inhibited the increase in [Na+]c induced by glutamate.
In AC, the changes in [Na+]c were monitored by following Sodium GreenTM fluorescence F480. Neurons were exposed to 25 μM glutamate (Glu, plus 10 μM glycine). Where indicated, neurons were treated with 10 μM MK801 (B) or 10 μM EIPA (C). The time scale shown in panel B is applicable to traces in A and C. In D, statistical analysis of glutamate-induced [Na+]c changes over time in dependence on the presence of MK801 and EIPA. Data are mean ± s.e.m., *p<0.01 compared to vehicle, n=3.
Next, we tested whether EIPA (10 μM) inhibited NMDAR and NCXrev and found that neither NMDA-induced nor gramicidin-induced increases in [Ca2+]c were affected by EIPA (Suppl. Fig. 9), suggesting that EIPA at the used concentration inhibited neither NMDAR nor NCXrev. Because the reversal of NCX requires an increase in [Na+]c, we hypothesized that inhibiting [Na+]c increase with EIPA should indirectly inhibit Ca2+ influx via NCXrev and thus EIPA could substitute for KB-R7943 in the combination with AP-5 (Fig. 5D). Indeed, whereas EIPA and AP-5 alone failed to prevent or attenuate glutamate-induced DCD (Fig. 8A–C), their combination completely prevented DCD (Fig. 8D). In these experiments, the EIPA was applied to neurons prior to glutamate to prevent [Na+]c increase. Thus, using three different experimental approaches to shut-off NCXrev we demonstrated that DCD in cultured hippocampal neurons exposed to glutamate depended on both NMDAR and NCXrev and that both mechanisms had to be inhibited to protect neurons against DCD.
Figure 8. Glutamate-induced sustained elevation in [Ca2+]c is inhibited by a combination of EIPA and AP-5 but not by individual inhibitors applied separately.
In A–D, cultured hippocampal neurons were exposed to 25 μM glutamate (Glu, plus 10 μM glycine). Where indicated, 10 μM EIPA (B) or 200 μM AP-5 (C), or a combination of 10 μM EIPA and 20 μM AP-5 (D) were applied. The time scale shown in panel D is applicable to all traces in A–C. In E, statistical analysis of glutamate-induced [Ca2+]c changes over time in dependence on the presence of different inhibitors. Data are mean ± s.e.m., *p<0.01 compared to vehicle, n=3.
DISCUSSION
The DCD is a critical event in glutamate excitotoxicity and therefore understanding of the mechanisms contributing to this phenomenon is of paramount importance. In the present paper, we found that the strong protection against DCD evoked by MK801 and memantine depended on inhibition of both NMDAR and NCXrev whereas inefficiency of AP-5 and KB-R7943 to protect against DCD correlated with their inability to inhibit either NCXrev or NMDAR. Thus, for the first time we demonstrated that both NMDAR and NCXrev play essential role in glutamate-induced DCD. Our results unify NMDAR and NCXrev hypotheses of DCD and explain the inefficiency of separate NMDAR and NCXrev inhibition in preventing DCD. We also demonstrated for the first time that NMDAR inhibitors, MK801 and memantine, are also potent NCXrev inhibitors. Importantly, the forward mode of NCX was not affected by these inhibitors (Brittain M.K., Brustovetsky, T., and Brustovetsky, N., unpublished data). The findings of this study add a new perspective to our understanding of the mechanisms of glutamate-induced DCD and provide a rationale for the inconsistency in neuroprotection afforded by different NMDAR inhibitors.
The prolonged exposure of neurons to glutamate causes overstimulation of glutamate receptors and produces a massive Ca2+ influx and significant elevation of [Ca2+]c (Tymianski et al., 1993b). Incoming Ca2+ can be extruded from the cytosol by Ca2+-ATPase in the plasma membrane or by NCX operating in the forward mode (Guerini et al., 2005). In addition, Ca2+ can be taken up by mitochondria (Bernardi, 1999). The Ca2+-ATPase has low transport capacity (Tymianski et al., 1993b), and therefore cannot compensate massive Ca2+ influx into neurons triggered by glutamate. The NCX, on the other hand, has a much greater Ca2+ transport capacity and therefore can more effectively clear elevated Ca2+ (Carafoli et al., 2001). However, glutamate-induced increase in [Na+]c and plasma membrane depolarization lead to a reversal of NCX that instead of extruding Ca2+ brings now more Ca2+ into the cell (Kiedrowski et al., 1994). In the cell, mitochondria play a significant role in clearing the elevated cytosolic Ca2+ (Herrington et al., 1996). However, mitochondrial Ca2+ uptake capacity is limited because of induction of the permeability transition pore (PTP) that precludes further Ca2+ accumulation (Chalmers and Nicholls, 2003; Li et al., 2009). Inhibition of the PTP induction by cyclosporin A or its non-immunosuppressive derivatives or by genetic ablation of the mitochondrial cyclophilin D defers calcium dysregulation and increases resistance of neurons to glutamate toxicity but cannot reliably protect neurons from DCD and cell death (Brustovetsky et al., 2009; Li et al., 2009). On the other hand, the extracellular space is practically an infinite source of Ca2+ and, therefore, attenuating Ca2+ entry from the outside of the cell seems the most effective way to protect neurons exposed to glutamate. However, this requires precise knowledge about Ca2+ entry routes into neurons exposed to glutamate and warrants extensive research in this direction.
In our study, inhibition of NMDAR and NCXrev appeared to be sufficient for preventing DCD and, therefore, we were focused on these mechanisms of Ca2+ influx into neurons exposed to glutamate. The stimulation of ionotropic glutamate receptors results in plasma membrane depolarization, eliminates voltage-dependent Mg2+ block of NMDAR, and maintains NMDAR activity permitting continuous divalent cation influx (Nowak et al., 1984; Planells-Cases et al., 2006). However, in electrophysiological experiments NMDAR is rapidly inactivated due to Ca2+-induced actin depolymerization (Legendre et al., 1993; Rosenmund and Westbrook, 1993) and therefore its role in DCD remains questionable. In addition to NMDAR, the NCXrev was proposed to significantly contribute to DCD by bringing Ca2+ into the cytosol in exchange for cytosolic Na+ (Hoyt et al., 1998; Kiedrowski et al., 1994). This hypothesis was based on the observations that glutamate considerably increases [Na+]c and depolarizes neurons, thus predisposing cells for NCX reversal. However, the extent to which NCXrev contributed to DCD in neurons exposed to glutamate was not completely elucidated. It was also not completely clear which of these two mechanisms – Ca2+ influx via NMDAR or via NCXrev – plays the major role in DCD.
In our previous study (Brustovetsky et al., 2010), MK801, a noncompetitive NMDAR inhibitor, prevented glutamate-induced DCD, consistent with the results from others (Stanika et al., 2009). These data suggested that NMDAR plays a key role in DCD. At the same time, these data strongly argued against NCXrev involvement in glutamate-induced DCD because MK801 is considered a selective NMDAR inhibitor. In the present paper, we found evidence that MK801 as well as memantine, both noncompetitive NMDAR inhibitors (Chen et al., 1992; Huettner and Bean, 1988), do in fact inhibit NCXrev, whereas AP-5, a competitive NMDAR inhibitor, did not. Moreover, inhibition of only one mechanism of Ca2+ entry into neurons – either NMDAR or NCXrev – appeared to be insufficient to prevent DCD, suggesting that both NMDAR and NCXrev play essential roles in the collapse of calcium homeostasis in neurons exposed to glutamate.
Sustained elevation in [Ca2+]c is causally linked to excitotoxic neuronal death (Kaku et al., 1993). Consequently, inhibition of sustained elevation in [Ca2+]c correlates with increased survival rate of neurons exposed to excitotoxic insult. For example, MK801 added after a short exposure to glutamate protected neurons from excitotoxic cell death (Brustovetsky et al., 2004), emphasizing NMDAR involvement in excitotoxicity. Memantine also has been successfully used in experiments to attenuate ischemia/reperfusion brain damage associated with excitotoxicity (Chen et al., 1992; Volbracht et al., 2006). In contrast to MK801 and memantine, AP-5, a competitive inhibitor of NMDAR, has been shown to be only moderately effective in neuroprotection against glutamate excitotoxicity (Levy and Lipton, 1990). Based on our data, this differential neuroprotective efficacy of NMDAR inhibitors could be due to the difference in their ability to inhibit NCXrev.
The NCXrev plays an important role not only in glutamate-induced DCD and excitotoxicity in neurons (Hoyt et al., 1998; Kiedrowski et al., 1994), but also in other pathological conditions such as ischemia/reperfusion heart injury (Feng et al., 2006; Ohtsuka et al., 2004) and traumatic spinal cord injury (Li et al., 2000). This exemplifies a significant need for potent and efficacious NCXrev inhibitors. However, since the introduction of KB-R7943 in 1996 (Iwamoto et al., 1996), only a few NCXrev inhibitors have been developed (Annunziato et al., 2004; Watanabe et al., 2006), warranting a more intense search for new NCXrev inhibitors. Our study serendipitously adds MK801 and memantine to the list of NCXrev inhibitors. Both MK801 and memantine appeared to be potent and efficacious in inhibiting NCXrev in neurons. It is likely that these agents will be also effective in inhibiting NCXrev in other cells (e.g. cardiomyocytes) and hence could be helpful not only in protecting neurons but other cells as well.
CONCLUSIONS
In the present study, we demonstrated that both NMDAR and NCXrev significantly contribute to DCD in glutamate-treated neurons. We found that strong protection against DCD evoked by MK801 and memantine correlates with strong inhibition of both NMDAR and NCXrev whereas inefficiency of AP-5 and KB-R7943 to protect against DCD correlates with their low efficacy to inhibit either NCXrev or NMDAR. Thus, our results unify NMDAR and NCXrev hypotheses of DCD and explain inefficiency of separate NMDAR and NCXrev inhibition in preventing DCD and protecting neurons against glutamate excitotoxicity.
Supplementary Material
Highlights.
AP-5 inhibits NMDA receptor but not reverse NCX in glutamate-treated neurons
MK801 and memantine inhibit both NMDAR and NCXrev in glutamate-exposed neurons
AP-5 does not prevent glutamate-induced delayed Ca dysregulation (DCD)
MK801 and memantine do prevent glutamate-induced DCD
Both NMDAR and NCXrev inhibition is necessary for preventing glutamate-induced DCD
Acknowledgments
This study was supported by the NIH/NINDS R01 NS 050131, by grant A70-0-079212 from Indiana State Department of Health - Indiana Spinal Cord and Brain Injury Research Fund, and by grant from Ralph W. and Grace M. Showalter foundation to NB. MKB was supported by AHA pre-doctoral fellowship 10PRE4300005 (Midwest Affiliate).
Footnotes
Abbreviations: BAPTA, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; DCD, delayed calcium dysregulation; NMDA, N-methyl-D-aspartate; NMDAR, NMDA receptor; NCX, Na+/Ca2+ exchanger; NCXrev, reverse NCX; EIPA, 5-(N-ethyl-N-isopropyl)amiloride; AP-5, D-(−)-2-amino-5-phosphonopentanoic acid; VGCC, voltage-gated calcium channels; AMPA, 2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl)propanoic acid; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; AUC, area under the curve; NHE, Na+/H+ exchanger; PTP, permeability transition pore; OGD, oxygen/glucose deprivation; 2-APB, 2-aminoethoxydiphenyl borate; TRP channels, transient potential receptor channels; ASICs, acid-sensing ion channels.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annunziato L, Pignataro G, Di Renzo GF. Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev. 2004;56:633–654. doi: 10.1124/pr.56.4.5. [DOI] [PubMed] [Google Scholar]
- Berliocchi L, Bano D, Nicotera P. Ca(2+) signals and death programmes in neurons. Philos Trans R Soc Lond B Biol Sci. 2005;360:2255–2258. doi: 10.1098/rstb.2005.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Brustovetsky T, Bolshakov A, Brustovetsky N. Calpain activation and Na(+)/Ca(2+) exchanger degradation occur downstream of calcium deregulation in hippocampal neurons exposed to excitotoxic glutamate. J Neurosci Res. 2010;88:1317–1328. doi: 10.1002/jnr.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustovetsky T, Brittain MK, Sheets PL, Cummins TR, Pinelis V, Brustovetsky N. KB-R7943, an inhibitor of the reverse Na+/Ca2+ exchanger, blocks N-methyl-D-aspartate receptor and inhibits mitochondrial complex I. Br J Pharmacol. 2011;162:255–270. doi: 10.1111/j.1476-5381.2010.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustovetsky T, Li V, Brustovetsky N. Stimulation of glutamate receptors in cultured hippocampal neurons causes Ca2+-dependent mitochondrial contraction. Cell Calcium. 2009;46:18–29. doi: 10.1016/j.ceca.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustovetsky T, Purl K, Young A, Shimizu K, Dubinsky JM. Dearth of glutamate transporters contributes to striatal excitotoxicity. Exp Neurol. 2004;189:222–230. doi: 10.1016/j.expneurol.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Budd SL, Nicholls DG. Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurochem. 1996;67:2282–2291. doi: 10.1046/j.1471-4159.1996.67062282.x. [DOI] [PubMed] [Google Scholar]
- Carafoli E, Santella L, Branca D, Brini M. Generation, control, and processing of cellular calcium signals. Crit Rev Biochem Mol Biol. 2001;36:107–260. doi: 10.1080/20014091074183. [DOI] [PubMed] [Google Scholar]
- Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem. 2003;278:19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- Chang DT, Rintoul GL, Pandipati S, Reynolds IJ. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol Dis. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Chen HS, Pellegrini JW, Aggarwal SK, Lei SZ, Warach S, Jensen FE, Lipton SA. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Clements JD, Westbrook GL. Kinetics of AP5 dissociation from NMDA receptors: evidence for two identical cooperative binding sites. J Neurophysiol. 1994;71:2566–2569. doi: 10.1152/jn.1994.71.6.2566. [DOI] [PubMed] [Google Scholar]
- Convery MK, Hancox JC. Comparison of Na+-Ca2+ exchange current elicited from isolated rabbit ventricular myocytes by voltage ramp and step protocols. Pflugers Arch. 1999;437:944–954. doi: 10.1007/s004240050866. [DOI] [PubMed] [Google Scholar]
- Czyz A, Baranauskas G, Kiedrowski L. Instrumental role of Na+ in NMDA excitotoxicity in glucose-deprived and depolarized cerebellar granule cells. J Neurochem. 2002;81:379–389. doi: 10.1046/j.1471-4159.2002.00851.x. [DOI] [PubMed] [Google Scholar]
- Dubinsky JM. Intracellular calcium levels during the period of delayed excitotoxicity. J Neurosci. 1993;13:623–631. doi: 10.1523/JNEUROSCI.13-02-00623.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky JM, Kristal BS, Elizondo-Fournier M. An obligate role for oxygen in the early stages of glutamate-induced, delayed neuronal death. J Neurosci. 1995;15:7071–7078. doi: 10.1523/JNEUROSCI.15-11-07071.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng NC, Satoh H, Urushida T, Katoh H, Terada H, Watanabe Y, Hayashi H. A selective inhibitor of Na+/Ca2+ exchanger, SEA0400, preserves cardiac function and high-energy phosphates against ischemia/reperfusion injury. J Cardiovasc Pharmacol. 2006;47:263–270. doi: 10.1097/01.fjc.0000202561.69291.ac. [DOI] [PubMed] [Google Scholar]
- Fromherz P, Hubener G, Kuhn B, Hinner MJ. ANNINE-6plus, a voltage-sensitive dye with good solubility, strong membrane binding and high sensitivity. Eur Biophys J. 2008;37:509–514. doi: 10.1007/s00249-007-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenamyre JT, MacKenzie G, Peng TI, Stephans SE. Mitochondrial dysfunction in Parkinson’s disease. Biochem Soc Symp. 1999;66:85–97. doi: 10.1042/bss0660085. [DOI] [PubMed] [Google Scholar]
- Grunewald T, Beal MF. Bioenergetics in Huntington’s disease. Ann N Y Acad Sci. 1999;893:203–213. doi: 10.1111/j.1749-6632.1999.tb07827.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Guerini D, Coletto L, Carafoli E. Exporting calcium from cells. Cell Calcium. 2005;38:281–289. doi: 10.1016/j.ceca.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996;16:219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- Hoyt KR, Arden SR, Aizenman E, Reynolds IJ. Reverse Na+/Ca2+ exchange contributes to glutamate-induced intracellular Ca2+ concentration increases in cultured rat forebrain neurons. Mol Pharmacol. 1998;53:742–749. [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem. 1996;271:22391–22397. doi: 10.1074/jbc.271.37.22391. [DOI] [PubMed] [Google Scholar]
- Kaku DA, Giffard RG, Choi DW. Neuroprotective effects of glutamate antagonists and extracellular acidity. Science. 1993;260:1516–1518. doi: 10.1126/science.8389056. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L. N-methyl-D-aspartate excitotoxicity: relationships among plasma membrane potential, Na(+)/Ca(2+) exchange, mitochondrial Ca(2+) overload, and cytoplasmic concentrations of Ca(2+), H(+), and K(+) Mol Pharmacol. 1999;56:619–632. doi: 10.1124/mol.56.3.619. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L, Brooker G, Costa E, Wroblewski JT. Glutamate impairs neuronal calcium extrusion while reducing sodium gradient. Neuron. 1994;12:295–300. doi: 10.1016/0896-6273(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Kimura J, Miyamae S, Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Rosenmund C, Westbrook GL. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci. 1993;13:674–684. doi: 10.1523/JNEUROSCI.13-02-00674.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DI, Lipton SA. Comparison of delayed administration of competitive and uncompetitive antagonists in preventing NMDA receptor-mediated neuronal death. Neurology. 1990;40:852–855. doi: 10.1212/wnl.40.5.852. [DOI] [PubMed] [Google Scholar]
- Li S, Jiang Q, Stys PK. Important role of reverse Na(+)-Ca(2+) exchange in spinal cord white matter injury at physiological temperature. J Neurophysiol. 2000;84:1116–1119. doi: 10.1152/jn.2000.84.2.1116. [DOI] [PubMed] [Google Scholar]
- Li V, Brustovetsky T, Brustovetsky N. Role of cyclophilin D-dependent mitochondrial permeability transition in glutamate-induced calcium deregulation and excitotoxic neuronal death. Exp Neurol. 2009;218:171–182. doi: 10.1016/j.expneurol.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004;1:101–110. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Chen H, Kintner DB, Shull GE, Sun D. Decreased neuronal death in Na+/H+ exchanger isoform 1-null mice after in vitro and in vivo ischemia. J Neurosci. 2005;25:11256–11268. doi: 10.1523/JNEUROSCI.3271-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H, Favaron M, Guidotti A, Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989;36:106–112. [PubMed] [Google Scholar]
- Matthews CC, Zielke HR, Parks DA, Fishman PS. Glutamate-pyruvate transaminase protects against glutamate toxicity in hippocampal slices. Brain Res. 2003;978:59–64. doi: 10.1016/s0006-8993(03)02765-3. [DOI] [PubMed] [Google Scholar]
- Matthews CC, Zielke HR, Wollack JB, Fishman PS. Enzymatic degradation protects neurons from glutamate excitotoxicity. J Neurochem. 2000;75:1045–1052. doi: 10.1046/j.1471-4159.2000.0751045.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers VB, Haydon DA. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972;274:313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- Newell EW, Stanley EF, Schlichter LC. Reversed Na+/Ca2+ exchange contributes to Ca2+ influx and respiratory burst in microglia. Channels (Austin) 2007;1:366–376. doi: 10.4161/chan.5391. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ohtsuka M, Takano H, Suzuki M, Zou Y, Akazawa H, Tamagawa M, Wakimoto K, Nakaya H, Komuro I. Role of Na+-Ca2+ exchanger in myocardial ischemia/reperfusion injury: evaluation using a heterozygous Na+-Ca2+ exchanger knockout mouse model. Biochem Biophys Res Commun. 2004;314:849–853. doi: 10.1016/j.bbrc.2003.12.165. [DOI] [PubMed] [Google Scholar]
- Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- Planells-Cases R, Lerma J, Ferrer-Montiel A. Pharmacological intervention at ionotropic glutamate receptor complexes. Curr Pharm Des. 2006;12:3583–3596. doi: 10.2174/138161206778522092. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Salinska E, Danysz W, Lazarewicz JW. The role of excitotoxicity in neurodegeneration. Folia Neuropathol. 2005;43:322–339. [PubMed] [Google Scholar]
- Smith GL, Elliott EE, Kettlewell S, Currie S, Quinn FR. Na(+)/Ca(2+) exchanger expression and function in a rabbit model of myocardial infarction. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S57–S63. doi: 10.1111/j.1540-8167.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI, Khodorov BI. Blockade of NMDA channels in acutely isolated rat hippocampal neurons by the Na+/Ca2+ exchange inhibitor KB-R7943. Neuropharmacology. 1999;38:1235–1242. doi: 10.1016/s0028-3908(99)00040-4. [DOI] [PubMed] [Google Scholar]
- Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc Natl Acad Sci U S A. 2009;106:9854–9859. doi: 10.1073/pnas.0903546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer SA, Miller RJ. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki K, Mochizuki S, Iino M, Mori H, Mishina M, Ozawa S. Ion permeation properties of the cloned mouse epsilon 2/zeta 1 NMDA receptor channel. Brain Res Mol Brain Res. 1994;26:37–46. doi: 10.1016/0169-328x(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Tymianski M, Charlton MP, Carlen PL, Tator CH. Secondary Ca2+ overload indicates early neuronal injury which precedes staining with viability indicators. Brain Res. 1993a;607:319–323. doi: 10.1016/0006-8993(93)91523-u. [DOI] [PubMed] [Google Scholar]
- Tymianski M, Charlton MP, Carlen PL, Tator CH. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J Neurosci. 1993b;13:2085–2104. doi: 10.1523/JNEUROSCI.13-05-02085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymianski M, Charlton MP, Carlen PL, Tator CH. Properties of neuroprotective cell-permeant Ca2+ chelators: effects on [Ca2+]i and glutamate neurotoxicity in vitro. J Neurophysiol. 1994;72:1973–1992. doi: 10.1152/jn.1994.72.4.1973. [DOI] [PubMed] [Google Scholar]
- Tymianski M, Wallace MC, Spigelman I, Uno M, Carlen PL, Tator CH, Charlton MP. Cell-permeant Ca2+ chelators reduce early excitotoxic and ischemic neuronal injury in vitro and in vivo. Neuron. 1993c;11:221–235. doi: 10.1016/0896-6273(93)90180-y. [DOI] [PubMed] [Google Scholar]
- Vigne P, Frelin C, Cragoe EJ, Jr, Lazdunski M. Ethylisopropyl-amiloride: a new and highly potent derivative of amiloride for the inhibition of the Na+/H+ exchange system in various cell types. Biochem Biophys Res Commun. 1983;116:86–90. doi: 10.1016/0006-291x(83)90384-4. [DOI] [PubMed] [Google Scholar]
- Volbracht C, van Beek J, Zhu C, Blomgren K, Leist M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur J Neurosci. 2006;23:2611–2622. doi: 10.1111/j.1460-9568.2006.04787.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Brittain JM, Wilson SM, Hingtgen CM, Khanna R. Altered calcium currents and axonal growth in Nf1 haploinsufficient mice. Transl Neurosci. 2010;1:106–114. doi: 10.2478/v10134-010-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Kimura J. Inhibitory effect of amiodarone on Na(+)/Ca(2+) exchange current in guinea-pig cardiac myocytes. Br J Pharmacol. 2000;131:80–84. doi: 10.1038/sj.bjp.0703527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Koide Y, Kimura J. Topics on the Na+/Ca2+ exchanger: pharmacological characterization of Na+/Ca2+ exchanger inhibitors. J Pharmacol Sci. 2006;102:7–16. doi: 10.1254/jphs.fmj06002x2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Ohmori H. Voltage-gated and chemically gated ionic channels in the cultured cochlear ganglion neuron of the chick. J Physiol. 1990;420:185–206. doi: 10.1113/jphysiol.1990.sp017907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GH, Melvin JE. Na+-dependent release of Mg2+ from an intracellular pool in rat sublingual mucous acini. J Biol Chem. 1996;271:29067–29072. doi: 10.1074/jbc.271.46.29067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.